Abstract
In the cosmetic industry there is an increasing demand for substances obtained from natural sources that can replace synthetic ones. Due to consumer demand for a protective filter with (SPF) labels in sunscreens, moisturizers, face make-up, and lipsticks worldwide, they produce tonnes of such products every year. Many species of cyanobacteria live in extreme environments, including sites with excessive doses of sunlight and drought. To survive in such extreme conditions, they produce compounds that allow both protection against ultraviolet radiation (UV), as well as the substances that are responsible for reducing oxidative stress. The aim of this study was to isolate, identify, and test the biological potential of the secondary metabolite scytonemin from the cyanobacterium Nostoc commune Vaucher ex Bornet et Flahault collected in Antarctica. The photoprotective effect was evaluated by the measurement of the sun protection factor (SPF) and the antioxidant activity was determined by two different assays including superoxide anion scavenging activity and free radical scavenging activity based on the amount of substance. An estimated SPF value of 33.34 ± 0.02 demonstrated that scytonemin might serve as a topically applicable ingredient for natural UV sunscreen cream.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Solar radiation is a significant environmental factor that contributes to skin aging and carcinogenesis (Brenner and Hearing 2008; Puizina-Ivic 2008; Singh et al. 2010). The importance of healthy skin leads to the creation of products designed to protect against harmful external and internal agents (Lodén 2014). Many of these products are classified as cosmetics and include different types such as skin creams, UV-protectors, anti-aging, and hypoallergenic products. Scientists are consistently confirming that cosmetic products made from natural compounds are useful and efficient in enhancing skin health (Mukherjee et al. 2011). They are also found to have fewer adverse effects and are environmentally sustainable (Cavinato et al. 2017).
In recent years, cyanobacteria have been considered an alternative supply of natural substances with applications in the cosmetic industry (Rastogi et al. 2015). Thanks to the comprehensive photosynthetic, adaptation, and defense system, cyanobacteria are able to produce various metabolites such as flavonoids, pigments (e.g. β-carotene, C-phycoerythrin, phycobiliproteins), phenols, saponins, steroids, tannins, terpenes and vitamins (Mulkidjanian et al. 2006; Stengel et al. 2011; Gangl et al. 2015). Cyanobacteria represent a great source of natural products that possess medicinal, industrial, and agricultural significance (Kiuru et al. 2014).
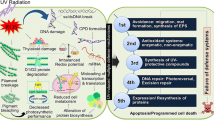
Cyanobacteria are photosynthetic prokaryotes with a long evolutionary history resulting in a wide range of species which can be found in different habitats (Whitton and Potts 2000). They appeared on Earth during the Precambrian era, approximately 2.8–3.5 billion years ago, and created the oxygen-rich environment necessary for the evolution of life. Many species of cyanobacteria are known to live in extreme environments, including regions with excessive sunlight exposure and drought. In order to survive in such harsh conditions, these organisms produce compounds that protect against ultraviolet radiation (UV) and reduce oxidative stress (Tamaru et al. 2005; Sinha and Häder 2008). Cyanobacteria have adapted to high levels of solar radiation by producing compounds such as mycosporine-like amino acids (MAAs) and scytonemin (SCY), which possess both photoprotective and antioxidant effects (Rastogi et al. 2010).
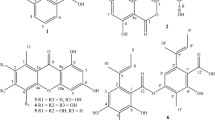
SCY is a yellow–brown dimeric molecule and it provides protection against UV radiation by absorbing light in the UV-B and UV-A ranges of the spectrum. SCY is a distinctive natural substance that consists of indolic and phenolic subunits—linked together by an olefinic carbon atom. This type of connection creates a novel ring structure, which has been named "the scytoneman skeleton" by (Proteau et al. 1993). In contrast to MAAs, SCY is exclusively produced by cyanobacteria and is located in the extracellular polysaccharide sheath (Rastogi et al. 2014). It is a stable compound that does not require additional energy to function, making it a useful defense mechanism for cyanobacteria when other protective methods are insufficient (Jones et al. 2011).
Solar ultraviolet radiation (UVR) consists of three types of UV radiation: UV-C (200–280 nm), UV-B (280–315 nm), and UV-A (315–400 nm). However, only UV-A and a fraction of UV-B can penetrate to the the Earth's surface (Vega et al. 2020). Of the three types, UV-B radiation is the most harmful since it causes mutations in the DNA of skin cells. On the other hand, UV-A radiation is not directly mutagenic but generates reactive oxygen species (ROS) that can cause mutations indirectly (Harrison and Young 2002; Ichihashi et al. 2003; Schuch et al. 2017). The skin has an internal mechanism called skin pigmentation that provides protection against damage from high levels of sun exposure (Brenner and Hearing 2008). Melanin is responsible for absorbing a wide range of UV radiation, and it also eliminates ROS, which are among the significant consequences of UV exposure on skin cells (Baumann 2005). As there is a growing demand for sunscreens to be included in lotions, moisturizers, facial makeup, and lipsticks (Daniel et al. 2004), global production of UV filters has increased dramatically each year (Manová et al. 2015).
