Abstract
The impact of growth conditions on the nitrogen, protein, colour and amino acid profiles of Palmaria palmata (from 2 locations) were investigated. Laboratory cultures of P. palmata were grown over a period of 18 days with and without nutrient addition, i.e., with culture medium (F/2) and urea (0.05 or 0.10 g L−1). The total nitrogen (TN) content of cultured P. palmata originating from longlines harvested from Cork increased with the addition of culture medium (F/2) and urea (0.05 g L−1) in comparison to the initial sample (4.15 ± 0.05 vs 1.73 ± 0.03%) while biomass from Galway increased with addition of culture medium (F/2) and urea (0.10 g L−1) in comparison to the initial sample (3.72 ± 0.05 vs 2.43 ± 0.06%). The protein nitrogen (PN) and non-protein nitrogen (NPN) of cultured P. palmata originating from Galway also increased when grown with the addition of nutrients. Nutrient enrichment during growth influenced the colour characteristics (lightness L*, redness a*, yellowness b* and colour difference ΔE) and the electrophoretic protein profile. Correlations between TN and ΔE (r = 0.945) for Cork originated sample, PN and ΔE (r =-0.944) and NPN and ΔE (r = 0.734) for Galway originated sample were observed. In-gel digestion followed by UPLC-MS/MS demonstrated that RuBiSCo, phycoerythrin and allophycocyanin expression in P. palmata was enhanced with nutrient addition. Amino acid profile and score analysis showed variation as a function of nutrient addition. Overall, nutrient inclusion in the growth medium provides a strategy for the enhanced production of high-quality protein by P. palmata.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is global interest in finding alternative protein sources for human consumption i.e., alternatives to animal sourced proteins (Nadeeshani et al. 2021). Numerous macroalgal species have been reported to contain significant levels of protein which are equivalent to some conventional protein rich foods such as eggs, fish, soybean and cereals (Harnedy and FitzGerald 2011). The protein quality and digestibility of macroalgal proteins has been reported to be on par with traditional protein sources (Bleakley and Hayes 2017). Hence, macroalgal protein may serve as a protein source for human consumption and therefore has potential for the development of protein-rich food ingredients.
Palmaria palmata (Linnaeus) Weber & Mohr, also known as dulse or dillisk, is a popular red macroalga consumed in the traditional cuisines of Ireland, Scotland, Iceland, Norway and the coastlines of Maine, USA and Nova Scotia, Canada due to its desirable flavour (Mouritsen et al. 2013). The protein content in P. palmata has been reported to vary between 8 to 35% on a dry weight (dw) basis (Bjarnadottir et al. 2018). Palmaria palmata can be harvested from the wild or cultivated on land or at sea to meet market demand (Edwards and Dring 2011; Grote 2019; Schmedes et al. 2019). In Ireland, for instance, the estimated annual harvest of both wild and cultivated P. palmata is approximately 100 tonnes (Walsh and Watson 2011; Grote 2019; Mac Monagail and Morrison 2020).
Most studies in relation to P. palmata cultivation focus on increasing biomass by utilising nutrient enriched seawater and/or by changing the irradiance of light or the salinity of water when grown on land (Schmedes and Nielsen 2020). Edwards and Dring (2011) investigated the impact of nutrient addition (culture medium, F/2) on the rate of growth and length of P. palmata sporelings cultured in the laboratory at different irradiances (5, 10, 25 and 50 μmol photons m−2 s−1). They showed that P. palmata sporeling length was longer at lower irradiances (5 and 10 μmol photons m−2 s−1) when the nutrient media (F/2) was enriched with an additional vitamin mixture (including vitamins B1, B12 and H) compared to P. palmata grown with F/2 without vitamin addition. Sanderson et al. (2012) investigated the impact of the nutrients in the effluent from salmon (Salmo salar) grown at a sea site in Scotland on the growth of P. palmata and Saccharina latissima. Cultivation near fish farms led to an increase in total nitrogen content, i.e., 4.6 ± 0.05 and 1.90 ± 0.18% in P. palmata and in S. latissima, compared to the reference samples, i.e., 2.3 ± 0.1 and 1.23 ± 0.06%, respectively. Schmedes and Nielsen (2020) subsequently reported that supplementation of the nutrient media (a vitamin-containing F/2 + nutrient stock solution) and a water salinity of 15% (w/v) improved the growth rate of young P. palmata fronds. Petridou (2020) investigated the effect of nutrient addition on the growth and nitrogen profiles of P. palmata tetraspores under laboratory conditions. Nutrient medium consisting of vitamins, nitrates and phosphates was added at different levels (no nutrients, half and full dose nutrients) during the culture of P. palmata. Treatments with half dose nutrients led to the highest protein content of 34.83% (dw, using a nitrogen to protein conversion factor of 4.7) when grown at 12˚C and a salinity of 20% (w/v) for 30 days. Despite the various investigations highlighted above, knowledge gaps exist which specifically compare the impact of nutrient addition using culture medium (F/2) with and without urea to cultured P. palmata. Furthermore, to our knowledge there is no study to date that investigates the impact of nutrient addition on the nitrogen, protein, colour and amino acid profile of cultured P. palmata. The hypothesis of this study was that nutrient supplementation of P. palmata with culture medium (F/2) alone or in combination with urea would increase its protein content. Therefore, the objective of this study was to examine the impact of the inclusion of culture medium (F/2) alone or in combination with urea (0.05 or 0.10 g L−1) on the nitrogen, protein, colour and amino acid profiles of cultured P. palmata obtained from biomass harvested from longlines from two separate locations off the west coast of Ireland (Cork and Galway) under controlled laboratory scale cultivation conditions.
