Abstract
Toxicity assessment of K2Cr2O7, ZnCl2 and NiCl2.6H2O focusing on the green unicellular alga Desmodesmus subspicatus showed that Ni was the most toxic (EC50 = 0.121 mg L−1), followed by Cr (EC50 = 0.357 mg L−1) and Zn (EC50 = 0.968 mg L−1). The alga was exposed to solutions of the individual metals and their mixtures (Zn + Ni, Zn + Ni + Cr). After 48, 72 and 96 h of exposure, the accumulation of Zn, Ni and Cr in algal cells was assessed. Moreover, the change in toxicity of the metals, the concentration of selected biogenic elements (Ca, Na, Mg, S, K, P, Mn) in algal cells and the rate of metal removal from the solutions were also analysed. In the case of single-metal solutions, for Zn and Cr, the toxicity and accumulation in algal cells decreased over the 96 h of the experiment, whereas for Ni as well as for the binary and ternary mixtures the toxicity and accumulation increased. Significant correlations between the amount of metals accumulated in the cells and the inhibition of growth rates were observed. The content of selected biogenic elements in algal cells decreased over time in all treatments with added metals as well as in the control group, and after 96 h was higher in all treatments with addition of Ni compared to the control group and single-metal solutions of Zn and Cr. The highest removal of the metal from the solution occurred in the case of the single metal solution of Zn, where 83.4% of Zn was removed from the solution in 96 h.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many anthropogenic activities such as industrial production, mining, transport, fossil fuel combustion, the use of paint and pigments, as well as agriculture contribute to environmental contamination by heavy metals. The danger of heavy metals is that they persist in the environment for a long time. In aquatic environments, they tend to accumulate in sediments and are taken up by aquatic plants and animals. Zn, Ni and Cr are among the metals that contribute significantly to the pollution of aquatic environments due to their good solubility in water. These metals were selected because they are frequent contaminants of the aquatic environment which enter from runoff water. In road traffic, Zn, Ni and Cr are significantly represented in engine oil emissions (28%, 25%, 33%) and emissions from brake wear (39%, 46%, 37%). Road abrasion and tire wear are also a source of these elements (Winther and Slentø 2010). In a study conducted into heavy metal contamination in urban soils, the highest average concentrations of Cr, Ni, Mn and Co were observed in road dust collected along the ring road, while the highest concentrations of Zn and U were found in dust collected along the motorway (Wei et al. 2010).
According to their importance for living organisms, metals can be divided into two groups: essential metals which are considered essential for life at low concentrations, and non-essential metals which have no function in the organism and are toxic even at low concentrations. Zn is essential for the mechanism of action of many enzymes involved in the metabolism of proteins and carbohydrates, but it is toxic at higher concentrations (Omar 2002). High Zn concentrations reduce the chlorophyll content and photosynthetic function of algae and inhibit growth and metabolic activity (Omar 2002; Al-Hasawi et al. 2020). Cr has no biological function in most organisms and is not necessary for any metabolic processes (Kováčik et al. 2015). It occurs most often in the two oxidation states which are most stable in the environment – trivalent Cr(III) and hexavalent Cr(VI), with hexavalent Cr being more mobile and more soluble and considered to be more toxic because it can easily penetrate the cell membrane (Leong and Chang 2020). This is due to the structural similarity between chromate and sulphate or phosphate anions (Aharchaou et al. 2017). Stress induced by Cr leads to a reduced rate of photosynthesis and respiration, disruption of chloroplast ultrastructure and changes in the cytoskeleton (Nowicka 2022). Cr(VI) causes serious cell damage by inducing rapid production of reactive oxygen species (ROS) and disruption of Fe homeostasis in cells (Volland et al. 2012). Ni is a micronutrient necessary for the proper functioning of some enzymes in plants and microorganisms (Nowicka 2022). It is essential only for cyanobacteria, for algae it is non-essential (Filová et al. 2021). Ni has a great affinity for biological structures that contain -SH groups such as proteins and enzymes, causing inhibition of growth, photosynthesis, respiration and other cellular processes (Dahmen-Ben Moussa et al. 2018).
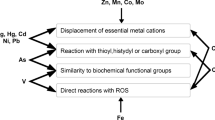
There are four main mechanisms of toxic action of heavy metal ions, specifically, by reaction with thioyl, histydyl and carboxyl protein groups, by displacement of essential metal cations, by a similarity with biochemical functional groups, especially phosphates, or by the generation of ROS (Nowicka 2022). Biochemical mechanisms responsible for changing the sensitivity of different algae types to metals include extracellular metal sorption on cell walls, metal complexion on algae exudates consisting of polysaccharides, intracellular sequestration of metals by phytochelatins and metallothioneins, and the excretion of intracellular metals (Fettweis et al. 2021).
Green algae are primary producers of aquatic ecosystems and are at the base of the food chain. These organisms are sensitive to increased concentrations of heavy metals in the aquatic environment and are able to absorb and to some extent accumulate these metals (Rai et al. 1992; Fargašová et al. 1997; Hörcsik and Balogh 2002; Komjarova and Blust 2009; Wang et al. 2012; Zeng et al. 2012; Aharchaou et al. 2017; Hamed et al. 2017; Ciurli et al. 2021; Filová et al. 2021), which leads to a biomagnification of these substances in the food chain. Metal toxicity, expressed mostly as growth reduction, has been studied on different types of algae (Vignati et al. 2010; Monteiro et al. 2011; Qian et al. 2013; Alharbi 2017; Fettweis et al. 2021; Filová et al. 2021; Macoustra et al. 2021). The green unicellular algae Desmodesmus subspicatus is a freshwater species and is often used for ecotoxicological tests as a model organism recommended by international standards (OECD 2011; ISO 2012).
Toxicological assessments are mainly based on monitoring the toxicity of one selected metal. However, in the aquatic environment, organisms are exposed to mixtures of metals the combined effect of which can significantly modify the resulting toxic effects of the studied metal. Additive, synergistic and antagonistic effects may be observed for various mixtures (Flouty and Khalaf 2015; Nys et al. 2017; Van Regenmortel and De Schamphelaere 2018). The influence of binary mixtures of Zn, Cu, Cd and Ni on the growth of the freshwater diatom Navicula pelliculosa has been studied (Nagai and De Schamphelaere 2016). Van Regenmortel and De Schamphelaere (2018) examined the effects of Cu-Ni binary mixtures and Cu-Ni-Zn ternary mixtures in various natural waters on the green algae Pseudokirchneriella subcapitata (synonym Raphidocelis subcapitata). The uptake of Ni, Pb and Cu by Chlamydomonas reinhardtii was quantified in single monometallic systems and binary metal mixtures (Flouty and Khalaf 2015). The sorption of Ni and Cu from binary mixtures by Chlorella vulgaris was studied in (Mehta and Gaur 2001). The toxicity of Ni, Cu and Zn has been tested on 8 freshwater algae species in (Fettweis et al. 2021). Komjarova and Blust (2009) examined the uptake of metals from a mixture of Cd, Cu, Ni, Pb and Zn by P. subcapitata and the water flea Daphnia magna. The effects of Ni-Zn-Cu-Cd and Ni-Zn-Cu mixtures on the growth rate of algae P. subcapitata and the reproduction of Ceriodaphnia dubia were evaluated by (Nys et al. 2017). Gebara et al. (2020) focused on testing the effects of Zn and Al mixtures on R. subcapitata. Franklin et al. (2002) investigated the combined effects of Cu, Cd and Zn on the cell division rate of the freshwater alga Chlorella sp. The effects of a mixture of 5 heavy metals (As, Cu, Pb, Ni, Zn) on R. subcapitata were analysed in (Expósito et al. 2017).