Sunscreens can be classified based on the route of administration topical or systemic. Topical sunscreens are divided based on the mechanism of protection into sunscreen with organic or inorganic substances (Rigel 2013). They have primarily two mechanisms of action: reflection and scattering of UV energy from the skin surface (inorganic sunscreens—titanium dioxide and zinc oxide) (Dransfield 2000) or absorption of the UV energy by converting it to heat energy (organic sunscreens for UV-A region are benzophenones, methyl anthranilanate, etc.; for UV-B are PABA and its derivative, cinnamates, salicylates, etc.; both UV-A and UV-B include besoctrizole, silatriazole) (Dransfield 2000; Lademann et al. 2005; Tuchinda et al. 2006; Serpone et al. 2007; Manaia et al. 2013). It is suspected that synthetic organic filters may have some adverse effects on humans, including allergic reactions, photo-toxicity, and endocrine disruptions (Wang et al. 2016). These UV filters can also accumulate in the aquatic environment, leading to negative impacts such as coral reef bleaching or hormone disorders in mammals (Tsui et al. 2014; Sánchez-Quiles and Tovar-Sánchez 2015). Therefore, there is a strong focus on developing broad-spectrum biological photoprotectors that can filter UV-B, UV-A, blue light, and infrared while also providing antioxidant activity (Grether-Beck et al. 2014). Researchers are actively exploring natural photoprotectors because they have lower toxicity and are biodegradable, making them more beneficial for health and the environment (Saewan and Jimtaisong 2015). It might be interesting to test the potential application of cyanobacteria in skin care products to reinforce their role as a source of different bioactive compounds. Furthermore, the increased understanding, participation, and concern of citizens regarding the negative impacts of synthetic compounds have made the research and development of natural compounds more appealing.
In view of the above, the aim of this study was to isolate and test the biological potential of the biomolecule SCY isolated from the cyanobacterium Nostoc commune Vaucher ex Bornet et Flahault, as well as develop of new sunscreen enriched with natural compound. Therefore, we prepared sunscreen with a SCY organic UV filter and focused on determining the stability of a potentially new scytonemin sunscreen as well as other selected properties.
Material and methods
Collection of cyanobacteria and isolation of scytonemin
Antarctic cyanobacterial samples of Nostoc commune were collected randomly (during January 2020) at James Ross Island, Antarctica close to the Czech Antarctic station of J. G. Mendel (63° 48′ 02″ S, 57° 52′ 57″ E).
Scytonemin (SCY) was extracted and isolated as described previously (Balskus et al. 2011). Briefly, 5 g (DW) of N. commune was dissolved in 100 mL acetone (CentralChem) and kept overnight at laboratory temperature on a magnetic stirrer. The acetone extract was filtered with a 42 μm nylon mesh filter to a round bottom flask and the supernatant was evaporated under reduced pressure at 40 °C by a rotary evaporator. The residue was washed with 2 mL of cyclohexane 6 times to remove carotenoids and chlorophylls; the resulting light green solution was removed and kept aside, while the remaining brown substance was dried and stored in a dark for further NMR analysis.
Nuclear magnetic resonance (NMR) spectroscopy
The structure of the compound (scytonemin) was verified by NMR spectra. Nuclear magnetic resonance data were collected on spectrometer Varian VNMRS 600 (USA) operating at 599.87 MHz for 1H and 150.84 MHz for 13C. Chemical shifts (δ in ppm) are given from the internal solvent and the partially deuterated residual: acetone-d5 2.05 ppm for 1H and acetone-d6 29.84 ppm for 13C. All data were analyzed using MNova 14.2.1 (2021) software. Due to the low photostability of scytonemin, it was not possible to measure 13C NMR spectrum and 2D experiments of sufficient quality.
Antioxidant activity
Free radical scavenging activity by the DPPH method
The free radical scavenging activity of scytonemin was measured using 1,1-diphenyl-2-picryl-hydrazil (DPPH). The antioxidant activity via this method is described in several studies, and here, we slightly modify it (Dorman et al. 2003; Ibañez et al. 2003; Kosanić et al. 2011). Scytonemin was dissolved in methanol to a concentration of 0.54 mg mL−1 (1 mM). Stock solution was subsequently diluted to desired concentrations (0.5, 0.25, 0.125, 0.0625 mM). The reaction mixture consisted of 2 mL of DPPH methanolic solution (0.1 mM) and 1 mL of scytonemin solution. The samples were incubated at laboratory in the dark for 30 min. After the incubation, the absorbance of the samples was measured at 517 nm (multi-detection microplate reader; the Synergy HT, BioTek). Ascorbic acid was used as a positive control. Methanol was used as a blank control. The DPPH radical concentration was calculated by the equation:
where A0 is the absorbance of negative control, and A1 is the absorbance of the reaction mixture of our samples. Half maximal effective concentration (EC50) was used to compare the radical scavenging activity.
Superoxide anion scavenging activity
The superoxide anion scavenging activity of scytonemin was measured according to the Nishimiki method (Nishikimi et al. 1972) as used by Ranković (2015) with slight modification. In brief, scytonemin was dissolved in 5% DMSO to a concentration of 0.54 mg mL−1 (1 mM). Stock solution was subsequently diluted to desired concentrations. A portion (100 μL) of each prepared sample was mixed with 1 mL NADH (468 μM nicotinamide adenine dinucleotide solution in 0.1 M phosphate buffer pH 7.4) and 1 mL of NBT (156 μM nitroblue tetrazolium solution in 0.1 M phosphate buffer pH 7.4). The reaction was started by adding 100 μL PMS (60 μM phenazine methosulfate solution dissolved in 0.1 M phosphate buffer pH 7.4). The mixture was incubated at laboratory temperature in the dark for 5 min. Then the absorbance was measured at 560 nm (multi-detection microplate reader; the Synergy HT, BioTek). The superoxide anion scavenging activity was calculated by the equation:
where A0 is the absorbance of negative control and A1 is the absorbance of the reaction mixture of our samples. Half maximal effective concentration (EC50) was used to compare the superoxide anion scavenging activity.