Materials and methods
Materials
Hydrochloric acid (HCl) and sodium hydroxide (NaOH) were from VWR (Ireland). Trichloroacetic acid (TCA), Kjeldahl tablets and phenol solution (equilibrated with 10 mM Tris HCl, pH 8.0, 1 mM ethylenediaminetetraacetic acid (EDTA), 1,4-dithiothreitol (DTT), iodoacetamide and wide range molecular weight markers (6.5 to 200 kDa) were from Sigma (Ireland). A commercial blend of culture medium F/2 (Cell-Hi F2P, powder version), based on Guillard’s F/2 medium was from Varicon Aqua Solutions Ltd. (UK). Agricultural grade urea granules (46–0-0) were from Goulding Chemicals Ltd (Ireland).
Tris–glycine sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) buffer (10 x) and Protein Loading Buffer Blue (2x) were from National Diagnostics (USA). Mini-protean TGX stain-free 8–16% gradient gels were from Bio-Rad (USA). Sequencing grade L-1-tosylamido-2-phenylethyl chloromethyl ketone (TPCK) treated trypsin (16,987 U mg−1) resistant to autolytic digestion was from Promega Corporation, (USA). The bicinchoninic acid (BCA) protein assay kits were from ThermoFisher Scientific (Ireland). All other reagents were of analytical grade.
Vegetative culture trials of P. palmata with different sources of nutrients
The concept of studying biomass arising from two different locations (Cork and Galway) was related to the fact that it is well established that geographical location and environmental conditions impacts biomass composition (Harnedy et al. 2014). In this study, two separate growth experiments were conducted using the P. palmata biomass harvested from longlines located at Cork (Bantry Bay) and Galway (Lehenagh Pool) trialling the effects of different sources of nutrients. All other culture conditions remained the same in each experiment. After harvesting from the longlines, 200 g of biomass from young P. palmata fronds was cleaned by rinsing with seawater and by the removal of any fouling organisms (mainly bryozoans) by gently scraping the surface of the fronds. Prior to the culture experiments, the biomass was acclimated under laboratory conditions for 4 days in a 250 L tank with UV filtered seawater. Acclimation parameters were a 12:12 Light:Dark (L:D) photoperiod at 60 μmol photons m−2 s−1 photosynthetically active radiation (PAR; measured with a PAR Quantum sensor SKP 215, Skye Instruments Ltd., UK) provided by two LED light sources (Spectron, Light Science, 20W 1.2 GB cool white, The Netherlands). Water was held at 11 ± 1 °C. Following acclimation, the culture trials consisted of the following treatments: i) natural sterilised seawater control, ii) complete (100%) replacement of culture medium containing F/2 (0.1 g L−1) once per week, iii) 100% F/2 and 0.05 g L−1 urea added once per week, iv) 100% F/2 and 0.10 g L−1 urea added once per week. The trials were carried out in 15 L tanks (n = 3). Other culture conditions involved a P. palmata tank stocking density of 4 g L−1 and a 16:8 L:D photoperiod at 100 µmol photons m−2 s−1 PAR (LED lights as already described). Agitation with aeration was maintained to ensure biomass circulated freely in the tank and seawater temperature was maintained at 11 ± 1 °C. Nutrients were incorporated into the seawater at day 0 and they were subsequently replaced after every seawater change which was done at day 6 and 12. Both trials (n = 3) ran for a total of 18 days. The position of the tanks in the light-controlled unit was changed every 3 days to ensure homogenisation of the lighting conditions. Blotted fresh weight biomass was weighed every 3 days and excess biomass was removed to ensure that the optimal stocking density was maintained in the tanks during the experimental period. Biomass samples were rinsed in seawater prior to N fraction determination in order to remove potential interference from residual urea in the growth medium. Thereafter, samples were freeze-dried (Labconco FreeZone12, USA), milled to an average size of < 3 mm (Krups F203 Grinder, USA) and stored at room temperature in an air-tight container for subsequent nitrogen, colour, protein and amino acid profile analyses.
