Abstract
The marine bacterium Vibrio parahaemolyticus is the leading cause of seafood-related food poisoning worldwide and a pathogen of marine species harvested in aquaculture. An outbreak of pathogenic V. parahaemolyticus within crustacean and bi-valve aquaculture facilities often results in significant loss of farmed product and in the spread of the pathogen into the human population. Evidence shows several marine bacteria and microalgae species have antibacterial effects against marine pathogens, including V. parahaemolyticus. This study explored the potential of combining possible mutualistic species as a consortium to enhance antibacterial properties against V. parahaemolyticus. Marine bacteria and microalgae were screened with the aim of identifying those able to successfully coexist while demonstrating growth suppression of multiple V. parahaemolyticus strains. This trial involved four screening phases to find effective inhibitor species and to gain insight into species-specific influences on cell growth. The combination of Tetraselmis sp. and Pseudoalteromonas peptidolytica achieved the greatest inhibition rate of V. parahaemolyticus of all combinations. This pairing resulted in the significant reduction of up to 24 ± 15% in Vibrio sp. copy number mL−1 day−1 in two of the five examined V. parahaemolyticus strains within five days, compared to control cultures absent of probiotic bacteria. Applying probiotic consortia such as this has the potential for use as a biocontrol technique within mariculture, but it will require additional research into the self-sustainability of successful consortia in natural aquatic conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pathogenic bacteria pose a continuous and global threat to food production in the aquaculture industry (Rodger 2016). Of particular concern are pathogens that bring significant economic loss through stock mortalities via infection, jeopardise industry reputation, and have the potential to induce severe gastrointestinal disease in human consumers (Wang et al. 2022a). Vibrio is a common genus of marine bacteria found within the planktonic phase, biofilms and in association with marine animals (Kalatzis et al. 2018). Within this genus are several species of notable pathogens, of which 12 species are considered pathogenic toward humans (Tarr et al. 2007). These species are the primary cause of the gastrointestinal disease vibriosis in human populations (Mohamad et al. 2019; de Souza Valente and Wan 2021). Vibrio parahaemolyticus is found ubiquitously in marine and brackish ecosystems (Baker‐Austin et al. 2010) and is a fast-growing bacterium (Miles et al. 1997) that presents in both pathogenic and non-pathogenic strains (Lopez-Joven et al. 2015). An infection from this species is described to represent “a significant threat to human health worldwide” (Letchumanan et al. 2019), however infection is often resultant from the consumption of infected seafood.
Pathogenic strains of V. parahaemolyticus are the cause of vibriosis infection in bi-valves, crustacea, and fish (Fang et al. 1987; Jakšić et al. 2002) and have been responsible for multiple downturns in aquaculture production by up to 40% depending on the region (Kumar et al. 2021). These strains of V. parahaemolyticus produce several toxins that can result in the development of Acute Hepatopancreatic Necrosis Disease (AHPND) in infected bi-valves and crustacea (López-León et al. 2016). Cases of high infection rates are typically found within aquaculture facilities where V. parahaemolyticus has an increased chance to proliferate due to certain undesirable conditions such as high density of farmed species, high-temperature environments, and high loading of organic matter (Song et al. 2017; Widiyanto et al. 2020).
AHPND has been recorded across multiple continents (Kumar et al. 2020), but is found primarily throughout Asia and South America, where farmed stock fatalities due to infection are estimated to have caused an accumulated economic loss in aquaculture industries of more than 43 billion US$ (Kumar et al. 2021). Hatchery facilities are notably vulnerable to Vibrio infection, as larval and juvenile stage organisms typically have lesser immune resistance than adults and are less likely to survive (Skjermo and Vadstein 1999). A recent approach to preventing the growth of pathogenic marine bacteria in the context of intensive/closed-system aquaculture involves utilising probiotic bacteria and/or microalgae as a method of biocontrol (Natrah et al. 2014). The introduction of probiotic species can reduce proliferation of the pathogenic species through resource competition or direct/indirect antagonism, and reduce the pathogen population size to a safer level (Fuentes et al. 2016).
In order to properly select a probiotic species, its ecological interactions in response to other organisms must be understood (Gullian et al. 2004). Relationships between microalgae and bacteria represent critical ecological associations within marine ecosystems (Natrah et al. 2014) and can span antagonistic to mutualistic interactions. These relationships are complex, species-specific, and can readily change based on available resources (Seymour et al. 2017). In antagonistic relationships, including parasitism, resource competition, and direct antagonism, either organism may release bioactive molecules to disrupt competitor cellular functions, commonly via membrane lysis, protein deactivation, and communication disruption (Kokou et al. 2012). The production of these molecules may be encouraged or suppressed by introducing signalling cues or by the selection of species present. These relationships can switch from antagonistic to mutualistic, or vice versa, in the presence of an additional organism and its accompanying metabolites and signalling molecules (Stock et al. 2019). In mutualistic relationships between microalgae and bacteria, microalgae will often release oxygen and organic carbon for uptake by the surrounding bacteria, who in return remineralize nutrients for uptake by the microalgae (Zhang et al. 2020). This interaction can also involve the exchange of bioactive metabolites and is often associated with algal growth factors (vitamins, phytohormones, siderophores) exuded by bacteria (Palacios et al. 2022). Conversely, microalgae can also promote bacterial growth by releasing various, largely under-investigated secondary metabolites into the phycosphere (Natrah et al. 2014).