Photoprotective activity
Determination of sun protection factor (SPF) of scytonemin
Scytonemin was dissolved in ethanol to a concentration of 1 mg mL−1 and analyzed for in vitro sun protection factor (SPF). Samples were analyzed for their absorption spectra (multi-detection microplate reader; the Synergy HT, BioTek) in the wavelength range of 290 to 320 nm, with ethanol serving as a blank. The absorption data were collected every 5 nm and five readings were taken at each wavelength. After obtaining the data, the SPF value was calculated using the equation of (Mansur et al. 1986):
where, EE: erythemal efficiency spectrum at wavelength λ; I: intensity of solar light at wavelength λ; Abs: absorbance of wavelength λ by a solution of the preparation; CF: correction factor (= 10). The values of EE x I are constants determined by Sayre et al. (1979) and are shown in Table 1
Preparation of the cream
The non-ionic cream base was chosen due to its compatibility with the tested active ingredient. The cream was reformulated according to the literature (Kovács et al. 2020) and occlusive components were replaced with semi-occlusive for better skin tolerance. This cream supported the barrier function of the skin without occlusion, had adequate consistency, a longer-lasting hydration effect, was easily spread and had a proper value of pH (Kovács et al. 2020).
The oil phase ingredients and water phase ingredients were separately heated to 70 ± 5 °C and homogenized. Then water phase was added to the oil phase and phases were stirred in a mortar until cooled. A preservative was added at the temperature of 30 ± 1 °C. All ingredients are listed in Table 2. Active ingredient scytonemin was at 0.1% concentration.
Cream stability study
Two samples were prepared for cream stability studies. One sample was a cream base (CB) and the second was a cream base with scytonemin ingredient (CB + SCY). These two samples are named as a set in the following text. Set of the creams was stored in a refrigerator at 8 ± 1 °C and 56% relative humidity (RH) in a closed vessel, was left in a laboratory at 22 ± 1 °C, 58% RH in a closed vessel, and was stored in an incubator at 40 ± 1 °C, 75% RH in an opened vessel. Subsequently, the set of creams was evaluated in terms of physical appearance, pH, viscosity, spreadability and phase separation at 3 time intervals (day of preparation, 2 weeks, and 1 month) during 30 days (Shantanu et al. 2011; Sah et al. 2017; Smaoui et al. 2017; ICH 2018).
Physical appearance and pH determination
The set of cream samples was organoleptically tested in terms of color and odor (Shantanu et al. 2011; Sah et al. 2017; Smaoui et al. 2017).
The pH of the cream formulations set was determined using a digital pH meter (Hanna HI 2211). Amount of 1.00 g cream base was dissolved in 25 mL deionized water. The pH values were measured at the temperature of 22 ± 1 °C after the sample preparation. Other samples from the set were similarly prepared. The first set was placed in a refrigerator (8 ± 1 °C and 56% RH), the second was left in the laboratory (22 ± 1 °C, 58% RH), and the third was placed in an incubator (40 ± 1 °C, 75% RH). The pH values of all sets were measured after two weeks and then after one month (Shantanu et al. 2011; Sah et al. 2017; Smaoui et al. 2017).
Viscosity measurement
The viscosity of the cream sets was measured at 22 ± 1 °C by a digital rotational viscometer (Fungilab ViscoLead One) with spindle R6 just after their preparation. Then, similar to the measurement of pH values, the first set was placed in a refrigerator (8 ± 1 °C and 56% RH), the second was left in the laboratory (22 ± 1 °C, 58% RH), and the third was placed in an incubator (40 ± 1 °C, 75% RH). The viscosity values of all sets were measured after two weeks and then after one month. After removal from the refrigerator and incubator, they were left at room temperature for approximately 15 min before each measurement. The spindle was rotated at 1, 2.5, 5, 10 and 20 RPM (Chen et al. 2016).
Spreadability test
An amount of 1.00 g base cream was applied between the two glass plates (20 × 20 cm) in a circular area with a 1 cm diameter. A 500 g weight was applied on the top of the glass plate. After 5 min, the change in the diameter was measured. Other samples from the set were similarly prepared. Then, similar to pH and viscosity measurements, the first set was placed in a refrigerator (8 ± 1 °C and 56% RH), the second was left in the laboratory (22 ± 1 °C, 58% RH), and the third was placed in an incubator (40 ± 1 °C, 75% RH). The spreadability values of all sets were measured after two weeks and then after one month. Spreadability, the area up to which the cream was spread, was calculated using the equation:
where S = spreadability [g cm s−1], l = area of cream spread on the glass plate [cm], m = weight [g] and t = time [s]) (Parija et al. 2012; Sah et al. 2017; Smaoui et al. 2017).
Centrifugation test
The amount of 1.00 g of each cream sample from the set in a 1.5 mL centrifuge tube was centrifuged at 4000 rpm for 10 min. After centrifugation, the occurence of phase separation was tested (Shantanu et al. 2011; Sah et al. 2017; Smaoui et al. 2017).
Results
Identification of scytonemin by NMR
The studied compound was identified as scytonemin, and the NMR data are as follows: full assignment of 1H and 13C resonances (Table 3 )and the chemical structure of scytonemin (Fig. 1).