Determination of Total Nitrogen (TN), Protein Nitrogen (PN) and Non-Protein Nitrogen (NPN)
The TN content (n = 2, due to limited sample availability) of the P. palmata samples from Cork and Galway was determined using the macro-Kjeldahl protocol as described by Connolly et al. (2013). The PN and NPN content of the macroalgal samples from Galway obtained at day 12 (n = 2, due to limited sample availability) and 18 (n = 3) were analysed using the protocol described by Stack et al. (2017). The units of concentration for the nitrogenous components were expressed throughout in terms of percentage (g nitrogen (100 g)−1 dry weight (dw) biomass).
Colour measurement
Colour determination (n = 3) in all milled P. palmata samples was carried out using a Konica Minolta CR-400 Chroma Meter (Minolta Camera Co., Japan) as described by Cermeno et al. (2019). The results were expressed using the reference CIELAB model, where L* represents lightness (the darkest being black at L* = 0, and the brightest being white at L = 100), a* represents the colour channel from red (positive values) to green (negative values) and b* represents the yellow (positive values) to blue (negative values) axis. The Chroma Meter was calibrated on the CIELAB colour space system using a white tile (Dc: L = 97.79, a = − 0.11, b = 2.69). The colour difference between the initial sample and the nutrient supplemented samples was determined as previously outlined by Cermeno et al. (2021) as follows:
where L1 = lightness/darkness of initial sample (reference), L2 = lightness/darkness of each treatment (seawater control, i.e., no nutrients, F/2 without or with urea (0.05 or 0.1 g L−1) sample, a1 = redness/greenness of initial sample (reference), a2 = redness of each treatment sample, b1 = yellowness/blueness of initial sample (reference) and b2 = yellowness/blueness of treatment samples.
Pearson correlation test was used to assess the relationship, if any, between ΔΕ and TN, PN and NPN of the P. palmata biomass.
Protein extraction and quantification for gel electrophoretic analysis
Protein extraction (n = 3) from the P. palmata samples was performed following the protocol described by Wang et al. (2006). The extracted protein pellet was dissolved at 25 mg mL-1 in Laemmli buffer (63 mM Trizma-base pH 6.8, 10% (v/v) glycerol and 2% (w/v) SDS). The protein content of the extracted sample was determined by diluting an aliquot of the sample 1:50 (v/v) with dH2O in order to avoid SDS interference with the BCA assay. A calibration curve of BSA (concentration range between 0–1000 µg mL−1) diluted in 1:50 (v/v) Laemmli buffer was used for protein determination.
Electrophoretic analysis of protein extracts
SDS polyacrylamide gel electrophoresis (SDS-PAGE) was performed on the P. palmata protein extracts of the biomass collected after 18 days cultivation for longline originated samples from Cork and at day 6, 12 and 18 cultivations for the longline originated samples from Galway. Samples were diluted with protein loading buffer blue 2X (1:1, v:v) to obtain a concentration of 1.5 mg mL−1 protein. After heating at 95 °C for 5 min and cooling to room temperature, the samples were loaded on 8–16% gradient Mini-Protean TGX stain-free gels placed in a Miniprotean Tetra Cell (Bio-Rad, UK) with Tris–Glycine-SDS 10X electrophoresis running buffer (1:10, v:v). Aliquots of 15 μg of protein (10 μL) were loaded into each well. Electrophoretic separation was carried out at 100 V for 2 h. The molecular weight markers used ranged from 6.5 to 200 kDa. Gel images were taken before and after Coomassie-staining with a Uvi-TEC biomolecular imager (UK). Images were captured using a UVITEC Cambridge Imaging system.