Studies of the relationships between microalgae and bacteria have explored their combined production of valuable bioactive molecules, leading to investigations of their benefits when cultured together (Perković et al. 2022). Phytoplankton-bacterial consortia have been examined as an alternative to antibiotics and have shown potential for disease control in shrimp mariculture (Wang et al. 2022b). One of the first studies to test the effect of a probiotic bacteria-microalgae consortium in aquaculture disease control (Chang et al. (2020) specifically investigated the use of consortia as a solution to V. parahaemolyticus infection in shrimp mariculture. The study found that a consortium of Picochlorum sp., a species of green microalgae, with one or more of its naturally associated bacteria, could significantly lower the cell density of total Vibrio over an 8-day period. While research in this area is limited, this study has highlighted the potential of this approach for intensive aquaculture practice, and we seek to expand upon this study with a wider array of microalgae and bacteria, to test the ability of non-associated microalgae and bacteria to grow successfully as consortia with the capacity to suppress the growth of V. parahaemolyticus.
Methods
Bacterial culture sourcing and maintenance
We examined the growth inhibition effects of 24 bacteria, spanning 13 genera (Tables 1 and SP 1), on five strains of Vibrio parahaemolyticus. The test bacteria were selected based on an extensive literature review of species that exhibit antimicrobial action against V. parahaemolyticus, and which of these species were available in the UTS, NSW (University of Technology Sydney, New South Wales, Australia) culture collection.
Each bacterial isolate was grown in marine broth (BD Marine Broth 2216), mixed with glycerol at a final concentration of 25%, snap-frozen with liquid nitrogen and stored at -80 °C. Before each experiment, new bacterial cultures were grown from the -80 °C stocks on marine agar to check for contamination before transfer to marine broth for overnight culture.
Microalgae culture sourcing and maintenance
Microalgal cultures were maintained in 500 mL aerated photobioreactors (Table 2). Cultures were supplemented with F/2 algal medium (Guillard and Ryther 1962) and incubated under 60—80 µmol photons m−2 s−1 light (Carvalho et al. 2009; Ihnken et al. 2011; Dammak et al. 2017) on a 12-h light cycle at 24 °C (Abdelaziz et al. 2014). Cultures were grown and kept on-site at UTS in Sydney, NSW (33.8688° S, 151.2093° E) and quality control performed regularly to ensure their integrity.
Microalgae enumeration
Cell abundance of microalgal cultures was determined using BD CytoFlex LX flow cytometer. Samples were fixed with 2% glutaraldehyde (pre-filtered at 0.2 µm), and refrigerated at 4 °C for a minimum of 20 min (Vaulot et al. 1989). Fixed samples were diluted using 33 ppt 0.22 µm filtered seawater sourced from Port Hacking NSW (34.0665° S, 151.1224° E). The seawater used for dilution was first filtered using MF-Millipore Membrane Filter, 0.22 µm pore size, and autoclaved at 121 °C for 20 min. Sample aliquots of 150 µL were dispensed into a 96 well plate for analysis. Microalgal cells were identified and enumerated according to red chlorophyll fluorescence using an excitation laser at wavelength 565 nm.
Growth of microalgae cultures was monitored during and between experimental phases using optical density (OD) measured on TECAN Spark microplate reader and Sparkcontrol software. Absorbance was measured between 400 to 750 nm, and chlorophyll fluorescence was measured at the excitation wavelength of 440 nm with 20 nm bandwidth, reading emission wavelength at 685 nm with 20 nm bandwidth (Jia et al. 2015). Manual gain set to 88 and z-position at 22,860 µm.
Bacterial Enumeration
For cell density measurement bacterial cultures were diluted at 1 × 104 with 33 ppt filtered seaweater, fixed using 2% glutaraldehyde solution and stored in the dark at 4 °C for 1 h. Samples were stained using SYBR Green I Nucleic Acid Gel Stain at 4 µL per 100 µL of sample and stored in the dark at 4 °C for 20 min. Bacterial abundance was quantified using a BD CytoFlex LX flow cytometer set to read SYBR Green stain fluorescence using an excitation laser at wavelength 535 nm.
Samples of the same bacterial cultures for OD measurement, with no initial dilution, were fixed using 2% glutaraldehyde solution and stored in the dark at 4 °C for 1 h. Fixed cultures were diluted to achieve a series of 5 samples between dilution ratios of 1:1 and 1:20 ascending of bacterial culture to 33 ppt FSW, at identical ratios to those made for the cell density measurement. The OD of these samples was taken using a TECAN Spark microplate reader and Sparkcontrol software, reading absorbance at 750 nm at 20 nm bandwidth, alongside blank samples containing the corresponding concentrations of marine broth and 33 ppt FSW present in the diluted bacterial cultures.
The cell density measurement of the bacterial cultures was plotted against the respective OD measurements for each dilution ratio, so OD may be used as a rapid proxy of bacterial cell density. This was repeated for all bacteria required for inoculation into algal cultures.
Sample collection and DNA extraction
During the final phase of the experiment, samples of 1 mL and 100 µL were collected from algal mother cultures and all experimental cultures and transferred to 1.5 mL tubes. 1 mL samples were centrifuged at 10,000 xg for 5 min, the supernatant removed, and pellet kept. All samples were stored at -30 °C for DNA extraction.
DNA was extracted using QIAGEN DNeasy UltraClean Microbial kit following manufacture instructions where sample DNA was eluted with 50 µL of elution buffer and kept at -30 °C. Three negative control extractions were performed to ensure the integrity of the DNA extraction kit.