Antioxidant activity of scytonemin
The results of the antioxidant activity are presented in Figs. 2 and 3. In general, it shows how the free radical and superoxide anion scavenging ability of extracted scytonemin changes depending on the amount used. At concentrations of 20.83 µM and 333.3 µM, the scavenging of free radicals of scytonemin increased by 9.28% and 62.84% respectively. The dose- dependent superoxide anion scavenging activity of scytonemin was 10.28% and 69.13% at concentrations of 2.84 µM and 45.46 µM. The EC50, which is the concentration at which 50% effectiveness is achieved, was determined to be 250.56 µM for free radical activity and 25.94 µM for the superoxide anion scavenging test.
Both antioxidant assays suggest that scytonemin is effective at removing radicals as compared to ascorbic acid used as a positive control. The free radical scavenging activity of ascorbic acid ranged between 30 and 98%, while superoxide anion scavenging activity ranged from 25 to 89%.
Photoprotective activity of scytonemin
As shown in Table 4, the scytonemin showed a high SPF (33.34 ± 0.02), with high absorbance values that ranged between 3.997 and 2.801 at λ = 290–320 nm.
Creams stability evaluation
Physical appearance
The results of cream samples appearance are summarized in Table 5. No visible changes were observed in the case of the cream base during the period of testing and it appeared to be stable. Nonetheless, the color of the cream containing scytonemin was slightly changed in dependence on the temperature and humidity conditions. The most significant change was in cream with scytonemin stored in the open vessel at 40 ± 1 °C; 75% RH already after two weeks. In all cream samples no odor was observed during the tested period.
pH
The pH of the tested creams pH was slightly changed in the acidic region from 4.73 to 5.98 (Fig. 4). These values match with the pH range of normal skin and it do not cause any skin irritation (Ambala and Vemula 2015). The pH values slightly varied depending on the active ingredient composition as well as within the testing period. While the cream base has a pH of around 5, the pH shifts to a higher value of around 6 for the sample containing CB + SCY. Moreover, the pH values were not significantly influenced by either temperature or humidity (Fig. 4).
Viscosity measurement
Viscosity is the ability of a fluid to keep its shape when a force is applied and provides important information about the colloidal structure of various chemical systems, mainly emulsions and particle dispersions. Thus, viscosity can be used to evaluate the colloidal stability of sunscreens (Saito et al. 2019). To evaluate the SCY influence on its sunscreen cream base colloidal stability, we determined the viscosity of the cream base and cream base with SCY ingredient. The viscosity of the creams decreases with increasing spindle RPM values (Fig. 5). Thus, all studied samples had pseudoplastic behaviour. A similar observation was described by Saito et al. (2019). The creams composition slightly influences the viscosity already immediately after their preparation (brown bars Fig. 6). Moreover, after two weeks storage of the cream sets in the refrigerator (8 °C, 56% RH), in a laboratory at room temperature (22 °C, 58% RH), and in an incubator (40 °C, 75% RH) the viscosity increased significantly in the case of the cream base as well as the CB + SCY cream (dark red, red and pink bars Fig. 6). A similar trend was observed in the case of cream sets stored for one month under the same conditions (temperature, humidity), with the exception of the cream stored in the laboratory at room temperature. In this case, the viscosity decreased (red bar Fig. 6) compare to this cream immediately after preparation (brown bar Fig. 6).
Spreadability test
Values of the spreadability varied from 10.42 to 6.67 g cm s−1 (Table 6). The cream spreadability containing SCY decreases immediately after preparation, and a decreasing trend is also observed during the testing period and in dependence on storage conditions. Surprisingly, the lowest values were observed in the CB + SCY cream at 22 ± 1 °C; 58% RH after two weeks.
Centrifugation test
The tested cream samples did not showed any separation of the phases during one month period of testing.
Discussion
Cyanobacteria are ubiquitous in both terrestrial and aquatic environments, even in extreme areas such as Antarctic dry valleys, thermophilic lakes, and caves (Steunou et al. 2006; Comte et al. 2007; Saw et al. 2013). In the course of their development, they have created several beneficial symbioses with other organisms (lichens, plants, protists) (Freeman and Thacker 2011). Recently, the significance of cyanobacterial metabolites has been recognized in biotechnology and industry, and they have been utilized by the pharmaceutical and cosmetic industries. Scytonemin, a small hydrophobic pigment molecule found in certain cyanobacteria, has been tested for various biological activities. This secondary metabolite can reduce the production of reactive oxygen species and the formation of DNA lesions (Proteau et al. 1993; Rastogi et al. 2015). It has dual kinase inhibitory activity that may be therapeutically important in acute and possibly chronic disorders (McInnes et al. 2005). Scytonemin has great pharmacological potential with anti-inflammatory and anti-proliferative activities (Stevenson et al. 2002; Takamatsu et al. 2003).
The antioxidant activity of scytonemin has only been investigated in a few studies. Pandey et al. (2020) tested scytonemin isolated from Lyngbya sp. for radical scavenging activity using the DPPH method. Scytonemin exhibited notable antioxidant activity of around 52% at a concentration of 0.8 mM, indicating that scytonemin acts as a radical scavenger. We chose to examine the antioxidant activity of scytonemin using the amount of substance calculation and provided superoxide anion scavenging, which has not been examined yet. Calculation by the amount of substance leads to a new consideration of antioxidant activity compared to many previous studies where the antioxidant effectiveness of substances is considered by weight (Elečko et al. 2022). This approach provides an accurate way based on the same amount of substance involved in radical as well as superoxide anion scavenging, calculated in micromoles rather than weight concentration. Concerning the results of free radical scavenging activity and superoxide anion scavenging activity, we found out that at our chosen concentration scytonemin exhibited significant antioxidant activity between 60 to 70% inhibition (Figs. 2, 3). Free radicals play a crucial role in cellular chemical reactions. Consequently, there is a effort to identify natural antioxidants without any adverse effects. Based on these findings, cyanobacteria could be promising candidates, as they contain substances like scytonemin, which possess confirmed antioxidant properties.