Exposure to heavy metals can affect the mineral composition of algae through ion exchange between heavy metal ions in a solution and elemental ions (e.g. Ca2+, Na+ and K+) that are bound to the cell surface (Salama et al. 2019). This issue has been addressed in only a few studies which focus only on some specific elements often with conflicting results (Hörcsik and Balogh 2002; Qian et al. 2013; Dahmen-Ben Moussa et al. 2018; Kováčik et al. 2018).
Although the impact of Ni and Zn mixtures on algae has been widely investigated, no information was found on the effect of Zn + Ni + Cr mixtures on green algae. These metals contribute significantly to the pollution of water and soils around roads. The aim of the present work was to compare the toxicity of the individual metals Zn, Ni, Cr and some of their mixtures (Zn + Ni, Zn + Ni + Cr) to the green alga D. subspicatus. Together with the toxicity assessment, the accumulation of individual metals in algae cells and changes in the mineral composition of cells were analysed over 3 time sections of the experiment (48, 72, 96 h). The percentage of the metals removed from the test solutions has also been determined for the specific time sections, because the information about the rate of metal removal can be used for bioremediation of wastewater.
Materials and methods
Tested organism
Desmodesmus subspicatus (BRINKMANN 1953/SAG 86,81) from the Culture Collection of Autotrophic Organisms (CCALA Třeboň, Czech Republic) was used for the ecotoxicological tests. The algae were maintained in a test medium, the composition of which is described in EN ISO 8692 (ISO 2012), in OECD TG 201 (OECD 2011) and in Online Resource 1.
Chemicals
The tested metals added to the algae were in the form of K2Cr2O7 (Penta s.r.o., Czech Republic, p.a.), ZnCl2 (Sigma Aldrich, ACS reagent) and NiCl2.6H2O (Sigma Aldrich, 99.999% trace metal basis). For each metal compound, its stock solution was mixed in a test medium, which was used to prepare the test concentrations of the individual metal compounds and their mixtures.
For each compound tested, a concentration series of 6 to 7 gradually increasing concentrations was first prepared to determine the value of EC50 after 72 h (concentration of the test sample at which the algae growth rate is reduced to 50% compared to the control group). K2Cr2O7 was tested in the range of 0.2 to 1.4 mg L−1, ZnCl2 in the range of 0.1 to 3.6 mg L−1, and NiCl2.6H2O in the range of 0.08 to 2 mg L−1.
Based on the toxicity information found for K2Cr2O7, ZnCl2, and NiCl2.6H2O, a concentration of 0.3 mg L−1 was selected to which each compound was diluted. This concentration corresponded to 106 µg of Cr, 144 µg of Zn and 74 µg of Ni in one L of solution. Next, a binary mixture was prepared containing 0.3 mg L−1 ZnCl2 and 0.3 mg L−1 NiCl2.6H2O and a ternary mixture containing 0.3 mg L−1 ZnCl2, 0.3 mg L−1 NiCl2.6H2O and 0.3 mg L−1 K2Cr2O7. The diluted compounds and mixtures were used in the time experiment to monitor changes in toxicity and, at the same time, bioaccumulation of Zn, Cr, Ni and changes in the content of the selected biogenic elements over time. The selected elements represented macro and micronutrients and they were Na, K, Ca, Mg, P, S, Mn.
The actual concentrations of the metals monitored and elements selected in the solutions tested were verified by ICP-MS/MS before addition of the algae.
Ecotoxicology test design
The ecotoxicology tests were carried out on the basis of EN ISO 8692 (ISO 2012). The testing itself took place in 50 mL polymethylpentene Erlenmeyer flasks, with a volume of the solution tested of 40 mL. The metal solutions were stabilized in the medium for 1 h before the addition of algae. Algae were added to each flask in such a volume that the initial cell density was 10,000 cells mL−1. The control groups contained only the test medium and the addition of algae. Incubation was carried out using a TS 606 CZ/2-Var thermostat (WTW, Czech Republic) at 23 ± 2 °C with constant shaking on an orbital shaker at 200 rpm (IKA KS260 basic, Germany) and constant illumination ranging from 68 to 94 µmol photons m−2 s−1.
Concentration Series — Determination of EC50
In the experiments to determine EC, individual concentrations from the concentration series were prepared in three repetitions and the control group concentration in six repetitions. Exposure of the algae to the test solutions lasted for 72 h; the algae cell density in each dilution and in the control group was then calculated in a Bürker chamber under a microscope. Cell density values were used to calculate growth rates and to calculate the inhibition of algal cell growth compared to the control group. The calculations were made according to the formulas given in EN ISO 8692 (ISO 2012).
Growth rates were calculated using the following equation:
where µ is the specific growth rate, nL is the cell density measured at the end of the test, n0 is the initial cell density, t0 is the time at the beginning of the test and tL is the time at the end of the test.
Growth inhibitions were calculated for each repetition according to the following equation:
where Ii is the growth inhibition for each of the concentrations tested and their replicates, µc is the mean growth rate of the control and µi is the growth rate for each replicate of the test concentrations.
Time Experiment — determination of toxicity and bioaccumulation
For the time experiment, each of the tested metals or their mixtures were prepared in nine repetitions; after 48, 72 and 96 h respectively, three Erlenmeyer flasks were removed from the shaker for both the control and the tested solutions. Cell density was determined under the microscope for all the solutions and was then used to calculate the inhibition of algae growth in individual time sections. The contents of each flask were then filtered through a nitrate cellulose filter with 0.45 µm pore size (Merck—Millipore, Germany). The filtrates were preserved with nitric acid and analysed using ICP-MS/MS. The filters used were then washed with ultra-pure water (30 mL), dried in air and placed in Teflon containers and digested in sub-boiled distilled nitric acid and suprapure hydrogen peroxide (Analytika, CZ) in a microwave digestion instrument (Berghof, Germany). Then the concentration of selected elements was determined using ICP-MS/MS (Agilent, Japan). Blank filters (clean filters washed with ultra-pure water) were digested and analysed in the same way. The resulting concentrations of the elements being determined were obtained by subtracting the blank filter values and, given that the cell density was known, they were recalculated to one algae cell. This value represents the total amount of elements that have been adsorbed on the surface of algal cells and accumulated directly in the cells.
Determination of selected elements using ICP-MS/MS
Filter samples after digestion and eluates were analysed using ICP-MS/MS (Agilent, Japan) with a collision/reaction cell to determine the content of selected elements (Ni, Zn, Cr, S, K, P, Mn, Mg, Ca, Na). Integration time for all isotopes was 0.3 s. The isotopes 58Ni, 53Cr, 65Zn, 55Mn, 24 Mg, 23Na, 44Ca and 39 K were analysed using He collision gas (4 mL min−1) measured on mass in a single quadrupole mode. Sulphur and phosphorus were analysed using the reaction mass shift MS/MS mode in O2 (0.29 mM min−1) on the masses of 32–48S and 31–37P reaction products. The Argon carrier gas flow rate was 1.07 L min−1, the forwarded RF power was 1,550 W. Calibration solutions were prepared from stock solutions of single-element standards with a concentration of 1 g L−1 (Analytika, CZ). The internal standard at a concentration of 10 µg L−1 was prepared from Agilent stock solution of 10 mg L−1 (Internal standard mix). Sub-boiled HNO3 (Berghof, Germany) and suprapure water (Merck—Milipore, Germany) were used to prepare all solutions.