Quantification of Vibrio with qPCR
To determine Vibrio abundance within our cultures, we used the Vibrio-specific 16S rRNA gene primers Vib1-F (5’-GGCGTAAAGCGCATGCAGGT-3’) and Vib2-R (5’-GAAATTCTACCCCCCTCTACAG-3’) (Gullian et al. 2004; Siboni et al. 2016), with counts quantified against a seven-point calibration curve prepared from a gene block standard. All qPCR runs were prepared with an epMotion 5075I Automated Liquid Handling System and performed on a Bio-Rad CFX384 Touch Real-Time PCR Detection System, with three technical replicates, a seven-point standard curve, and negative controls. A melting curve was also added to the end of the Vibrio-specific assay to confirm the presence of a single PCR product. The reaction mixture included: 2.5 µL iTaq Universal SYBR Green SMX (Bio-Rad), 0.2 µL of each 10 µM forward and 10 µM reverse primer, 1 µL of template DNA, and 1.1 µL of sterile water for a final reaction volume of 5 µL.
Vibrio-specific 16S rRNA qPCR was performed using the following cycling conditions: 95 °C for 3 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min, followed by a melting curve. For quality control, the percent coefficient of variation (%CV) was calculated for the qPCR technical triplicates, and where necessary, triplicates with a %CV of greater than 2% had a technical replicate removed from the analysis.
Experimental design
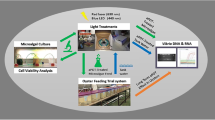
The study was separated into four experimental phases (Fig. 1) for two purposes: (i) to screen for algal and bacterial species that inhibited the growth of V. parahaemolyticus; (ii) to understand how the groups of microorganisms (microalgae, test bacteria, and V. parahaemolyticus) react to each other’s presence in terms of changes in growth rate, to more clearly define species-specific interactions.
Screening Phases
Phase 1
A well diffusion plate assay was used to test for antagonism of probiotic test bacteria against V. parahaemolyticus strains. This assay was conducted as per methodology described by (Raina et al. 2016) using 5 biological replicates per test bacteria species. The diffusion agar composition was amended to suit V. parahaemolyticus as follows: 1.00% bacteriological agar (Agar No. 1, Oxoid), 3.00% evaporated sea salt, 0.50% cenozoic acid, 0.10% glucose solution, and 0.2 µm filtered reverse osmosis water. The inhibition zone diameter of each well inoculated with test bacteria was measured using a non-digital calliper in 0.5 mm increments. Probiotic species demonstrating at least one inhibition zone with a diameter greater than 0.0 mm were considered successful.
Phase 2
Co-culture experiments to determine the effect of each species of test bacteria on microalgal growth were performed in Falcon 12-well Clear Flat Bottom TC-treated Multiwell Cell Culture Plates (Abdelaziz et al. 2014; Temkin et al. 2019). All parent cultures were diluted with F/2 Guillard’s algal medium at 2:1 algal culture to medium ratio one week preceding bacterial inoculation to ensure microalgal cultures were in exponential phase for better determination of change to the exponential growth rate. Following this, 100 µL of algal culture was plated on Vibrio selective agar (TCBS) to confirm the absence of Vibrio spp. in algal cultures before inoculation (Di Pinto et al. 2011). Test bacteria species were inoculated into respective wells at a 1:10 algae to bacteria cell ratio in a randomised arrangement with 3 biological replicates. Cell density measurements were taken before and after 48 h incubation at 24 °C with shaking at 150 rpm, and light at 60—80 µmol photons m−2 s−1 on a 12-h light cycle (Han et al. 2016).
Phase 3
Co-culture experiments to determine the effect of each strain of V. parahaemolyticus on microalgal growth were performed in 12-well clear flat-bottom plates, whereby the methodology was identical to Phase 2, except probiotic test bacteria species were substituted for V. parahaemolyticus strains. The V. parahaemolyticus strains that had the greatest influence on microalgal growth were chosen as suitable candidates for Phase 4 experiments.
Selection for Phase 4 bacteria candidates
Vibrio parahaemolyticus and test bacteria candidates for Phase 4 were chosen as follows; from Phase 1 by selecting test bacteria responsible for the highest levels of growth inhibition of V. parahaemolyticus. From Phase 2 by selecting test bacteria for greatest algal growth promotion, as these species would be the most likely to form successful co-cultures. From Phase 3 by selecting V. parahaemolyticus strains for the greatest algal growth reduction, to use the most impactful strains.
Phase 4 – Co-culture of microalgae, V. parahaemolyticus, and probiotic bacteria
The growth rates of each microalgae species and V. parahaemolyticus strain were monitored over six-day period to determine any changes brought on by the presence of probiotic bacteria, in comparison to the following negative controls; negative control Type 1 – microalgae only, in F/2 medium to ensure proper growth and health of starting culture; negative control Type 2 – microalgae and V. parahaemolyticus co-culture, with strains V#1 and V#5 separately. A 24-h acclimatisation period was used following each microalgal and bacterial inoculation. Microalgae parent cultures were diluted with F/2 medium, at a 2:1 algl culture to medium ratio, one week before the experimental day zero. The experimental co-cultures were grouped by species of microalgae, each species designated three 12-well plates, giving 3 biological replicates of each co-culture. For inoculation, overnight cultures of bacteria were diluted with 33 ppt FSW and inoculated into well-plates to achieve a 1:10 microalgae to bacteria cell ratio in 100 µL of bacterial inoculum, customised to the algal cell density of each well individually. Followed by 100 µL of marine broth diluted with 33 ppt FSW to account for variation in trace marine broth in each culture. Ensuring the concentration of marine broth across all wells was equal, including in negative controls. Well plates were sealed with Parafilm M to limit evaporation, cross-contamination within the plate, and allow for gas exchange.
On day zero, 1 mL of parent culture from each microalgae species was prepared for later DNA extraction and qPCR, to confirm 0 Vibrio sp. copy numbers per mL present in each parent culture before V. parahaemolyticus inoculation. Parent cultures were enumerated through flow cytometry, and diluted with F/2 medium, then inoculated into the designated 12-well plates (Qiao, 2021) at 1.6 mL for a total of 2.0 mL on the addition to all bacterial cultures. The plates were measured for OD and chlorophyll fluorescence and then sealed with one layer of Parafilm M. Plates were placed into Eppendorf Innova S44i shaking incubator at 24 °C, 50 rpm orbital shaking, and 60—80 µmol photons m−2 s−1 of light on a 12-h light cycle. Algal cultures were allowed 24 h for acclimatisation.