The photoprotective role of scytonemin has been studied in cyanobacteria inhabiting various ecological niches (Garcia-Pichel and Castenholz 1991; Garcia-Pichel et al. 1992; Sinha et al. 1999). However, the UV sunscreen role of scytonemin in N. commune from Antarctica has not been tested yet. It is worth noting that the content of scytonemin in species from Antarctica is three times higher than that in species from Europe (unpublished data). Furthermore, the Sun Protection Factor (SPF) of scytonemin has never been determined. The SPF is a quantitative measurement of the effectiveness of a sunscreen formulation. There are two ways to determine the level of photoprotection provided by sunscreen compounds: through in vivo or in vitro testing. In vivo, type of determination has been used for many years and although useful and precise, is a time-consuming process, expensive, and involves human volunteers (Dutra et al. 2004). Therefore, researchers have focused on developing in vitro methods to evaluate the effectiveness of sunscreen compounds. There are two main types of in vitro methods for measuring the SPF. One involves measuring UV radiation absorption or transmission through sunscreen films on quartz plates or biomembranes. The other involves determining the absorption characteristics of the sunscreen agents using spectrophotometric analysis of dilute solutions. Previous research has used various methods (Mansur et al. 1986; Gordon 1993; Walters et al. 1997; Fourneron et al. 1999; Pissavini et al. 2003). In this study, the SPF of scytonemin was evaluated using UV spectrophotometry and the Mansur equation (Mansur et al. 1986). Scytonemin presented a calculated SPF value higher than 30, which is considered to have very good sunscreen activity. It indicates that scytonemin can be a good candidate for sunscreen cream and for cosmeceutical purposes.
Due to consumer demand for a protective filter with (SPF) labels on sunscreens, moisturizers, face make-up, and lipsticks worldwide, they produce tons of such types of products every year (Manová et al. 2015). Sunscreen products available in the market contain synthetic UV filters. Inorganic UV filters are physical blockers that have mineral particles like titanium dioxide (TiO2) and zinc oxide (ZnO) (Serpone et al. 2007). Even though these filters absorb a significant amount of UV radiation, they also produce highly oxidizing radicals (Serpone et al. 2007). Although these particles are used as nanoparticles, they have been found to cause toxicity in human dermal fibroblasts because they can penetrate the cell membrane (Pan et al. 2009). Furthermore, synthetic UV filters have been detected in surface waters and river sediments (Balmer et al. 2005; Zhang et al. 2015), and studies have reported high toxicity for various organisms (Wang et al. 2016). Therefore, although synthetic UV filters are effective against ultraviolet radiation, their use in sunscreens and cosmetics can have harmful effects on humans and ecosystems (Morone et al. 2019). In this aspect, cyanobacteria with the content of their bioactive substances offer a considerable number of advantages, namely in the low demands for cultivation and the possibility of increasing production of compounds by manipulation of cultivation conditions. The evidence described above, although based on studies only at the laboratory scale, indicates the potential for the use of cyanobacteria for the production of natural substances, due to the high degree of sustainability of biomass.
As mentioned previously, a non-ionic cream base was chosen due to its compatibility with the tested active ingredient. The cream was reformulated according to the literature (Kovács et al. 2020) and occlusive components were replaced with semi-occlusive for better skin tolerance. Active SCY ingredient was incorporated in an oil phase in order to prepare the sunscreen creams by the emulsification process described by Amnuaikit and Boonme (2013). The cream base, in contrast to the cream containing SCY, remained white during the whole tested period as well as under all selected conditions. On the contrary, the cream labeled CB + SCY was already yellow-green immediately after the preparation. After stability tests under the studied conditions (change in temperature and humidity) the color remained stable after two weeks of storage in a refrigerator. However it changed color to different shades of brown (from gray-brown to light brown) when changing the storage conditions.
In addition, the pH of all samples was in the range of 4.7 to 6.0 indicating that they are safe for application on the skin and will not cause skin irritation and no phase separation and change in odor were observed in all samples after the stability test. The viscosity of the creams decreased with increasing spindle RPM values, which points to their pseudoelastic behavior and indicates that the decrease in viscosity is due to changes in the relaxation properties of these colloidal systems as a result deformation of dispersed molecules or particles (Saito et al. 2019). The presence of SCY (potential organic filter) in a cream base (CB + SCY) caused an increase in viscosity (except storage at 22 °C after a month), which contributed to the colloidal stability of the creams. On the other hand, it is interesting that both time and storage conditions increased viscosity, which points to their potential for sunscreen application even at 40 °C. It also should be noted that the viscosity of creams can also be influenced by the content of water phase ingredients, which can change during the selected storage conditions.
Conclusion
Scytonemin is unique among natural products due to its special structure, location in a cell, as well as strong absorption maxima in UV-A in addition to the violet–blue region. This study demonstrated that scytonemin might serve as a topically applicable ingredient for a natural UV sunscreen cream with an SPF value higher than 30. From the point of view of the potential of SCY as an organic UV filter in sunscreens, the selected cream base is suitable for this active ingredient type. In addition, cream stability tests confirmed that the cream containing SCY can be stored at room temperature and even at higher and lower temperatures, but in a vessel with a closed lid.