Relative quantification of the protein bands in the stain free SDS-PAGE gels (n = 3) of the P. palmata protein extracts after 18 days cultivation from the biomass collected from longlines originated samples from Cork was performed by densitometry using a Uvi-Tec imaging system and UViBand Software. The relative protein content in individual bands was expressed as the percentage volume of each band in relation to the total volume of the initial sample at day 0.
Identification of the proteins in the electrophoretic bands using ultra-performance liquid chromatography mass spectrophotometry (UPLC-MS/MS)
Electrophoretic bands stained with Coomassie Blue were manually excised and destained with 100 μL of 25 mM NH4HCO3: acetonitrile (1:1; v:v), as previously described by Shevchenko et al. (2006) with some modifications (Santos-Hernandez et al. 2021). In-gel digestion was performed at 37 °C overnight with 12.5 µg μL−1 of trypsin in 25 mM NH4HCO3. The peptide profile of the reconstituted samples was analysed using a UPLC/Q-TOF with a BEH C18 column, 130 Å, (2.1 mm × 50 mm × 1.7 μm) using an Acquity UPLC (Waters, Ireland) connected to an Impact HD ultra-high resolution Q-TOF mass spectrometer (Bruker Daltonics, Germany) equipped with an electrospray ionization source. The separation gradient and the ion source parameters were as previously described by Santos-Hernandez et al. (2021). Q-TOF Control (version 3.4, Bruker Daltonics) was employed to control the MS and MS/MS analyses. Scans for auto MS/MS were carried out between 100 and 2500 m/z. Analysis of MS/MS data and peptide sequence identification was achieved using PEAKS Studio software version 8.0 (Bioinformatics Solutions Inc., Canada). An in-house database of the main P. palmata proteins extracted from Swissprot was used for peptide sequencing chains in PEAKS Studio analysis. Precursor error mass was set at 0.5 Da and the score threshold for peptides (-10 lgP) was set at 20. The peptide sequences obtained were then manually verified using Data Analysis 4.0 (Bruker Daltonics).
Amino acid profile
Amino acid analysis (n = 3) was performed on the P. palmata samples originating from longlines in Galway (analysis was not performed on samples originating from longlines in Cork due to a limitation in sample availability). Analysis was carried out using the external accredited service of the Center for Biological Research (Madrid, Spain) following the protocol outlined in Friedman (2004). During acid hydrolysis, asparagine and glutamine were converted to aspartic and glutamic acid, respectively and tryptophan was destroyed. The results were expressed as g of amino acid per 100 g dw.
The amino acid score (AAS) of P. palmata samples that originated from longlines in Galway was calculated as mg of amino acid per g of protein divided by the mg of the same amino acid per g of the reference protein for the essential amino acids (FAO 2013; Harnedy-Rothwell et al. 2021). The protein content in P. palmata was estimated using the PN content (w/w) in the test samples multiplied by a nitrogen conversion factor of 4.7 following Bjarnadottir et al. (2018). The reference protein was based on the AAS values provided by the Food and Agriculture Organization (FAO) where the reference requirement is indicated for two population cohorts, i.e., (1) children (from 6 to 36 months old) and (2) older children (older than 36 months), adolescents, and adults (FAO 2013).
Statistical analysis
Data was presented as the mean ± standard deviation (SD). Normal distribution and homogeneity of variance in the data was confirmed by the Shapiro–Wilk test and the Levene’s test of equality of variances, respectively. Data were analysed by one-way analysis of variance (ANOVA) with Turkey post hoc comparison to test at a significance level of p = 0.05 using SPSS (Version 26, IBM Inc., USA) and GraphPad Prism version 5.0 (GraphPad Software IncUSA), where applicable. Correlations between ΔΕ and TN, PN and NPN were determined with the Pearson’s correlation test using SPSS.