On day one, the cell density of each microalgae culture was measured, and overnight marine broth cultures of probiotic bacteria were enumerated through rapid OD proxy. Overnight cultures of probiotic bacteria were diluted accordingly with 33 ppt FSW and inoculated into the designated well-plates.
On day two, the algae cell density of each co-culture was measured through flow cytometry, and overnight marine broth cultures of V. parahaemolyticus enumerated through rapid OD proxy. V. parahaemolyticus cultures were diluted accordingly with 33 ppt FSW and inoculated into the 12-well plates. One 100 µL sample from each well was collected and frozen for later DNA extraction and qPCR analysis.
On day four, the algal cell density of each co-culture was measured through flow cytometry.
On day six the algal cell density of each co-culture was measured through flow cytometry. 1.0 mL (all remaining culture) from each well was centrifuged at 3000 rpm for 10 min, the supernatant removed, and frozen at -80 °C for later DNA extraction and QPCR.
Statistical analyses
All analyses were performed using R script v4.2.1 (Team 2023). All data first passed tests of normality and homoscedasticity.
Phase 1 data analysis was performed only on data from tested bacteria that demonstrated successful inhibition. Data passed PERMDISP test of homogeneity of multivariate variances (PERMDISP, F-value 0.715, p-value 0.5834), and PERMANOVA test was performed, followed by pairwise analysis between probiotic bacteria, and between V. parahaemolyticus strains.
For the Phase 2 and 3 experiments, data analysis was performed on each species of microalgae separately as no cross-species comparison was required. Cell density data underwent Log2 transformation to model for comparative proportional differences between control cultures and trial cultures, and to distinguish the degree of positive or negative difference. All data met assumptions of normality, homoscedasticity and independent sampling and was tested by one-factor ANOVA followed by post-hoc Tukey–Kramer HSD analysis.
Phase 4 data was separated into non-normalised and normalised data to test for both total and comparative differences respectively. One-factor ANOVA were performed on data divided into various groups depending on the test parameter, with each test analysed independently and no cross-comparisons made between tests. All data met assumptions of normality, homoscedasticity and independent sampling.
Results
Phase 1—V . parahaemolyticus and probiotic bacteria antagonism assay
During the well plate inhibition assay, bacteria T#1, T#3, T#4, T#5, and T#7 showed successful inhibition against the growth of one or more strains of V. parahaemolyticus (Table 3). PERMANOVA analysis (n = 5) showed the interaction between the V. parahaemolyticus and probiotic bacteria as significant (PERMANOVA: F(4, 16) = 9.465, p = 0.001). In the pairwise analysis, T#1, and T#3 showed significant difference in inhibition from all other test bacteria, and V#1 showed a significant difference in inhibition from all other V. parahaemolyticus strains (Fig. 2).
Inhibition of V. parahaemolyticus (V#X) strains by successful test bacteria (T#X). *** = 0.001, ** = 0.01, * = 0.1 (PERMANOVA pairwise analysis, positive significance only). Refer to Table 1 for code references on species names
Phase 2—Microalgae and probiotic bacteria co-culture
Data on cell count µL−1 day−1 for each microalgae species underwent log2 transformation to determine any significant difference from the negative controls. Both positive and negative impacts on algal growth were observed depending on the bacterial species and the microalgae species (Fig. 3, Table SP 2). However, no single bacterial species caused a significant response in cell growth across all three microalgae species, where significant negative responses only occurred in C. muelleri co-cultures containing T#1 and T#3 (Table SP 4).
Both C. muelleri and P. lutheri exhibited their strongest negative growth rate in response to co-culture with bacteria T#3 at log2 = -1.21 and log2 = -1.52 respectively (Table SP 2) (log2 = 0 indicates no change in comparison to Type 2 negative controls). However, the presence of T#3 caused an increase in growth rate for Tetraselmis sp. at log2 = 1.20. A similar trend was seen in algal responses to T#1 and T#4. In all cases, Tetraselmis sp. showed a reduced negative response in comparison to other algae species (Fig. 3).
Phase 3—Microalgae and V. parahaemolyticus co-culture
One-factor ANOVA for each separate alga showed significance only for C. muelleri (ANOVA: F(6, 14) = 123.2, p = < 0.001). The addition of V. parahaemolyticus strains resulted in negative growth (uL−1 day−1) for all trial cultures (Fig. 4, Table SP 5), yet no V. parahaemolyticus strains demonstrated a consistent significant change across all three microalgae species.
Despite significant negative results only appearing in C. muelleri co-cultures, overall this species responded with a reduced negative response compared with P. lutheri and Tetraselmis sp. co-cultures. C. muelleri co-cultures with V#2 and V#3 had negligible change to growth rate (log2 > -0.2), whereas all other co-cultures demonstrate decreased growth (log2 between -0.6 and -2.55). Of the algal species, Tetraselmis sp. responded with the highest proportion of negative change in growth in response to V#4 and V#5 at log2 = -2.55 and log2 = -2.47 respectively (Fig. 4, Table SP 3).
Selection of Phase 4 candidate strains
Of the test bacteria from Phase 1, species T#1 and T#3 were selected, as post-hoc pairwise analysis showed these to cause significant inhibition of all V. parahaemolyticus strains. Of the remaining species, as Phase 2 results presented no significant responses in algal growth, T#7 was selected as it induced the greatest frequency of positive responses across the microalgae species.