Removal of metals from solution
For each metal and the individual treatments in which it was present, the percentage of metal removal by algae was calculated from the initial concentration of the metal in the solution and its concentration in the filtrate at each time section according to the relationship:
where c0 was the concentration of the metal in the solution at the beginning of the experiment and cF was the concentration of the metal in the filtrate over a certain period of time (48, 72 and 96 h).
Statistical analysis
Statistical analyses were carried out in GraphPad Prism 6 (GraphPad Software, Inc., USA). A nonlinear regression method was used to calculate EC50 values. One-way analysis of variance (ANOVA) was used to determine the significance of differences in concentrations of elements in cells between treatments and controls in individual time sections. Values were considered statistically significant if the probability was P < 0.05. For each treatment, differences between means were assessed by Dunnett’s multiple range test.
Results
EC50 values estimated for the tested compounds (K2Cr2O7, ZnCl2 and NiCl2.6H2O)
From the calculated inhibition values for the individual dilutions of the tested compounds, EC50 values for the tested metals were estimated with their 95% confidence intervals, specifically 0.357 mg L−1 (0.318 to 0.396 mg L−1) for K2Cr2O7; 0.968 mg L−1 (0.587 to 1.59 mg L−1) for ZnCl2 and 0.121 mg L−1 (0.112—0.131 mg L−1) for NiCl2.6H2O. The results show that among the metals Ni was the most toxic to algae, followed by Cr; the least toxic was Zn. EC50 values for the tested salts and individual metals are given in Online Resource 2.
Development of toxicity of Zn, Cr, Ni and their mixtures over 96 h
The ecotoxicology tests were carried out in 5 treatments for one selected concentration of the tested compounds of 0.3 mg L−1; in the mixtures the concentration of the individual compounds was also 0.3 mg L−1. Treatment 1 represented the addition of ZnCl2 only (designated as Zn), treatment 2 the addition of K2Cr2O7 (designated as Cr), treatment 3 the addition of NiCl2.6H2O (designated as Ni), treatment 4 the addition of ZnCl2 and NiCl2.6H2O (designated as Zn + Ni), treatment 5 the addition of ZnCl2, NiCl2.6H2O and K2Cr2O7 (designated as Zn + Ni + Cr). Following dilution of all the compounds and their mixtures, the actual concentrations of Zn, Cr and Ni in the solutions tested were determined by means of ICP-MS/MS at the beginning of the ecotoxicology tests. The average values from the ecotoxicology tests for each treatment are given in Table 1. The selected higher Zn:Ni ratio in the mixtures corresponds to the real environmental ratio of these metals in contaminated flows (Fettweis et al. 2021).
Figure 1 shows the changes in toxicity, expressed as inhibition of growth rates compared to the control group, for each treatment and at time sections of 48, 72 and 96 h, respectively. The toxicity of metals and mixtures changed over time and varied for individual metals and mixtures. Although the Zn concentration in the solutions was approximately 2 times higher and the Cr concentration was approximately 1.3 times higher than the Ni concentration at the beginning of the tests (Table 1), a significant decrease in toxicity was observed over 96 h for the Zn and Cr treatments, whereas in the treatment with Ni and the metal mixtures the opposite trend was observed. After 48 h, the highest toxicity was observed in the Cr treatment (36.6% inhibition of growth rates) and the lowest toxicity in Zn and Ni (17.7% inhibition); after 72 h the lowest growth inhibition was observed for Cr (14.4%) and the highest for Ni (40.2%). At the end of the experiment, after 96 h, the lowest toxicity was observed in the Zn (1.5%) and Cr (4.7%) treatments; however, if these metals were present in the mixture, the toxicity was significantly higher and comparable for both mixtures (Zn + Ni 34.0%, Zn + Ni + Cr 34.6%). The most toxic treatment was Ni (38.5%), the toxicity of which was slightly higher than the toxicity of the treatments containing Ni in mixtures. Average values of cell densities, growth rates and inhibitions for the individual tested treatments during 96 h ecotoxicological tests are shown in Online Resource 3.
Bioaccumulation of metals
With the addition of Zn, Cr and Ni, significant differences (p < 0.05) in the concentration of these metals in algal cells were observed in all the treatments and time sections tested compared to the control group (Fig. 2). These findings thus point to the accumulation of these metals in algal cells. The concentration of Zn in the cells was highest in the treatment with the addition of Zn only and an exposure duration of 48 h, where it reached 0.148 pg cell−1 (Fig. 2a). The amount of Zn in the cells decreased rapidly over the subsequent course of the experiment, after 96 h it was 0.034 pg cell−1, which corresponds to a 4.4-fold decrease in 2 days. In contrast, the concentration of Zn in the cells exposed to Zn + Ni and Zn + Ni + Cr mixtures increased with time, from 0.055 and 0.073 pg cell−1 (48 h) to 0.099 and 0.111 pg cell−1 (96 h); i.e., the concentrations increased 1.8 and 1.5 times, respectively (Fig. 2a).
Bioaccumulation of Zn, Cr and Ni by algae over 48 to 96 h in different test treatments. The data represents the average value ± SD yielded by three repetitions (n = 3). Statistical significance (one-way ANOVA) is at *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 level. The values on the left y-axis of the graphs show how the metal concentrations in the cells differed between the treatments compared to the control group (bar graphs, treatments labelled with the letter F), the values on the right y-axis show the changes in metal concentrations in the solutions over 96 h (dot plots, treatments labelled with the letter S)
As with Zn, the amount of Cr in the cells (treatment with the addition of Cr) decreased over time from 0.076 pg cell−1 (48 h) to 0.023 pg cell−1 (96 h), i.e., to 3.3 times lower the value (Fig. 2b). A decrease was also observed in the mixture with the addition of Cr, from 0.054 to 0.047 pg cell−1 at the end of the test (1.1-fold decrease), where the concentration after 72 h was lower than after 96 h (Fig. 2b).
The amount of Ni in the cells increased with time in all treatments in which Ni accumulation was assessed, i.e., treatment with addition of Ni or in the mixtures with Zn and Cr (Fig. 2c). Its concentration increased from 0.039 to 0.068 pg cell−1 (1.7 times more) in the treatment with Ni. In the mixtures, the increase of Ni content was slower, it increased 1.5 times in the treatment with the Ni + Zn mixture (from 0.019 to 0.029 pg cell −1) after 48 h, and 1.3 times (from 0.023 to 0.028 pg cell −1) in the treatment with the Ni + Zn + Cr mixture. In the Ni + Zn + Cr mixture, there was a slight decrease in Ni concentration in the cells after 72 h, as was observed in the same treatment for Cr concentration in the cells.