References
Ambala R, Vemula S (2015) Formulation and characterization of ketoprofen emulgels. J Appl Pharmaceut Sci 5:112–117
Amnuaikit T, Boonme P (2013) Formulation and characterization of sunscreen creams with synergistic efficacy on SPF by combination of UV filters. J Appl Pharmaceut Sci 3:1–5
Balmer ME, Buser H-R, Müller MD, Poiger T (2005) Occurrence of some organic UV filters in wastewater, in surface waters, and in fish from Swiss lakes. Environ Sci Technol 39:953–962
Balskus E, Case R, Walsh C (2011) The biosynthesis of cyanobacterial sunscreen scytonemin in intertidal microbial mat communities. FEMS Microbiol Ecol 77:322–332
Baumann L (2005) How to prevent photoaging? J Invest Dermatol 125:xii–xiii
Brenner M, Hearing VJ (2008) The protective role of melanin against UV damage in human skin. Photochem Photobiol 84:539–549
Cavinato M, Waltenberger B, Baraldo G, Grade CV, Stuppner H, Jansen-Dürr P (2017) Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 18:499–516
Chen M, Alexander K, Baki G (2016) Formulation and evaluation of antibacterial creams and gels containing metal ions for topical application. J Pharmaceut 2016:5754349
Comte K, Šabacká M, Carré-Mlouka A, Elster J, Komárek J (2007) Relationships between the Arctic and the Antarctic cyanobacteria; three Phormidium-like strains evaluated by a polyphasic approach. FEMS Microbiol Ecol 59:366–376
Daniel S, Cornelia S, Fred Z (2004) UV-A sunscreen from red algae for protection against premature skin aging. Cosmet Toilet Manuf Worldw 129:139–143
Dorman H, Peltoketo A, Hiltunen R, Tikkanen M (2003) Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem 83:255–262
Dransfield G (2000) Inorganic sunscreens. Radiat Prot Dosimetry 91:271–273
Dutra E, Oliveira D, Kedor-Hackmann E, Santoro M (2004) Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Rev Bras Cienc Farmaceut 40:381–385
Elečko J, Vilková M, Frenák R, Routray D, Ručová D, Bačkor M, Goga M (2022) A comparative study of isolated secondary metabolites from lichens and their antioxidative properties. Plants 11:1077
Fourneron J-D, Faraud F, Fourneron A (1999) Sur la mesure in vitro de la protection solaire de crièmes cosmétiques. Compt Rend L’Acad Sci IIC 2:421–427
Freeman CJ, Thacker RW (2011) Complex interactions between marine sponges and their symbiotic microbial communities. Limnol Oceanogr 56:1577–1586
Gangl D, Zedler JA, Rajakumar PD, Martinez EMR, Riseley A, Włodarczyk A, Purton S, Sakuragi Y, Howe CJ, Jensen PE (2015) Biotechnological exploitation of microalgae. J Exp Bot 66:6975–6990
Garcia-Pichel F, Castenholz RW (1991) Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment. J Phycol 27:395–409
Garcia-Pichel F, Sherry ND, Castenholz RW (1992) Evidence for an ultraviolet sunscreen role of the extracellular pigment scytonemin in the terrestrial cyanobacterium Chlorogloeopsis sp. Photochem Photobiol 56:17–23
Gordon V (1993) Evaluation du facteur de protection solaire. Parfums, Cosmétiques, Arômes 112:62–65
Grether-Beck S, Marini A, Jaenicke T, Krutmann J (2014) Photoprotection of human skin beyond ultraviolet radiation. Photodermatol Photoimmunol Photomed 30:167–174
Harrison GI, Young AR (2002) Ultraviolet radiation-induced erythema in human skin. Methods 28:14–19
Ibañez E, Kubátová A, Señoráns FJ, Cavero S, Reglero G, Hawthorne SB (2003) Subcritical water extraction of antioxidant compounds from rosemary plants. J Agric Food Chem 51:375–382
ICH (2018) Stability testing of active pharmaceutical ingredients and finished pharmaceutical products. Annex 10. WHO Tech Rep Ser 1010:309–351
Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T (2003) UV-induced skin damage. Toxicology 189:21–39
Jones CS, Esquenazi E, Dorrestein PC, Gerwick WH (2011) Probing the in vivo biosynthesis of scytonemin, a cyanobacterial ultraviolet radiation sunscreen, through small scale stable isotope incubation studies and MALDI-TOF mass spectrometry. Bioorg Med Chem 19:6620–6627
Kiuru P, DʼAuria MV, Muller CD, Tammela P, Vuorela H, Yli-Kauhaluoma J (2014) Exploring marine resources for bioactive compounds. Planta Medica 80:1234-1246
Kosanić M, Ranković B, Vukojević J (2011) Antioxidant properties of some lichen species. J Food Sci Technol 48:584–590
Kovács A, Péter-Héderi D, Perei K, Budai-Szűcs M, Léber A, Gácsi A, Csányi E, Berkó S (2020) Effects of formulation excipients on skin barrier function in creams used in pediatric care. Pharmaceutics 12:729
Lademann J, Schanzer S, Jacobi U, Schaefer H, Pflu¨ cker F, Driller H, Beck J, Meinke M, Roggan A, Sterry W (2005) Synergy effects between organic and inorganic UV filters in sunscreens. J Biomed Opt 10:14008