To find the V. parahaemolyticus strains most inhibitory to microalgal growth in Phase 3, selection was based on those strains showing the highest frequency of greatest significant decrease in cell density of all algal species. As no significant effects were observed, V. parahaemolyticus strains V#1 and V#5 were selected due to the frequency and magnitude of negative responses caused.
Phase 4—Co-culture of microalgae, V. parahaemolyticus, and probiotic bacteria
Co-cultures containing the microalgae species Tetraselmis sp., including Type 2 negative controls, demonstrated a reduction in Vibrio sp. copy number (mL−1 day−1) between Day 2 and Day 6. The Vibrio sp. copy numbers increased in all other trials and Type 2 control cultures not containing Tetraselmis sp. A more complex pattern was found when all data was normalised to the Type 2 negative controls using the Log2 normalising equation, in which case the independent variable was restricted to the test bacteria species.
The co-culture of Tetraselmis sp. + P. peptidolytica (T#3), demonstrated significant Vibrio sp. copy number mL−1 reduction (Fig. 5, Table 4) between Day 2 to Day 6. 24 ± 15% (AV ± SE) reduction observed in V#1 co-cultures, and 6.33 ± 0.6% reduction in V#5 co-cultures, both in comparison to Type 2 negative controls. Additionally, a 157 ± 9% increase in algal growth was observed in cultures of Tetraselmis sp. in contrast to type 2 negative controls.
The bacterial species P. peptidolytica (T#3) (Fig. 6) was found to have the greatest inhibitory effect on V. parahaemolyticus (ANOVA: F(2,43) = 11.26, p < 0.0001), where it caused a reduction in Vibrio sp. copy number mL−1 for all microalgae species and V. parahaemolyticus strains.
Change in microalgae cellular growth was assessed using Log2 normalised data comparative to Type 1 negative controls in a one-way ANOVA: F(2, 51) = 38.01, p < 0.0001, followed by post-hoc Tukey–Kramer HSD pairwise analysis. The pairwise analysis demonstrated that with the addition of both probiotic bacteria and V. parahaemolyticus, the cell density (µL−1 day−1) of Tetraselmis sp. cultures increased in comparison to C. muelleri and P. lutheri cultures (Fig. 7) showed only negative change in cell abundance.
Tetraselmis sp. (Fig. 8) was found to be the best performing microalgae species overall using one-factor ANOVA: F(2,43) = 11.284, p = 0.001, followed by post-hoc Tukey–Kramer HSD pairwise analysis, causing a significant negative growth rate of Vibrio sp. copy number mL−1 across all microalgae species. Pairwise analysis showed the change in Vibrio sp. copy number mL−1 day−1 in Tetraselmis sp. cultures were significantly different to P. muelleri.
Discussion
Response of V. parahaemolyticus strains
Vibrio parahaemolyticus strains showed variable susceptibility to inhibition by the test bacteria (between 0—10.2 ± 0.3 cm) (Table 3). Variable resistance to antibacterial secondary metabolites between the strains may be linked to their expression of virulence factors; however, the extent of the variation in inhibition generally depends on the producer of the antibacterial metabolites (Soto‐Rodriguez et al. 2022). The differences in inhibition were consistent and species-specific, with strains V#1 showing the greatest susceptibility during the Phase 1 well diffusion assay. This could be due to environmental factors related to the geographical origin of the strain. The geographical origin of the strain and seasonal variations in the local environment, may impact gene expression of marine Vibrio species (Takemura et al. 2014; Turner et al. 2014), and thus affect their susceptibility to inhibition. During Phase 3, V. parahaemolyticus strains sourced from diseased individuals in the Darwin 2022 outbreak suppressed microalgal growth more-so than environmentally sourced strains (Fig. 4). This suggests that bacteria-bacteria and algae-bacteria interactions can be specific to fine bacterial taxonomic variations rather than just on the species level. When cultured with Pseudovibrio dentrificans (T#1) in Phase 1, strong growth inhibition was observed within all V. parahaemolyticus strains (Fig. 2). This may be due to the inhibitory metabolites produced by T#1, as species of this genus are known to show strong broad-spectrum antimicrobial activity (Aranda et al. 2012; Raina et al. 2016).
Inhibition potential of probiotic bacteria
During Phase 1, Pseudovibrio dentrificans (T#1) exhibited the greatest inhibition of all V. parahaemolyticus strains (Fig. 2). In a previous study this isolate demonstrated strong, broad-spectrum inhibition against several species of marine Vibrio, due to the production of tropodithietic acid (TDA) (Raina et al. 2016), a broad-spectrum tropolone compound with strong antibiotic properties (Rasmussen et al. 2016). It is currently unknown how TDA functions as an antibacterial; however, Henriksen et al. (2022) and Rabe et al. (2014) have suggested TDA causes oxidative stress via disruption of the mitochondrial membrane. All other species of probiotic bacteria trialled in this study caused moderate inhibition in comparison and are known to produce various antimicrobial secondary metabolites, including fatty acids, alkaloids and peptides (Palomo et al. 2013; Offret et al. 2016).
Micrococcus yunnanensis (T#5) caused the weakest levels of inhibition overall. Out of the five strains of V. parahaemolyticus, only inhibiting V#1 may contribute to the geographic variation between V#1 and the remaining V. parahaemolyticus strains. During Phase 2, interactions between probiotic bacteria and microalgae are complex, as the algal growth rates vary in a combination-specific manner (Fig. 3). To design sustainable consortia, compatibility between bacterial and algal species must be carefully considered, likely as the unique phycosphere conditions of each microalgae may influence the variety of bacterial species able to coexist in that space (Fuentes et al. 2016; Han et al. 2016). During Phase 4, Pseudoalteromonas peptidolytica (T#3) presented as the predominant inhibitior, demonstrating a significant effect against both V#1 and V#5 strains of V. parahaemolyticus, indicating T#3 as the best performing probiotic bacteria. The ability of P. peptidolytica to inhibit the growth V. parahaemolyticus is congruent with a recent study by Aranda et al. (2012), where it is discussed that many species of Pseudoalteromonas exhibit strong inhibition toward a variety of marine Vibrios, including V. parahaemolyticus strains that tested both positive and negative for major virulence factors.