For the treatments with one tested metal, the accumulation of the tested metals was higher than for the mixtures only after 48 h and 72 h of exposure; for Ni also after 96 h of exposure. The reduction in the concentration of metals in the cells is related to the toxicity of each treatment. For Zn and Cr treatments, the toxicity decreased significantly after 96 h, while for Ni treatment and the mixtures (Zn + Ni, Zn + Ni + Cr), the inhibition of growth rates ranged between 30 to 40% (Fig. 1).
The graphs in Fig. 3 show how the accumulation of metals by algal cells varied at different time intervals, depending on whether the tested metals were present in the solutions alone or in the tested mixtures. Over time, the representation of metals in the cells in the treatments changed as described above. After 48 h, the accumulation of Zn in the mixtures by the algal cells was approximately halved compared to the single-metal Zn solution (Fig. 3a). After 72 h, the accumulation of Zn by the cells in the solutions treated with the mixtures decreased even more (Fig. 3b), but the differences were no longer large, and after 96 h, a significant increase in accumulation of Zn in the cells was observed (about 3 times higher) compared to the solution with Zn alone (Fig. 3c). A similar pattern was observed for Cr, but in that case the differences between Cr accumulation by the cells exposed to the mixture and those treated with the single-metal solution were not as pronounced as in the case of Zn. To the contrary, in the case of Ni, its accumulation by the cells was lower at all time sections in the solutions with the metal mixtures, compared to the solution containing only Ni. In the cells exposed to the mixtures of the metals tested, Zn accounted for the largest proportion of the accumulated metals, followed by Cr and Ni (Fig. 3).
Removal of metals from solution
For all the metals studied, a gradual decrease in their concentrations in the solution remaining after the algae filtration was observed with increasing time (Fig. 2, right y-axis). The calculated metal removal values are presented in Table 2. Figure 2 and Table 2 show that the highest and fastest uptake by algae occurred in the treatment with Zn alone, since after 96 h the algae removed 83.4% of Zn from the solution. The rate of removal of Zn from the solution was approximately equal for both mixtures after 96 h and 2 times lower than that of the solution with Zn alone (Table 2). If Cr or Ni alone were added to the solution, the removal rate of these metals after 96 h was significantly lower than in the case of the addition of Zn alone, specifically 28.5% for Cr and 33.3% for Ni. In the Zn + Ni + Cr mixture, the removal of Cr and Ni from the solution after 96 h was lower compared to the solutions with single-metal addition, specifically 2.3 times and 1.3 times lower, respectively. For the Zn + Ni mixture, the Ni removal rate was 1.7 times lower than in the case of the solution with Ni alone (Table 2). In the case of Ni, the graph (Fig. 2c) shows that the removal rate was similar for treatments with Ni alone and for those treated with the Ni + Zn + Cr mixture, while the removal of Ni from the solution was lower for the binary mixture at all three time intervals (Fig. 2c). For the solution with the Zn + Cr + Ni mixture, Zn was removed in the highest amount, followed by Ni and then Cr for all time intervals. After 48 h, Zn was removed in the highest amount (treatment with the addition of Zn alone); on the other hand, Cr was removed minimally both in the solution with Cr alone and with the addition of Cr mixtures. After 96 h, although low Zn accumulation in the cells was observed, a high percentage of Zn removal from the solution was also observed, which is related to the essentiality of this metal and the low inhibition of growth rates and thus higher cell density, which was, for example compared to the Ni solution, approximately 9 times higher, i.e., a higher amount of cells removed more Zn. More algae remove more metals since there are more ligand sites for the metals.
Correlation between growth rate inhibition and metal concentration in cells
Significant correlations were observed for all metals between the metal content in the algal cells and inhibition of growth rates (Fig. 4). For the treatments with addition of Zn, a positive correlation was observed (Fig. 4a) between Zn content in the algal cells and the inhibition rates, the Pearson correlation coefficient was 0.547 (P = 0.0032). The graph shows that Zn alone causes a lower inhibition of growth rate at the same concentration of Zn in the algal cells than when Zn was added in the mixtures. This trend is not as significant for Cr (Fig. 4b), where the correlation coefficient was 0.74 (P = 0.004). The distortion could have been caused by a smaller amount of data for the mixtures with the addition of Cr. Figure 4c shows the relationship between Ni concentration in the cells and the inhibition of growth rates with a correlation coefficient of 0.669 (P < 0.0001); a lower accumulation of Ni in the cells was observed for the mixtures than for the addition of Ni alone, and even with low accumulation, a significant increase in growth rate inhibition was observed.
Changes in concentrations of selected biogenic elements in algae
During the time experiment, concentrations of selected biogenic elements in the algal cells were determined and the individual test treatments were compared to a control group of organisms. The test medium used to prepare the solutions analysed contained both macronutrients and trace elements important for algae growth. Of the selected nutrients, the concentration of which was measured at the beginning of the test prior to the addition of algae, the element contained at the highest concentration was Na (mean value of 14 mg L−1), followed by Ca (5 mg L−1), Mg (3 mg L−1), S (1.54 mg L−1), K (0.55 mg L−1) and P (0.15 mg L−1). Mn (109 µg L −1) and Zn (4.92 µg L−1) were present in trace amounts. Figure 5 shows changes in the concentrations of the selected biogenic elements in the algae cells for each treatment tested and in the control group over 96 h. Figure 5a, b, c clearly show that the content of the selected elements in the algae cells decreased over time in all treatments as well as in the control group. After 48 h, the highest content of all the elements monitored was observed in the treatment with the addition of Cr, which showed the highest toxicity and also the highest Cr concentrations in the cells (Fig. 5a). After 72 h, the differences in concentrations of the biogenic elements between the control group, the low toxicity treatments (Zn, Cr) and the more toxic treatments (Ni, Zn + Ni, Zn + Ni + Cr) became more pronounced (Fig. 5b). The highest content of all the biogenic elements monitored was observed in the cells affected by the addition of Ni, which showed the highest toxicity after 72 h of all the treatments as well as a high accumulation of Ni in the cells. After 96 h, the lowest concentrations of all the biogenic elements monitored were measured in the control group and the treatment with the addition of Zn, followed by the treatment with Cr; the highest concentrations were in the treatments with Ni and in the mixture of Zn + Ni + Cr, which were the most toxic. Significantly higher concentrations of P, K, S, Mn, Mg and Na in the cells were observed for all treatments with the addition of Ni after 96 h compared to the control group, in the ternary mixture even the concentration of Ca was higher.
Due to the large differences between the concentrations of all the biogenic elements monitored in the treatments and the control group (Fig. 5), for better comparison the concentrations of the biogenic elements in the cells were expressed as percentages of the total amount of the monitored biogenic elements (Fig. 6). The individual treatments were compared to the control group and the percentage changes in the representation of the monitored biogenic elements over time were observed. Figure 6 shows that the percentage of K was higher in the control group when compared to the other treatments, while a very low percentage of K was observed in all treatments with Ni after 48 h of exposure. In the toxic treatments with metals, a higher percentage of P was observed than in the control group cells. After 96 h, a similarity in the mineral composition between the control group and the almost non-toxic treatment with Zn is evident. The treatment with Cr, which also showed low toxicity, differed from the control group and the treatment with the addition of Zn mainly by the presence of a higher percentage of Ca and a lower percentage of K and Na. A similar percentage of the biogenic elements is evident for the treatment with the addition of Ni and the mixture of Ni + Zn, which differed from the control group mainly by a lower content of K and Ca and a higher content of P, Mn and Na. Compared to other treatments with Ni, the solution with Zn + Ni + Cr had a higher percentage of Ca and Na and a lower percentage of Mn and K. The analysis of the graphs in Fig. 6 shows that the content of the biogenic elements in the cells changed over time.