Results
Total nitrogen (%) content of P. palmata biomass
The effect of different culture conditions on TN was assessed for the P. palmata samples collected from longlines in Cork and which were subsequently grown for 18 days under laboratory conditions but under different nutrient regimes. The TN content ranged between 1.10 ± 0.03 to 4.15 ± 0.05% among the different nutrient regimes (Table 1). Differences in the TN of the starting materials was observed with TN values of 1.89 ± 0.03% for the initial sample before cleaning (data not included) and after cleaning (1.73 ± 0.03%, Table 1). Cleaning of seaweed with seawater leads to the removal of detritus, epiphytes, filamentous endophytes and other particulate matter which may interfere with the analysis of their chemical composition (Kambey et al. 2021). There was an increase in TN during growth in the presence of culture medium (F/2 at 0.1 g L−1) alone or in combination with urea at both concentrations tested. Nutrient enrichment of seawater with F/2 and urea at both 0.05 and 0.10 g L−1 resulted in the highest TN values of 4.15 ± 0.05 and 4.11 ± 0.01%, respectively. The TN for the F/2 and urea grown samples was higher than with F/2 alone (3.90 ± 0.06%) which indicates the beneficial impact of augmenting the algal culture medium with additional fertilizer (urea) to increase P. palmata nitrogen content. The lowest TN after 18 days culture occurred in the control sample with seawater alone (1.10 ± 0.03%).
The TN in the P. palmata samples harvested from longlines in Galway and subsequently grown for 18 days under laboratory conditions under different nutrient regimes was also determined. After 18 days growth the P. palmata samples showed higher TN values, i.e., 3.18 ± 0.05, 3.64 ± 0.05 and 3.72 ± 0.05 (%) when cultured with F/2, F/2 + 0.05 g L−1 and F/2 + 0.10 g L−1 urea, respectively, in comparison with the initial sample (day 0) which had a TN content of 2.43 ± 0.06% (Table 1). This agrees with the observations for the Cork origin samples above where an increase in TN during growth in the presence of culture medium (F/2) alone or in combination with urea at both concentrations tested was also observed. This indicates the possibility of increasing the TN values of the Galway origin sample with an algal culture medium containing additional fertilizer (urea) during an 18-day culture period. The lowest TN after 18 days culture occurred in the control sample (no nutrients) with seawater alone was 1.21 ± 0.03%.
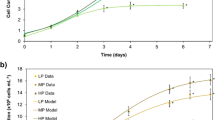
PN and NPN of the P. palmata biomass
Due to a limitation in sample supply, the PN and NPN values were only determined for the Galway origin samples during 12 and 18 days culture. Furthermore, analysis of PN and NPN for the control day 12 samples was not conducted due to limited sample availability. The highest mean PN value was obtained for F/2 with 0.10 g L−1 urea (3.24 ± 0.03%) when cultured for 18 days (Fig. 1A). However, there was no statistically significant differences in PN between this sample and the samples cultured with F/2 and 0.10 g L−1 urea at day 12 (3.23 ± 0.03%), F/2 and 0.05 g L−1 at day 18 (3.20 ± 0.04%) and F/2 only at day 12 (3.17 ± 0.03%) (P > 0.05). A significant decrease in PN content was observed in P. palmata cultured with F/2 only at day 18 (2.81 ± 0.02%) in comparison with day 12 (3.17 ± 0.03%) (P < 0.05). Meanwhile, a significant increase in PN was observed for the sample cultured with F/2 and 0.05 g L−1 urea at day 18 (3.20 ± 0.04%) in comparison with day 12 (2.82 ± 0.04%) (P < 0.05).
Protein nitrogen (PN) (A) and non-protein nitrogen (NPN) (B) contents of Palmaria palmata biomass harvested from longlines located at Galway and subsequently cultured for 12 (empty bars) and 18 days (full bars) in sterile seawater with and without F/2 (culture medium) with and without urea at 10 ˚C and a light:darkness photoperiod of 16:8 h. Values are mean ± SD, day 12 (n = 2) and day 18 (n = 3). Error bars represent 95% confidence limits. Statistical analyses were performed by one-way ANOVA followed by Tukey post hoc test comparing all groups (P ≤ 0.05). Values with different letters represent significant differences (P < 0.05)
Data on NPN content showed an increase in NPN in all samples grown in nutrient enriched medium in comparison with the control without nutrients and the initial sample for the Galway originated sample (Fig. 1B). The highest mean NPN values were obtained during growth in the presence of F/2 with 0.05 g L−1 urea (1.10 ± 0.01%) followed by F/2 with 0.10 g L−1 urea (0.87 ± 0.01%) when cultured for 12 days. The results also showed a decrease in NPN at day 18 in comparison with the same conditions at day 12 for samples cultured with F/2 only or in combination with urea (0.05 or 0.10 g L−1).