Responses of microalgae species
Similarities were observed between C. muelleri and P. lutheri in Phase 2 and 3 co-culture experiments regarding algal growth rates. During Phase 4, co-cultures containing either C. muelleri or P. lutheri elicited similar inhibition levels for V. parahaemolyticus strains. In these cases, Tetraselmis sp. responds with greater algal cell growth or Vibrio inhibition. Soto‐Rodriguez et al. (2022) discussed that both virulent and non-virulent strains of V. parahaemolyticus exhibit high susceptibility to growth inhibition by Tetraselmis sp. through the production of bioactive peptides. Tetraselmis sp. cells exuded a sticky mucus-like substance causing clumping and bio-film formation. This behaviour has been described in other studies (Fon-Sing and Borowitzka 2016; Isdepsky and Borowitzka 2019) and may be linked to the observed high rates of V. parahaemolyticus inhibition. It has been reported that bacterial metabolism may also introduce a beneficial environment, regarding available nutrients, for algal growth in a species-specific manner (Han et al. 2016). In this case, it was likely to be influenced by the probiotic bacteria, as it was shown in Phase 3 that all microalgae responded negatively to the presence of V. parahaemolyticus.
During Phase 4, C. muelleri and Tetraselmis sp. caused significantly greater V. parahaemolyticus inhibition in comparison to P. lutheri (Fig. 8), both with and without probiotic bacteria, indicating that the species of microalgae is influential factor in the performance of a consortium as an effective inhibitor. Tetraselmis sp. cultures demonstrated effective inhibition, with significantly greater V. parahaemolyticus inhibition overall and increased algal cell density in all cultures.
Complex interactions of microalgae, probiotic bacteria, and V. parahaemolyticus
Observed interactions during Phase 4 were primarily unique to specific combinations of microorganisms. V#1 and V#5 show similar levels of susceptibility to inhibition across all combinations except when in culture with T#1, where the inhibition rate changes between V. parahaemolyticus strains depending on the microalgae species present. Regardless of its strong capacity for inhibition, T#1 may be sensitive to the changes in algal phycosphere conditions brought on by the presence of either V#1 or V#5. This was again observed between Phase 1 and Phase 4 where both T#1 and T#3 demonstrated greater inhibition toward all tested strains of V. parahaemolyticus, except in Tetraselmis sp. cultures where T#1 has almost no effect on both strains of V. parahaemolyticus. This again suggests sensitivity in T#1 toward the phycosphere conditions of Tetraselmis sp.
A correlation between increased algal cell density and higher inhibition rates of V. parahaemolyticus was only observed in cultures of Tetraselmis sp. While C. muelleri cultures experienced significantly greater inhibition of V. parahaemolyticus compared to P. lutheri, the growth rates of the two species remained statistically similar. The high cell growth and inhibitory capacity of Tetraselmis sp. in conjunction with P. peptidolytica (T#3) in the presence of either V#1 or V#5 indicated this pairing as the best performing probiotic consortia. These more complex interactions are supported by the idea presented by Fuentes et al. (2016) that bacteria are highly sensitive to the conditions of the algal phycosphere, showing the need to identify relevant species-specific interactions before a successful consortium can be created (Han et al. 2016).
Conclusion
The principal goal of this study was to find a consortium of probiotic microalgae and bacteria that can sustain itself and inhibit the growth of V. parahaemolyticus. The combination of Tetraselmis sp. and Pseudoalteromonas peptidolytica produced a consortium that successfully suppressed the growth of V. parahaemolyticus over 7 days. The self-sustainability of this consortium could only be measured in the growth of the microalgae, and in this Tetraselmis sp. was the only microalgae to exhibit positive growth compared to its negative control monoculture. Both species also improved the inhibition efficacy of all other co-cultures in which either were present, further supporting their strength as successful inhibitors and potential probiotics. These would be ideal candidates for additional trials to further test the probiotic potential of these species as individuals or in consortia, which may unveil better probiotic solutions for pathogenic Vibrio outbreaks. Given the fact that a vast majority of aquaculture species rely on living algae culture as a feed in early life stages, administering these consortia or individual species as a biocontrol in hatcheries has the potential to reduce the impact of Vibrio outbreaks on the aquaculture industry significantly.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abdelaziz AE, Leite GB, Belhaj MA, Hallenbeck PC (2014) Screening microalgae native to Quebec for wastewater treatment and biodiesel production. Bioresour Technol 157:140–148