Discussion
Our study found that Zn was the least toxic metal when compared to Cr and Ni, which can be explained by the fact that Zn is one of the trace elements that are necessary for algae growth in small amounts and are therefore contained at low concentrations also in the test medium, but may act as an inhibitor at high concentrations as has been observed before (Juneja et al. 2013). The specific EC50 values depend on the organisms tested and the test conditions. EC50 of the green alga Chlorella sp. after 72 h was 120 µg Ni L−1 (Macoustra et al. 2021). Filová et al. (2021) studied the effect of ZnCl2 and NiCl2.6H2O on R. subcapitata and reported EC50 for these compounds after 96 h equal to 0.20 and 0.5 mg L−1, respectively. A lower toxicity of Ni than Zn for P. subcapitata is also reported by Nys et al. (2017). On the other hand, in many cases the toxicity of Ni for algae was observed to be higher than the toxicity of Zn (Nagai and De Schamphelaere 2016; Expósito et al. 2017; Al-Hasawi et al. 2020; Fettweis et al. 2021). For Ni and Desmodesmus sp., EC50 values were determined to be 570 µg Ni L−1 and 236 µg L−1 in hard and moderately hard water, respectively (Deleebeeck et al. 2009). These values roughly correspond to the value we obtained in the present study. Monteiro et al. (2011) reported EC50 for Zn after 96 h equal to 16.99 mg L−1 for Scenedesmus obliquus and 4.87 mg L−1 for Desmodesmus pleomorphus. EC50 of 153 ± 17.9 µg L−1 was determined for P. subcapitata and K2Cr2O7 (Vignati et al. 2010). The value of EC50 for Cr of Chlorella pyrenoidosa was 1.6 mg L−1 and an increase in the Cr concentration caused a significant reduction in the growth of Chlorella cells (Hörcsik and Balogh 2002).
The decreasing toxicity of Zn and Cr in the time course experiment for the solutions containing each metal corresponded to their decreasing accumulation in the cells and may have been caused by the fact that, unlike Ni, these metals were added to the solutions at concentrations well below their corresponding EC values. In contrast, in cells exposed to Ni, both toxicity and Ni accumulation increased with time. Zeng et al (2012) observed increased metal toxicity to cells depending on time due to increased intracellular metal concentrations. Gorbi et al. (2004) report that the toxic effect of Cr(VI) on algae may be related to the rate of bioaccumulation and the reduction of effect appears to be caused by the interaction between Cr(VI), exudates and algae cells. Cr(VI) suppressed the viability and autofluorescence of chlorophyll of Scenedesmus quadricauda, while Cr(III) had a higher stimulating effect on the formation of ROS and lipid peroxidation (Kováčik et al. 2015). Rai et al. (2013) observed a decrease in the growth, chlorophyll and protein content of C. vulgaris after its exposure to Cr; moreover, an increase in Cr concentration caused a significant decrease in cell density. Exposure of R. subcapitata to Cr significantly reduced algal growth and the mean cell fluorescence of chlorophyll a at the highest tested concentrations (15.36 – 19.2 µM of Cr) (Alho et al. 2022). Lower concentrations of Zn increased dry weight, chlorophyll a, b, carotenoids and total amino acid content in S. obliquus and S. quadricauda. On the other hand, higher Zn concentrations inhibited growth and other metabolic activities (Omar 2002). Ni in cells produces ROS, which can damage lipids on cell membranes; Ni can also replace the basic metals in metalloenzymes, causing disruption of metabolic pathways (Expósito et al. 2017).
In our experiment, both binary (Zn + Ni) and ternary (Zn + Ni + Cr) mixtures showed higher toxicity after 96 h of exposure compared to the toxicity of single-metal solutions in which only Zn or Cr was present, but lower toxicity compared to a solution containing only Ni. For both mixtures (Zn + Ni, Zn + Ni + Cr), antagonistic effects were observed. Nys et al. (2017) studied the antagonistic effects of ternary (Ni-Zn-Cu) and quaternary (Ni-Zn-Cu-Cd) mixtures on the growth of P. subcapitata. The mixture of Zn and Al has synergistic effects on algae at low doses and antagonistic effects at high doses (Gebara et al. 2020). The effect of an equitoxic mixture of Cu + Cd to the growth of Chlorella sp. corresponds more to concentration additive (synergistic interaction), while the combinations of Cu + Zn, Cd + Zn and Cu + Cd + Zn were all antagonistic (Franklin et al. 2002). Natural waters with different chemical characteristics were used for testing Cu-Ni and Cu-Ni-Zn mixtures on the growth of P. subcapitata. Only in one case, in water with a lower amount of ions, the Cu-Ni-Zn mixture had an antagonistic effect on algal growth (Van Regenmortel and De Schamphelaere 2018).
In the 96 h experiments, a decreasing accumulation of Zn and Cr by D. subspicatus cells was observed but an increasing Ni accumulation occurred, when these metals were present in the solutions individually. On the other hand, in the mixtures, the accumulation of Zn and Ni in the cells increased with time. In 72 h experiments with Microcystis cells, intracellular concentrations of Cd increased with exposure time, while for Zn they always reached a plateau, because Zn is an essential element which is maintained in homeostasis, i.e., even if new Zn enters the cells during exposure, the equivalent amount of Zn may be excluded from the cell. Cd, on the other hand, is a non-essential metal, bound in the cell to metallothionein and its excretion from the cytoplasm is not easy, therefore the intracellular concentration of Cd increased with the increasing time of exposure (Zeng et al. 2012). Ciurli et al. (2021) report a significant increase in the Zn content (32 mg Zn (100 g DW)−1) in Chlorella after the first week of growth in a solution with Zn (4 mg L−1), which continued until the third week when the level of Zn in the algae reached 484 mg (100 g DW)−1. Zn2+ was found to be adsorbed more on the algae surface, e.g., the cell surface of the green alga C. reinhardtii adsorbed 6 times more Zn2+ than was adsorbed inside the cells (Wang et al. 2012).
Accumulation of Cr in Scenedesmus acutus was noticeable already after 2 days of exposure (Gorbi et al. 2001). Maximum Cr accumulation by C. vulgaris was recorded after 96 h at a Cr concentration of 100 µg mL−1 (3,579.01 µg g−1 dw). After 120 h, the accumulation rate was lower than for treatments with shorter exposure times (Rai et al. 2013). With increasing concentrations of Cr and Zn in the solution, the concentration of these metals in D. subspicatus cells increased, with a significant increase in accumulation observed from the moment the metal concentration in the solution corresponded to EC50 (Bucková et al. 2022). For C. reinhardtii, after 1 h of exposure, the main part of the accumulated Cr (Cr VI and III) was found in the adsorbed fraction and after 72 h the main part was found in the intracellular (absorbed) fraction (Aharchaou et al. 2017). Hörcsik and Balogh (2002) also report that a large portion of Cr (70%) was localised in the cell wall of C. pyrenoidosa, while the membrane and the soluble fraction contained a smaller portion of the total Cr. Cr was absorbed by C. vulgaris cells, where it disrupted the structure of chloroplast and a part of the Cr absorbed by the cells was transported into a vacuole (Qian et al. 2013).