Colour parameters of P. palmata biomass
The effect of the addition of F/2 with and without urea (0.05 and 0.10 g L−1) on P. palmata originating from longlines from two locations (Cork and Galway) was assessed in terms of the colour of the biomass produced. Table 2 shows the L*, a* and b* values for the cultured biomass originating from longlines in Cork after 18 days culture. It was observed that the L* value decreased significantly for the control (53.16 ± 0.94) and after culturing (34.30 ± 1.57—34.94 ± 0.98) with the addition of F/2 with and without urea (0.05 and 0.10 g L−1) in comparison with the initial sample which exhibited the highest L* value (59.53 ± 2.46) (P < 0.05). The positive value of a* for the F/2 cultured sample (3.79 ± 0.21) indicates a red colour, with no statistically significant difference being observed (P > 0.05) when the samples were cultured with F/2 and urea (3.91 ± 0.43 and 3.98 ± 0.49 for F/2 with 0.05 and 0.10 g L−1 of urea, respectively). The negative values of a* for the initial (-0.20 ± 0.95) and control (-2.44 ± 1.67) samples indicate a green colour. The positive values for the b* of the samples indicates a yellow colour. Similar b* values were observed in P. palmata samples cultured with F/2 (6.46 ± 0.07) and with F/2 and urea (6.29 ± 0.43 and 6.00 ± 0.77 for F/2 with 0.05 and 0.10 g L−1 of urea, respectively) (P > 0.05). The highest b* value was observed for the control sample (29.39 ± 0.94) and the lowest value (6.00 ± 0.77) for the sample cultured with F/2 and 0.10 g L−1 urea (Table 2). The ΔE values of the Cork longline originated cultured samples were also calculated (Table 2).
The colour parameters of the P. palmata samples harvested from longlines grown in Galway and subsequently cultured between 6, 12 and 18 days in the laboratory were also analysed. Nutrient enrichment of P. palmata impacted the colour characteristics (L*, a* and b*) of all cultured samples (Fig. 2A, B and C). A decrease in L* value was observed during all sampling days, i.e., day 6, 12 and 18 when compared to the initial sample (day 0, Fig. 2A) in agreement with what was observed for the Cork longline harvested seaweed samples. An increase in a* (redness) value was observed on inclusion of F/2 in the culture medium with and without urea for day 6, 12 and 18 in comparison to the initial sample (Fig. 2B). However, the control sample without nutrient showed a decrease in a* value for day 6 and 12 with day 18 showing a large negative a* value (-2.00 ± 1.6). A decrease in b* value (yellowness) was observed in samples grown with F/2 with and without urea (Fig. 2C). Inversely, the b* value of the control sample increased on day 6, 12 and 18. Changes in ΔE were observed for all samples and culture times (Fig. 2D).
Colour parameters of Palmaria palmata biomass originally harvested from longlines in Galway, Ireland and subsequently cultured with nutrient supplementation at 10˚C with a lightness:darkness photoperiod of 16:8 h. Control no nutrient was the culture medium with only seawater, F/2 was the culture medium, F/2 + 0.05 and F/2 + 0.10 was the culture medium with 0.05 and 0.10 g L−1 urea, respectively. Values represent mean ± SD (n = 3). Panel labels indicate different growth conditions. Growth period: 0 represent starting/initial sample used for laboratory culture, while 6, 12 and 18 represent the length in days during which the samples were cultured in the laboratory. Initial sample was used as reference to calculate ΔE of the cultured Palmaria palmata
Pearson correlation analysis
Pearson’s correlation indicated a statistically positive (r = 0.945, p = 0.016) correlation between TN and colour difference for the Cork sourced samples while a negative (r =-0.896, p = 0.006) correlation was observed between TN and ΔE for P. palmata samples that originated from Galway. A statistically significant negative correlation between ΔE and PN and a positive correlation between ΔE and NPN was observed for the Galway samples (Table 3).