Aranda CP, Valenzuela C, Barrientos J, Paredes J, Leal P, Maldonado M, Godoy FA, Osorio CG (2012) Bacteriostatic anti-Vibrio parahaemolyticus activity of Pseudoalteromonas sp. strains DIT09, DIT44 and DIT46 isolated from Southern Chilean intertidal Perumytilus purpuratus. World J Microbiol Biotechnol 28:2365–2374
Baker-Austin C, Stockley L, Rangdale R, Martinez-Urtaza J (2010) Environmental occurrence and clinical impact of Vibrio vulnificus and Vibrio parahaemolyticus: a European perspective. Environ Microbiol Rep 2:7–18
Carvalho AP, Monteiro CM, Malcata FX (2009) Simultaneous effect of irradiance and temperature on biochemical composition of the microalga Pavlova lutheri. J Appl Phycol 21:543–552
Chang Y-H, Kuo W-C, Wang H-C, Chen Y-M (2020) Biocontrol of acute hepatopancreatic necrosis disease (AHPND) in shrimp using a microalgal-bacterial consortium. Aquaculture 521:734990
Dammak M, Hadrich B, Miladi R, Barkallah M, Hentati F, Hachicha R, Laroche C, Michaud P, Fendri I, Abdelkafi S (2017) Effects of nutritional conditions on growth and biochemical composition of Tetraselmis sp. Lipids Health Dis 16:41
de Souza VC, Wan AH (2021) Vibrio and major commercially important vibriosis diseases in decapod crustaceans. J Invertebr Pathol 181:107527
Di Pinto A, Terio V, Novello L, Tantillo G (2011) Comparison between thiosulphate-citrate-bile salt sucrose (TCBS) agar and CHROMagar Vibrio for isolating Vibrio parahaemolyticus. Food Control 22:124–127
Fang S, Huang W, Chen L (1987) Contamination of seafood by Vibrio parahaemolyticus in Taiwan. Chin J Microbiol Immunol 20:140–147
Fon-Sing S, Borowitzka M (2016) Isolation and screening of euryhaline Tetraselmis spp. suitable for large-scale outdoor culture in hypersaline media for biofuels. J Appl Phycol 28:1–14
Fuentes JL, Garbayo I, Cuaresma M, Montero Z, González-del-Valle M, Vílchez C (2016) Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar Drugs 14:100
Guillard RR, Ryther JH (1962) Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Gullian M, Thompson F, Rodriguez J (2004) Selection of probiotic bacteria and study of their immunostimulatory effect in Penaeus vannamei. Aquaculture 233:1–14
Han J, Zhang L, Wang S, Yang G, Zhao L, Pan K (2016) Co-culturing bacteria and microalgae in organic carbon containing medium. J Biol Res-Thessaloniki 23:1–9
Henriksen NN, Lindqvist LL, Wibowo M, Sonnenschein EC, Bentzon-Tilia M, Gram L (2022) Role is in the eye of the beholder—the multiple functions of the antibacterial compound tropodithietic acid produced by marine Rhodobacteraceae. FEMS Microbiol Rev 46:fuac007
Ihnken S, Roberts S, Beardall J (2011) Differential responses of growth and photosynthesis in the marine diatom Chaetoceros muelleri to CO2 and light availability. Phycologia 50:182–193
Isdepsky A, Borowitzka MA (2019) In-pond strain selection of euryhaline Tetraselmis sp. strains for reliable long-term outdoor culture as potential sources of biofuel and other products. J Appl Phycol 31:3359–3370
Jakšić S, Uhitil S, Petrak T, Bažulić D, Karolyi LG (2002) Occurrence of Vibrio spp. in sea fish, shrimps and bivalve molluscs harvested from Adriatic sea. Food Control 13:491–493
Jia F, Kacira M, Ogden KL (2015) Multi-wavelength based optical density sensor for autonomous monitoring of microalgae. Sensors 15:22234–22248
Kalatzis PG, Castillo D, Katharios P, Middelboe M (2018) Bacteriophage interactions with marine pathogenic vibrios: implications for phage therapy. Antibiotics 7:15
Kokou F, Makridis P, Kentouri M, Divanach P (2012) Antibacterial activity in microalgae cultures. Aquacult Res 43:1520–1527
Kumar R, Ng TH, Wang HC (2020) Acute hepatopancreatic necrosis disease in penaeid shrimp. Rev Aquacult 12:1867–1880
Kumar V, Roy S, Behera BK, Bossier P, Das BK (2021) Acute hepatopancreatic necrosis disease (AHPND): virulence, pathogenesis and mitigation strategies in shrimp aquaculture. Toxins 13:524
Letchumanan V, Loo K-Y, Law JW-F, Wong SH, Goh B-H, Ab Mutalib N-S, Lee L-H (2019) Vibrio parahaemolyticus: The protagonist of foodborne diseases. Prog Microb Mol Biol 2:a29
Lopez-Joven C, de Blas I, Furones MD, Roque A (2015) Prevalences of pathogenic and non-pathogenic Vibrio parahaemolyticus in mollusks from the Spanish Mediterranean Coast. Front Microbiol 6:736
López-León P, Luna-González A, Escamilla-Montes R, del Carmen F-M, Fierro-Coronado JA, Álvarez-Ruiz P, Diarte-Plata G (2016) Isolation and characterization of infectious Vibrio parahaemolyticus, the causative agent of AHPND, from the whiteleg shrimp (Litopenaeus vannamei). Latin Am J Aquat Res 44:470–479
Miles DW, Ross T, Olley J, McMeekin TA (1997) Development and evaluation of a predictive model for the effect of temperature and water activity on the growth rate of Vibrio parahaemolyticus. Int J Food Microbiol 38:133–142
Mohamad N, Amal MNA, Yasin ISM, Saad MZ, Nasruddin NS, Al-saari N, Mino S, Sawabe T (2019) Vibriosis in cultured marine fishes: a review. Aquaculture 512:734289
Natrah FM, Bossier P, Sorgeloos P, Yusoff FM, Defoirdt T (2014) Significance of microalgal–bacterial interactions for aquaculture. Rev Aquacult 6:48–61
Offret C, Desriac F, Le Chevalier P, Mounier J, Jégou C, Fleury Y (2016) Spotlight on antimicrobial metabolites from the marine bacteria Pseudoalteromonas: chemodiversity and ecological significance. Mar Drugs 14:129