Lodén M (2014) Interactions between the stratum corneum and topically applied products: Regulatory, instrumental and formulation issues with focus on moisturizers. Br J Dermatol 171:38–44
Manaia EB, Kaminski RCK, Corrêa MA, Chiavacci LA (2013) Inorganic UV filters. Braz J Pharmaceut Sci 49:201–209
Manová E, von Goetz N, Hungerbuehler K (2015) Aggregate consumer exposure to UV filter ethylhexyl methoxycinnamate via personal care products. Environ Int 74:249–257
Mansur JdS, Breder MNR, Mansur MCdA, Azulay RD (1986) Determinaçäo do fator de proteçäo solar por espectrofotometria. Ann Bras Dermatol 121–124
McInnes C, Mezna M, Fischer PM (2005) Progress in the discovery of polo-like kinase inhibitors. Curr Top Med Chem 5:181–197
Morone J, Alfeus A, Vasconcelos V, Martins R (2019) Revealing the potential of cyanobacteria in cosmetics and cosmeceuticals—A new bioactive approach. Algal Res 41:101541
Mukherjee PK, Maity N, Nema NK, Sarkar BK (2011) Bioactive compounds from natural resources against skin aging. Phytomedicine 19:64–73
Mulkidjanian AY, Koonin EV, Makarova KS, Mekhedov SL, Sorokin A, Wolf YI, Dufresne A, Partensky F, Burd H, Kaznadzey D (2006) The cyanobacterial genome core and the origin of photosynthesis. Proc Nat Acad Sci 103:13126–13131
Nishikimi M, Appaji Rao N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854
Pan Z, Li W, Slutsky L, Clark R, Pernodet N, Rafailovich M (2009) Adverse effects of titanium dioxide nanoparticles on human dermal fibroblasts and how to protect cells. Small 5:511–520
Pandey A, Pathak J, Singh DK, Ahmed H, Singh V, Kumar D, Sinha RP (2020) Photoprotective role of UV-screening pigment scytonemin against UV-B-induced damages in the heterocyst-forming cyanobacterium Nostoc sp. strain HKAR-2. Braz J Bot 43:67–80
Parija S, Sahoo A, Sundeep Kumar H, Mishra B (2012) Formulation development and evaluation of triple combination cream of hydroquinone, tretinoin and mometasone furoate for the treatment of skin disorders. Int J Pharm Ind Res 2:2231–3468
Pissavini M, Ferrero L, Alard V, Heinrich U, Tronnier H, Kockott D, Lutz D, Tournier V, Zambonin M, Meloni M (2003) Determination of the in vitro SPF. Cosmet Toiletries 118:63–72
Proteau P, Gerwick W, Garcia-Pichel F, Castenholz R (1993) The structure of scytonemin, an ultraviolet sunscreen pigment from the sheaths of cyanobacteria. Experientia 49:825–829
Puizina-Ivic N (2008) Skin aging. Acta Dermatovenerol Alp Panonica Adriat 17:47–54
Ranković B (ed) (2015) Lichen secondary metabolites: Bioactive properties and pharmaceutical potential. Springer, Cham, Switzerland
Rastogi RP, Sonani RR, Madamvar D (2015) Cyanobacterial sunscreen scytonemin: Role in photoprotection and biomedical research. Appl Biochem Biotech 176:1551–1563
Rastogi RP, Richa SRP, Singh SP, Haeder D-P (2010) Photoprotective compounds from marine organisms. J Indust Microbiol Biotechnol 37:537–558
Rastogi RP, Sinha RP, Moh SH, Lee TK, Kottuparambil S, Kim Y-J, Rhee J-S, Choi E-M, Brown MT, Häder D-P (2014) Ultraviolet radiation and cyanobacteria. J Photochem Photobiol B 141:154–169
Rigel DS (2013) Sun protection and self‐tanners. In: Farris PK (ed) Cosmeceuticals and Cosmetic Practice. Wiley Blackwell, Chichester, pp 252–260
Saewan N, Jimtaisong A (2015) Natural products as photoprotection. J Cosmet Dermatol 14:47–63
Sah SK, Badola A, Mukhopadhyay S (2017) Development and evaluation of tioconazole loaded emulgel. Int J Appl Pharm 9:83–90
Saito GP, Bizari M, Cebim MA, Correa MA, Junior MJ, Davolos MR (2019) Study of the colloidal stability and optical properties of sunscreen creams. Eclética Quím 44:26–36
Sánchez-Quiles D, Tovar-Sánchez A (2015) Are sunscreens a new environmental risk associated with coastal tourism? Environ Int 83:158–170
Saw JH, Schatz M, Brown MV, Kunkel DD, Foster JS, Shick H, Christensen S, Hou S, Wan X, Donachie SP (2013) Cultivation and complete genome sequencing of Gloeobacter kilaueensis sp. nov., from a lava cave in Kīlauea Caldera, Hawai'i. PLoS One 8:e76376
Sayre RM, Agin PP, LeVee GJ, Marlowe E (1979) A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem Photobiol 29:559–566
Schuch AP, Moreno NC, Schuch NJ, Menck CFM, Garcia CCM (2017) Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic Biol Med 107:110–124
Serpone N, Dondi D, Albini A (2007) Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorg Chim Acta 360:794–802
Shantanu K, Snehal B, Megha G, Vaibhav U, Amol R (2011) Formulation and in vitro evaluation for sun protection factor of lutein ester extracted from Tagetes erecta Linn flower (Family-Asteraceae) sunscreen creams. Res J Pharmaceut Biol Chem Sci 2:947–955
Singh SP, Häder D-P, Sinha RP (2010) Cyanobacteria and ultraviolet radiation (UVR) stress: mitigation strategies. Ageing Res Rev 9:79–90