Electrophoretic profile of P. palmata biomass
The protein profiles of 18 day cultured P. palmata originating from longlines grown in Cork were analysed using SDS-PAGE (Supplementary Fig. 1). A total of 7 bands (labelled 1–7) were observed with molecular masses corresponding to 55, 48, 33, 26, 20, 18 and 10 kDa, respectively. A similar protein band profile was observed for P. palmata samples originating from longlines grown in Galway after 6 and 18 days of culture (Supplementary Fig. 2). However, the intensity of the protein bands was different for both groups of samples depending on the nutrient supplements received during growth. Bands 1, 6 and 7 were more intense in the biomass cultured with F/2 both with and without urea (0.05 or 0.10 g L−1 urea, Supplementary Fig. 1 (lanes c-e) and Supplementary Fig. 2 (lanes d-i)). Additional faint bands corresponding to 33 and 20 kDa i.e., bands 3 and 5, respectively, were also observed in Supplementary Fig. 1 (c-e) and Supplementary Fig. 2 (lanes d-i).
Relative quantitative analysis, using densitometry, was carried out for the protein electrophoretic bands from biomass harvested from longlines in Cork following 18 days cultivation (Fig. 3). Differences in relative protein expression were observed depending on the culture conditions, e.g., the relative protein band volume for bands 1, 3 and 6 were significantly higher for the samples cultured with F/2 alone or with F/2 + urea (0.05 and 0.10 g L−1) in comparison to the protein band distribution in the initial sample, except for band 3 in the sample grown with F/2 + urea (0.05). No culture medium composition related differences appeared to exist in protein band volume/intensity for bands 2, 4, 5 and 7.
Comparative individual protein band intensity obtained using densitometry analysis of sodium dodecyl sulphate polyacrylamide gel electrophoresis profiles of Palmaria palmata protein extracts harvested from longlines in Cork and subsequently cultured for 18 days at 10 °C with a lightness to darkness photoperiod of 16:8 h. P. palmata control grown in sterilised seawater (Initial sample, day 0); P. palmata grown without F/2 and urea grown in sterilised seawater (control); P. palmata grown with culture medium (F/2 only); P. palmata grown with culture medium F/2 and 0.05 g L−1 urea (F/2 + 0.05 g L−1 urea); and P. palmata grown with culture medium F/2 and 0.10 g L−1 urea (F/2 + 0.10 g L.−1 urea). Error bars represent 95% confidence limits. Different letters denote significant differences within each protein band for the different culture conditions (P < 0.05). Statistical analyses were performed by one-way ANOVA followed by Tukey post hoc test comparing all groups (P ≤ 0.05)
In-gel digestion of selected protein bands
In-gel digestion was employed to identify the proteins present in electrophoretic bands 1, 6 and 7 in the Cork origin samples (Supplementary Fig. 1) in combination with UPLC-MS/MS. The MS analysis of the band 1 tryptic digest yielded molecular ions for 44 peptides which are present in ribulose bisphosphate carboxylase (Rubisco) large chains which has a molecular mass of 54,029 and 48,005 Da (Table 4). The MS analysis of band 6 herein identified R-phycoerythrin subunits α and β, and B- phycoerythrin with intact molecular masses of 17,627 and 18,552, and 17,696 Da, respectively. MS analysis of band 7 identified peptides arising from ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit and the allophycocyanin alpha-subunit with intact molecular masses of 15.663, 17.459 and 17.564 Da, respectively.
Amino acid profile of P. palmata biomass
Due to sample availability limitations, amino acid analysis was only determined for the Galway origin samples cultured for 12 and 18 days. The essential amino acid (EAA) content of the Galway originated sample ranged between 3.12 ± 0.11 for control without F/2 and urea (day 18) to 18.98 ± 5.75 g (100 g)−1 of sample for F/2 with 0.05 g L−1 urea while the EAA content of the initial sample was 7.47 ± 0.69 (g (100 g)−1 of sample) on a dry weight basis (Table 5). Nutrient addition led to a significant increase in the EAA content of P. palmata cultured with F/2 only (14.30 ± 2.40 g (100 g)−1 of sample), F/2 + 0.05 g L−1 urea (18.98 ± 5.75 g (100 g)−1 dw) and F/2 + 0.10 g L−1 urea (12.92 ± 2.15 g (100 g)−1 of sample) obtained at day 18. In comparison, in the day 12 samples the EAA content in P. palmata cultured with F/2 only (3.87 ± 0.65 g (100 g−1 dw), F/2 + 0.05 g L−1 urea (5.76 ± 0.87 g 100 g−1 dw) and F/2 + 0.10 gL−1 urea (5.24 ± 0.85 g 100 g)−1 dw) were significantly lower (P < 0.05) than in the day 18 samples. The EAA values constituted between 28.96 to 43.76% of the total amino acid (TAAs).