Palacios OA, López BR, de-Bashan LE, (2022) Microalga Growth-Promoting Bacteria (MGPB): A formal term proposed for beneficial bacteria involved in microalgal–bacterial interactions. Algal Res 61:102585
Palomo S, González I, De la Cruz M, Martín J, Tormo JR, Anderson M, Hill RT, Vicente F, Reyes F, Genilloud O (2013) Sponge-derived Kocuria and Micrococcus spp. as sources of the new thiazolyl peptide antibiotic kocurin. Mar Drugs 11:1071–1086
Perković L, Djedović E, Vujović T, Baković M, Paradžik T, Čož-Rakovac R (2022) Biotechnological enhancement of probiotics through co-cultivation with algae: Future or a trend? Mar Drugs 20:142
Rabe P, Klapschinski TA, Brock NL, Citron CA, D’Alvise P, Gram L, Dickschat JS (2014) Synthesis and bioactivity of analogues of the marine antibiotic tropodithietic acid. Beilstein J Org Chem 10:1796–1801
Raina J-B, Tapiolas D, Motti CA, Foret S, Seemann T, Tebben J, Willis BL, Bourne DG (2016) Isolation of an antimicrobial compound produced by bacteria associated with reef-building corals. PeerJ 4:e2275
Rasmussen BB, Grotkjær T, D’Alvise PW, Yin G, Zhang F, Bunk B, Spröer C, Bentzon-Tilia M, Gram L (2016) Vibrio anguillarum is genetically and phenotypically unaffected by long-term continuous exposure to the antibacterial compound tropodithietic acid. Appl Environ Microbiol 82:4802–4810
Rodger HD (2016) Fish disease causing economic impact in global aquaculture. In: Adams A (ed) Fish vaccines. Springer, Basel, pp 1–34
Seymour JR, Amin SA, Raina J-B, Stocker R (2017) Zooming in on the phycosphere: the ecological interface for phytoplankton–bacteria relationships. Nat Microbiol 2:17065
Skjermo J, Vadstein O (1999) Techniques for microbial control in the intensive rearing of marine larvae. Aquaculture 177:333–343
Song X, Ma Y, Fu J, Zhao A, Guo Z, Malakar PK, Pan Y, Zhao Y (2017) Effect of temperature on pathogenic and non-pathogenic Vibrio parahaemolyticus biofilm formation. Food Control 73:485–491
Soto-Rodriguez SA, Magallón-Servín P, López-Vela M, Nieves Soto M (2022) Inhibitory effect of marine microalgae used in shrimp hatcheries on Vibrio parahaemolyticus responsible for acute hepatopancreatic necrosis disease. Aquacult Res 53:1337–1347
Stock W, Blommaert L, De Troch M, Mangelinckx S, Willems A, Vyverman W, Sabbe K (2019) Host specificity in diatom–bacteria interactions alleviates antagonistic effects. FEMS Microbiol Ecol 95:fiz171
Takemura AF, Chien DM, Polz MF (2014) Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Front Microbiol 5:38
Tarr CL, Patel JS, Puhr ND, Sowers EG, Bopp CA, Strockbine NA (2007) Identification of Vibrio isolates by a multiplex PCR assay and rpoB sequence determination. J Clin Microbiol 45:134–140
Team RDC (2023) R: A language and environment for statistical programming. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Temkin MI, Carlson CM, Stubbendieck AL, Currie CR, Stubbendieck RM (2019) High-throughput co-culture assays for the investigation of microbial interactions. JoVE:e60275
Turner JW, Malayil L, Guadagnoli D, Cole D, Lipp EK (2014) Detection of Vibrio parahaemolyticus, Vibrio vulnificus and Vibrio cholerae with respect to seasonal fluctuations in temperature and plankton abundance. Env Microbiol 16:1019–1028
Vaulot D, Courties C, Partensky F (1989) A simple method to preserve oceanic phytoplankton for flow cytometric analyses. Cytometry 10:629–635
Wang D, Flint SH, Palmer JS, Gagic D, Fletcher GC, On SL (2022a) Global expansion of Vibrio parahaemolyticus threatens the seafood industry: Perspective on controlling its biofilm formation. LWT 158:113182
Wang Z, Chu Y, Chang H, Xie P, Zhang C, Li F, Ho S-H (2022) Advanced insights on removal of antibiotics by microalgae-bacteria consortia: A state-of-the-art review and emerging prospects. Chemosphere 307:136117
Widiyanto T, Rusmana I, Febrianti D, Shohihah H, Triana A, Mardiati Y (2020) Profiles of Vibrio and heterotrophic bacteria in the intensive Vanamae shrimp culture using bioremediation technique in Karawang. IOP Conf Ser: Earth Environ Sci 535:012019
Zhang B, Li W, Guo Y, Zhang Z, Shi W, Cui F, Lens PN, Tay JH (2020) Microalgal-bacterial consortia: from interspecies interactions to biotechnological applications. Renewable and Sustainable Energy Rev 118:109563
Acknowledgements
We would like to express our thanks to Jean-Baptiste Raina, Nine Le Reun, and Abeeha Khalil from the University of Technology Sydney, NSW for advisory input and specimen donations. Also to Territory Pathology via CDU for specimen donations.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This study was funded by a Climate Change Cluster Honours Scholarship, University of Technology Sydney.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. Material preparation, data collection and analysis were performed by BO, molecular analysis BO and NS, manuscript was written by BO, NS, MP.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Osborne, B., Siboni, N., Seymour, J.R. et al. Exploring the potential of algae-bacteria interactions in the biocontrol of the marine pathogen Vibrio parahaemolyticus. J Appl Phycol 35, 2731–2743 (2023). https://doi.org/10.1007/s10811-023-03071-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03071-3