Filová et al. (2021) report that the amount of Ni accumulated in algae cells increased with an increasing concentration of Ni in the solution and corresponded to 0.023 and 0.496 µg mg−1 dry weight for concentrations of 0.25 mg L−1 and 0.90 mg L−1, respectively. The green alga Dunaliella sp. was able to accumulate 0.005 pg cell−1 of Ni within 1 day for concentrations of 100 mg Ni L−1 and after 7 days these values increased to 0.05 pg cell−1, while a rapid increase was observed for the total amount of Ni in the algal cells (adsorbed and intracellular) during day 1, followed by a decrease which was more pronounced mainly for higher Ni concentrations, and from day 5 onwards, an increase in Ni concentration in the cells was again observed (Dahmen-Ben Moussa et al. 2018). In our experiment, an increase in Ni accumulation in the cells was observed from 0.039 (day 2) to 0.068 pg cell−1 (day 4) at a Ni concentration of 80 µg L−1. Biosorption of Chlorella cells was rapid within the first few minutes, then it slowed down, and after 1 h of the Ni action, no apparent increase in Ni cell absorption was observed (Wong et al. 2000). The accumulation of Cu, Cd, Ni, Pb and Zn by P. subcapitata reached steady state within 24 to 48 h, with the rate of uptake of Cu being higher than for other metals and the lowest for Ni (Komjarova and Blust 2009).
Essential and nonessential metals can share the same uptake pathways and interact with each other, which affects their uptake, bioaccumulation and toxicity, and leads to different effects (from antagonistic to synergistic action) (Flouty and Khalaf 2015). In our experiment, the accumulation of Zn, Cr and Ni by the algal cells in both mixtures was lower after 48 and 72 h than if the algae were exposed to the metals individually. However, there was a change after 96 h of exposure when Zn and Cr accumulation was higher in cells exposed to binary and ternary mixtures compared to solutions of single metal. For Ni the effect was the opposite. Franklin et al. (2002) observed a decrease in the extracellular and intracellular concentrations of Zn in Chlorella sp. cells with the simultaneous presence of Cu in the medium compared to Zn alone, which was caused by competition between Cu and Zn ions for cell binding sites and led to a decrease in growth inhibition. On the other hand, Zn had no noticeable effect on Cu intake by algal cells after 48 and 72 h. Mehta and Gaur (2001) state that the total metal (Ni + Cu) sorbed from the binary metal solution by C. vulgaris cells was lower than the total sorption of individual metals from their respective single-metal solutions, indicating competition between Ni and Cu for common binding points on Chlorella, with the algae having a greater affinity for Cu than Ni. It was shown that in C. reinhardtii Cu2+ competed strongly with Ni in absorption because the alga had a higher binding affinity for Ni transport sites and reduced Ni uptake at high concentrations. On the other hand, Ni had no effect on the uptake of Cu or Pb (Flouty and Khalaf 2015). When Ni has been tested in combination with other metals (Cu2+, Cu+, Mn2+, Mo6+, V5+), its accumulation in S. quadricauda cells has been reduced in all cases. This means that all the metals tested had an inhibitory effect on Ni accumulation (Fargašová et al. 1997). Franklin et al. (2002) state that toxicity expressed as inhibition of growth was related to both extracellular and intracellular Cu concentrations and, if Zn was added to Cu, the toxicity of the mixture was also affected by the intracellular concentration of Zn.
The ability of algae to remove metals is used in the bioremediation of wastewater. Removal of Zn, Cr and Ni by D.subspicatus cells was significantly higher if the metals were found in single-metal solutions, not in mixtures, where the rate of removal of Zn was the highest, followed by removal of Ni and Cr. Alharbi (2017) reported that within 12 days, Chroococcus minutus was able to remove 68% of Zn2+ from a solution of 10 mg L−1 Zn2+ and Chlorococcum aegyptiacum removed 35%. Chlorella removed 4.5% of Zn after 7 days and 28–30% of Zn after 2 weeks, while 60% of Mn was removed within 1 week (Ciurli et al. 2021).
The high amount of biogenic elements observed in all treatments with the addition of Ni (Ni, Zn + Ni, Zn + Ni + Cr), which showed higher toxicity, could be explained by the fact that toxic metals can change cell morphology, its shape and size, as well as cell wall permeability. Franklin et al. (2002) state that the size of Chlorella sp. cells increased with increasing concentrations of Cu, Cd and Zn in the medium with a maximum increase after 48 h; the largest increase in size was caused by Cu (51% after 72 h), Zn caused an increase of 18% after 72 h. Also, binary metal combinations caused similar increases in the size of Chlorella cells, up to 50% of cells were enlarged compared to control cells. Changes in cell size and cell complexity of R. subcapitata due to Zn and Al are also reported in Gebara et al. (2020). Exposure of algae to Cr caused cell volume to increase, but the size of chloroplast reduced and the size of the vacuole increased (Qian et al. 2013). On the other hand, Dahmen-Ben Moussa et al. (2018) state that cells defend themselves against oxidative stress caused by the presence of Ni by reducing cell size, which limits the exchange with the external medium and induces a decrease in Ni absorption and it may also cause damage to the cell membrane. Zn, Pb and Cd caused a significant change in the shape of the cell wall, damage to chloroplasts, complete disorganisation of cellular components and formation of granules, cell wall breakdown and cell death in the case of the green alga C. aegyptiacum (Alharbi 2017).
In our study, the content of the biogenic elements in the cells changed over time and was probably influenced by the toxicity of the metals, their accumulation in the algal cells, as well as by whether they were essential or non-essential metals. The toxic action of Ni can be caused by competition of Ni2+ with other metal cations. Ni can replace Mg, Ca, Zn, Cu (Dahmen-Ben Moussa et al. 2018; Nowicka 2022), because it enters the cell by the same transport systems that are used for these physiologically important metal cations (Expósito et al. 2017). Flouty and Khalaf (2015) mention the interference of Ni with Mg and Na transporters. The inhibitory effect of Zn2+ may be associated with the replacement of Mn2+ and Ca2+. Zn causes deficiency of Fe2+, Mn2+ and Cu2+ (Nowicka 2022). In our experiment, a lower percentage of Ca was observed for the treatments with Zn after 48 and 72 h, when Zn showed toxic effects on algae and also for treatments with Ni (72 h, 96 h). The explanation may be the same mechanism of the toxic effect of Zn and Ni on cells, namely displacement of essential metal cations (Fig. 6). Kováčik et al. (2018) reported that cells exposed to metals actively retain Na, unlike cells of the control group that release Na. The same was observed after 96 h of exposure in all treatments with Ni (Fig. 6). The low percentage of K observed in cells treated with Ni can be explained by its leakage due to a change in the permeability of the plasma membrane (Alharbi 2017).