Sinha RP, Häder D-P (2008) UV-protectants in cyanobacteria. Plant Sci 174:278–289
Sinha RP, Klisch M, Vaishampayan A, Häder D-P (1999) Biochemical and spectroscopic characterization of the cyanobacterium Lyngbya sp. inhabiting Mango (Mangifera indica) trees: presence of an ultraviolet-absorbing pigment, scytonemin. Acta Protozool 38:291–298
Smaoui S, Ben Hlima H, Ben Chobba I, Kadri A (2017) Development and stability studies of sunscreen cream formulations containing three photo-protective filters. Arab J Chem 10:S1216–S1222
Stengel D, Connan S, Popper Z (2011) Algal Chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol Adv 29:483–501
Steunou A-S, Bhaya D, Bateson MM, Melendrez MC, Ward DM, Brecht E, Peters JW, Kühl M, Grossman AR (2006) In situ analysis of nitrogen fixation and metabolic switching in unicellular thermophilic cyanobacteria inhabiting hot spring microbial mats. Proc Nat Acad Sci 103:2398–2403
Stevenson CS, Capper EA, Roshak AK, Marquez B, Eichman C, Jackson JR, Mattern M, Gerwick WH, Jacobs RS, Marshall LA (2002) The identification and characterization of the marine natural product scytonemin as a novel antiproliferative pharmacophore. J Pharmacol Exp Therapeut 303:858–866
Takamatsu S, Hodges TW, Rajbhandari I, Gerwick WH, Hamann MT, Nagle DG (2003) Marine natural products as novel antioxidant prototypes. J Nat Prod 66:605–608
Tamaru Y, Takani Y, Yoshida T, Sakamoto T (2005) Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. Appl Environ Microbiol 71:7327–7333
Tsui MM, Leung H, Wai T-C, Yamashita N, Taniyasu S, Liu W, Lam PK, Murphy MB (2014) Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface waters from different countries. Water Res 67:55–65
Tuchinda C, Lim HW, Osterwalder U, Rougier A (2006) Novel emerging sunscreen technologies. Dermatol. Clinics 24:105–117
Vega J, Bonomi-Barufi J, Gómez-Pinchetti JL, Figueroa FL (2020) Cyanobacteria and red macroalgae as potential sources of antioxidants and UV radiation-absorbing compounds for cosmeceutical applications. Mar Drugs 18:659
Walters C, Keeney A, Wigal CT, Johnston CR, Cornelius RD (1997) The spectrophotometric analysis and modeling of sunscreens. J Chem Ed 74:99
Wang J, Pan L, Wu S, Lu L, Xu Y, Zhu Y, Guo M, Zhuang S (2016) Recent advances on endocrine disrupting effects of UV filters. Int J Environ Res Public Health 13:782
Whitton B, Potts M (2000) Introduction to the cyanobacteria. In: Whitton BA, Potts M (eds) The Ecology of Cyanobacteria: Their Diversity in Time and Space. Kluwer, Dordrecht, pp 1–11
Zhang N-S, Liu Y-S, Van den Brink PJ, Price OR, Ying G-G (2015) Ecological risks of home and personal care products in the riverine environment of a rural region in South China without domestic wastewater treatment facilities. Ecotoxicol Environ Safety 122:417–425
Acknowledgements
We would like to thank Pragya Singh, PhD student at the Department of Botany, University of Pavol Jozef Šafárik, Košice, Slovakia, for the assistance with spectrophotometric analyses that greatly helped the research. Additionally, we would like to express our gratitude to Anna Uhrinová from the Department of Chemistry, Biochemistry, and Biophysics, University of Veterinary Medicine and Pharmacy, Košice, Slovakia, for her help with the measurements of creams. Thanks to Stanislav Tanecka for proofreading the article.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic This study was supported by the Slovak Grant Agency KEGA (009UPJŠ-4/2023 and 008SPU-4/2023) and by the Slovak Research and Development Agency under contract No. APVV-21–0289.
Author information
Authors and Affiliations
Contributions
D.R. (Dajana Ručová), S.S (Simona Sovová), Z.V. (Zuzana Vargová) wrote the paper and designed the experiments; M.B. (Martin Bačkor) collected the cyanobacteria during expedition; D.R. (Dajana Ručová), and D.R. (Deepti Routray) isolated scytonemin from cyanobacteria; M.V. (Mária Vilková) performed Nuclear Magnetic Resonance (NMR); R.F. (Richard Frenák) performed antioxidant activities; S.S (Simona Sovová) and Z.K. (Zuzana Kostecká) prepared creams and tested their selected properties; M.B. (Martin Bačkor) and D.R. (Dajana Ručová) approved the final version of the manuscript and supervised experiments; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ručová, D., Vilková, M., Sovová, S. et al. Photoprotective and antioxidant properties of scytonemin isolated from Antarctic cyanobacterium Nostoc commune Vaucher ex Bornet & Flahault and its potential as sunscreen ingredient. J Appl Phycol 35, 2839–2850 (2023). https://doi.org/10.1007/s10811-023-03109-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03109-6