Exposure of C. vulgaris to Cr increased the uptake of Mg, Ca and Na (Qian et al. 2013). On the other hand, Hörcsik and Balogh (2002) state that the high concentration of Cr in C. pyrenoidosa cells caused higher Ca concentrations and lower concentrations of Mg and Fe in cells due to ion exchange. In our experiment (Cr treatment) we found that after 48 h of exposure, when the Cr concentration in the cells was highest, a higher percentage of Na and a lower percentage of Mg and Ca were observed compared to the control group. After 96 h the cells in Cr treatment contained a significantly higher percentage of Ca than control group but the amount of Mg and Na was comparable to the control group (Fig. 6). The high amount of Ca in Cr treated cells can be explained by the different transport mechanism of Cr compared to Ni and Zn, whereby Cr may be taken up in algal cells instead of Fe (Volland et al. 2012).
Conclusion
Based on 72 h tests with the concentration series of K2Cr2O7, NiCl2.6H2O and ZnCl2, it was found that Ni was the most toxic to the green alga D. subspicatus, followed by Cr and Zn. The lowest toxicity of Zn is due to the fact that Zn belongs among the essential elements necessary for algae growth. In the case of single-metal solutions (Zn Cr), the accumulation of these metals by the cells and the toxicity of these solutions were found to decrease over 96 h. On the other hand in the case of solutions containing Ni (Ni, Zn + Ni, Zn + Ni + Cr), their toxicity and the accumulation of Ni by the cells increased. For all three metals tested, significant correlations were found between the amount of the metal accumulated in the cells and the inhibition of growth rates. After 96 h of exposure, the presence of another metal in the mixture caused a higher accumulation of Zn and Cr compared to the single-metal solutions. On the contrary, the accumulation of Ni by the cells was lower in the solutions treated with the mixtures at all time sections of the experiment than if the algae were exposed to the solution containing only Ni; this finding points to inhibition of Ni uptake due to the presence of other metals.
When the metals were present in the single-metal solutions, there was a significantly higher removal of the metal from the solution than in the case of the mixtures. The highest rate of metal removal from the solution occurred in the case of Zn.
The content of the selected biogenic elements (Na, K, Ca, Mg, P, S, Mn) in the algal cells decreased over time in all treatments with metals as well as in the control group. After 96 h the concentration of the biogenic elements was significantly higher in all treatments with the addition of Ni compared to the control group and treatments with the single-metal solutions of Zn and Cr. This could be caused, for example, by larger cell sizes or cell wall disruptions. In algae exposed to Cr, both individually and in a mixture, the percentage of Ca in the cells increased after 96 h of exposure. On the other hand, in the case of Ni, a decrease in the percentage of Ca was observed with increasing exposure time. This may be explained by different transport mechanisms for Cr and Ni, where Ni may undergo ion exchange between Ni and Ca ions. Due to the low toxicity of Zn, no significant changes in Ca concentrations after 96 h were observed.
Green algae are important organisms in aquatic ecosystems and they are found at the base of the food chain and are capable of accumulating toxic metals. Therefore, it is important to know their interaction with toxic metals and the mixtures thereof, which can be used both in risk assessment but also in bioremediation of wastewater.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aharchaou I, Rosabal M, Liu F, Battaglia E, Vignati DAL, Fortin C (2017) Bioaccumulation and subcellular partitioning of Cr(III) and Cr(VI) in the freshwater green alga Chlamydomonas reinhardtii. Aquat Toxicol 182:49–57
Al‐Hasawi ZM, Abdel‐Hamid MI, Almutairi AW, Touliabah HE (2020) Response of Pseudokirchneriella subcapitata in free and alginate immobilized cells to heavy metals toxicity. Molecules 25:2847
Alharbi RM (2017) Toxicity and bioaccumulation of lead, cadmium and zinc in Chroococcus minutus and Chlorococcum aegyptiacum. Int J Pharm Res Allied Sci 6:290–300
Alho L de OG, Gebara RC, Mansano A da S, Rocha GS, Melão M da GG (2022) Individual and combined effects of manganese and chromium on a freshwater Chlorophyceae. Environ Toxicol Chem 41:1004–1015
Bucková M, Hegrová J, Jandová V, Svoboda J, Ličbinský R (2022) Evaluation of toxicity and bioaccumulation of metals from transport and their effect on the mineral composition of green alga Desmodesmus subspicatus using ICP MS/MS. J Appl Phycol 34:2735–2742
Ciurli A, Di Baccio D, Scartazza A, Grifoni M, Pezzarossa B, Chiellini C, Mariotti L, Pardossi A (2021) Influence of zinc and manganese enrichments on growth, biosorption and photosynthetic efficiency of Chlorella sp. Environ Sci Pollut Res 28:8539–8555
Dahmen-Ben Moussa I, Athmouni K, Chtourou H, Ayadi H, Sayadi S, Dhouib A (2018) Phycoremediation potential, physiological, and biochemical response of Amphora subtropica and Dunaliella sp. to nickel pollution. J Appl Phycol 30:931–941
Deleebeeck NME, De Laender F, Chepurnov VA, Vyverman W, Janssen CR, De Schamphelaere KAC (2009) A single bioavailability model can accurately predict Ni toxicity to green microalgae in soft and hard surface waters. Water Res 43:1935–1947
Expósito N, Kumar V, Sierra J, Schuhmacher M, Giménez Papiol G (2017) Performance of Raphidocelis subcapitata exposed to heavy metal mixtures. Sci Total Environ 601–602:865–873
Fargašová A, Bumbálová A, Havránek E (1997) Metal bioaccumulation by the freshwater alga Scenedesmus quadricauda. J Radioanal Nucl Chem 218:107–110
Fettweis A, Bergen B, Hansul S, De Schamphelaere K, Smolders E (2021) Correlated Ni, Cu, and Zn sensitivities of 8 freshwater algal species and consequences for low-level metal mixture effects. Environ Toxicol Chem 40:2015–2025
Filová A, Fargašová A, Molnárová M (2021) Cu, Ni, and Zn effects on basic physiological and stress parameters of Raphidocelis subcapitata algae. Environ Sci Pollut Res 28:58426–58441
Flouty R, Khalaf G (2015) Role of Cu and Pb on Ni bioaccumulation by Chlamydomonas reinhardtii: Validation of the biotic ligand model in binary metal mixtures. Ecotoxicol Environ Saf 113:79–86
Franklin NM, Stauber JL, Lim RP, Petocz P (2002) Toxicity of metal mixtures to a tropical freshwater alga (Chlorella sp.): The effect of interactions between copper, cadmium, and zinc on metal cell binding and uptake. Environ Toxicol Chem 21:2412–2422
Gebara RC, Alho L de OG, Rocha GS, Mansano A da S, Melão M da GG (2020) Zinc and aluminum mixtures have synergic effects to the algae Raphidocelis subcapitata at environmental concentrations. Chemosphere 242:125231
Gorbi G, Corradi MG, Invidia M, Bassi M (2001) Light intensity influences chromium bioaccumulation and toxicity in Scenedesmus acutus (Chlorophyceae). Ecotoxicol Environ Saf 48:36–42
Gorbi G, Invidia M, Zanni C, Torelli A, Corradi MG (2004) Bioavailability, bioaccumulation and tolerance of chromium: Consequences in the food chain of freshwater ecosystems. Ann Chim 94:505–513
Hamed SM, Zinta G, Klöck G, Asard H, Selim S, AbdElgawad H (2017) Zinc-induced differential oxidative stress and antioxidant responses in Chlorella sorokiniana and Scenedesmus acuminatus. Ecotoxicol Environ Saf 140:256–263
Hörcsik ZT, Balogh Á (2002) Intracellular distribution of chromium and toxicity on growth in Chlorella pyrenoidosa. Acta Biol Szeged 46:57–58
ISO (2012) International Organization for Standarization. ISO 8692. Water Quality - Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae. Geneva, Switzerland
Juneja A, Ceballos RM, Murthy GS (2013) Effects of environmental factors and nutrient availability on the biochemical composition of algae for biofuels production: A review. Energies 6:4607–4638
Komjarova I, Blust R (2009) Application of a stable isotope technique to determine the simultaneous uptake of cadmium, copper, nickel, lead, and zinc by the water flea Daphnia magna from water and the green algae Pseudokirchneriella subcapitata. Environ Toxicol Chem 28:1739–1748
Kováčik J, Babula P, Hedbavny J, Kryštofová O, Provaznik I (2015) Physiology and methodology of chromium toxicity using alga Scenedesmus quadricauda as model object. Chemosphere 120:23–30
Kováčik J, Bujdoš M, Babula P (2018) Impact of humic acid on the accumulation of metals by microalgae. Environ Sci Pollut Res 25:10792–10798
Leong YK, Chang JS (2020) Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour Technol 303:122886
Macoustra GK, Jolley DF, Stauber JL, Koppel DJ, Holland A (2021) Speciation of nickel and its toxicity to Chlorella sp. in the presence of three distinct dissolved organic matter (DOM). Chemosphere 273:128454
Mehta SK, Gaur JP (2001) Removal of Ni and Cu from single and binary metal solutions by free and immobilized Chlorella vulgaris. Eur J Protistol 37:261–271
Monteiro CM, Fonseca SC, Castro PML, Malcata FX (2011) Toxicity of cadmium and zinc on two microalgae, Scenedesmus obliquus and Desmodesmus pleiomorphus, from Northern Portugal. J Appl Phycol 23:97–103
Nagai T, De Schamphelaere KAC (2016) The effect of binary mixtures of zinc, copper, cadmium, and nickel on the growth of the freshwater diatom Navicula pelliculosa and comparison with mixture toxicity model predictions. Environ Toxicol Chem 35:2765–2773
Nowicka B (2022) Heavy metal–induced stress in eukaryotic algae—mechanisms of heavy metal toxicity and tolerance with particular emphasis on oxidative stress in exposed cells and the role of antioxidant response. Environ Sci Pollut Res 29:16860–16911
Nys C, Van Regenmortel T, Janssen CR, Blust R, Smolders E, De Schamphelaere KAC (2017) Comparison of chronic mixture toxicity of nickel-zinc-copper and nickel-zinc-copper-cadmium mixtures between Ceriodaphnia dubia and Pseudokirchneriella subcapitata. Environ Toxicol Chem 36:1056–1066
OECD (2011) Test guideline no. 201. Freshwater alga and cyanobacteria, growth inhibition test. Organization for Economic Cooperation and Development, Paris
Omar HH (2002) Bioremoval of zinc ions by Scenedesmus obliquus and Scenedesmus quadricauda and its effect on growth and metabolism. Int Biodeterior Biodegrad 50:95–100
Qian H, Sun Z, Sun L, Jiang Y, Wei Y, Xie J, Fu Z (2013) Phosphorus availability changes chromium toxicity in the freshwater alga Chlorella vulgaris. Chemosphere 93:885–891
Rai UN, Singh NK, Upadhyay AK, Verma S (2013) Chromate tolerance and accumulation in Chlorella vulgaris L.: Role of antioxidant enzymes and biochemical changes in detoxification of metals. Bioresour Technol 136:604–609
Rai UN, Tripathi RD, Kumar N (1992) Bioaccumulation of chromium and toxicity on growth, photosynthetic pigments, photosynthesis, in vivo nitrate reductase activity and protein content in a chlorococcalean green alga Glaucocystis nostochinearum Itzigsohn. Chemosphere 25:1721–1732
Salama ES, Roh HS, Dev S, Khan MA, Abou-Shanab RAI, Chang SW, Jeon BH (2019) Algae as a green technology for heavy metals removal from various wastewater. World J Microbiol Biotechnol 35:75
Van Regenmortel T, De Schamphelaere KAC (2018) Mixtures of Cu, Ni, and Zn act mostly noninteractively on Pseudokirchneriella subcapitata growth in natural waters. Environ Toxicol Chem 37:587–598
Vignati DAL, Dominik J, Beye ML, Pettine M, Ferrari BJD (2010) Chromium(VI) is more toxic than chromium(III) to freshwater algae: A paradigm to revise? Ecotoxicol Environ Saf 73:743–749
Volland S, Lütz C, Michalke B, Lütz-Meindl U (2012) Intracellular chromium localization and cell physiological response in the unicellular alga Micrasterias. Aquat Toxicol 109:59–69
Wang NX, Zhang XY, Wu J, Xiao L, Yin Y, Miao AJ, Ji R, Yang LY (2012) Effects of microcystin-LR on the metal bioaccumulation and toxicity in Chlamydomonas reinhardtii. Water Res 46:369–377
Wei B, Jiang F, Li X, Mu S (2010) Contamination levels assessment of potential toxic metals in road dust deposited in different types of urban environment. Environ Earth Sci 61:1187–1196
Winther M, Slentø E (2010) Heavy metal emissions for danish road transport. National Environmental Research Institute, Aarhus University, Denmark. pp 99– NERI Technical Report no. 780. http://www.dmu.dk/Pub/FR780.pdf
Wong JPK, Wong YS, Tam NFY (2000) Nickel biosorption by two Chlorella species, C. vulgaris (a commercial species) and C. miniata (a local isolate). Bioresour Technol 73:133–137
Zeng J, Zhao DY, Ji YB, Wu QL (2012) Comparison of heavy metal accumulation by a bloom-forming cyanobacterium, Microcystis aeruginosa. Chin Sci Bull 57:3790–3797
Acknowledgements
Authors acknowledge to Ministry of Transport of Czech Republic for the financial support.
Funding
Open access publishing supported by the National Technical Library in Prague. This article was produced with the financial support of the Ministry of Transport within the programme of long-term conceptual development of research institutions.
Author information
Authors and Affiliations
Contributions
Martina Bucková: Conceptualization, Methodology, Data curation, Formal analysis and investigation, Writing – original draft, Writing – review & editing. Jitka Hegrová: Conceptualization, Methodology, Data curation, Formal analysis, and investigation, Writing – original draft, Writing – review & editing. Vilma Jandová: Formal analysis and investigation, Writing – review & editing. Josef Svoboda: Formal analysis and Investigation, Writing – review & editing. Jiří Huzlík: Data curation, Writing – review & editing. Roman Ličbinský: Supervision, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bucková, M., Hegrová, J., Jandová, V. et al. Study of bioaccumulation of Cr, Ni and Zn by the green alga Desmodesmus subspicatus and assessment of the toxic effect of selected elements and their mixtures on algae cells. J Appl Phycol 35, 2241–2256 (2023). https://doi.org/10.1007/s10811-023-03072-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03072-2