The non-essential amino acid (NEAA) content ranged between 4.01 ± 0.04 g (100 g)−1 dw for control no nutrient (day 18) to 24.76 ± 7.86 g (100 g)−1 dw for F/2 + 0.05 g L−1 urea (day 18). The study herein shows significant (P < 0.05) increases in the NEAA content of P. palmata cultured with nutrient addition specifically in F/2 alone, F/2 + 0.05 g L−1 urea and F/2 ± 0.10 g L−1 urea (19.24 ± 3.33, 24.76 ± 7.86 and 17.09 ± 2.28, g (100 g)−1 dw, respectively) for samples obtained at day 18 (Table 5).
The branched chain amino acids (BCAA) content ranged between 1.11 ± 0.01 g (100 g)−1 dw for the control no nutrient (day 18) to 6.64 ± 1.01 g (100 g)−1 dw in F/2 + 0.05 g L−1 urea (day 18). The total amino acid (TAA) content ranged between 7.13 ± 0.12 g (100 g)−1 for the control no nutrient to 43.74 ± 13.62 g (100 g)−1 for the F/2 + 0.05 g L−1 urea sample all obtained at day 18.
Amino acid scores for the EAA for different age categories
The amino acid scores, an indication of protein quality, of cultured P. palmata biomass that originated from longlines in Galway are shown in Fig. 4. The calculated amino acid score ranged from 0.48 to 5.86 and 0.51 to 7.55 per residue when referenced against the recommended amino acid requirement for (i) children from 6 months to 3 years and (ii) in older child, adolescent and adult population cohorts, respectively (FAO 2013). The study herein showed that the sulphur containing amino acid (SAA) score of the cultured P. palmata ranged from 2.56 to 7.55 (Fig. 4).
Calculated amino acid (AA) scores for essential AAs for different age categories from cultured P. palmata samples that harvested from longlines located in Galway, Ireland and which were subsequently cultured for 12 and 18 days at 10 °C with a lightness to darkness photoperiod of 16:8 h. AA residues are represented by their 3-letter code. SAA: sulphur amino acids (cysteine + methionine), AAA: aromatic amino acids (tyrosine + phenylalanine). Tryptophan was not determined (nd). F/2 represents nutrient supplemented culture medium, F/2 + 0.05 and F/2 + 0.10 represent culture medium supplemented with F/2 and 0.05 and 0.10 g L−1 urea, respectively. AA score was calculated as mg of amino acid per g of protein divided by the mg of the same amino acid per g of the reference protein for the essential AAs. Values are mean ± SD, (n = 2). Error bars represent 95% confidence limits. The dotted line delineates an AA score of 1.0. An AA score > 1.00 is an indication that it meets the dietary requirement for the specific population cohort. An AA score < 1 is an indication that it does not meet the dietary requirement for the population cohort. The two selected population cohorts considered were: children (from 6 months to 3 years old) and older children (older than 3 years old), adolescents and adults, according to the Food and Agriculture Organisation
Discussion
Nitrogen profiles of the cultured P. palmata samples
Nitrogen is an essential element that is required to support the growth of seaweed (Xu et al. 2020a) and plays a vital role in plant metabolism as it is a constituent of proteins, chlorophylls, coenzymes, nucleic acids, phytohormones and secondary metabolites (Taiz and Zeiger 2010; Xu et al. 2020a, b). The study herein involved the use of culture medium (F/2) in addition with urea (at concentrations of 0.05 or 0.10 g L−1) to investigate their efficacy in increasing P. palmata nitrogen/protein content. F/2 contains nitrates, phosphates and vitamins which support the growth and accumulation of proteins in macroalgae (Kim and Jeune 2009; Ashokkumar et al. 2015). Edwards and Dring (2011) reported an increase in the biomass of P. palmata sporelings cultured in the laboratory using culture medium F/2. Urea is also a source of nitrogen for macroalgal growth (Smith et al. 2018), although it is rarely used. However, no previous studies appear to report on the impact of a combination of urea and F/2 on the TN of P. palmata. Tyler et al. (2005) reported the effect of urea addition in supporting the growth of the macroalga Ulva lactuca (Chlorophyta) and Gracilaria vermiculophylla (Rhodophyta) thalli growth in comparison with those grown without urea at 20 °C with a light intensity of 550 μmol photons m−2 s−1 and a L:D photoperiod of 16:8. The nitrogen content increased in G. vermiculophylla by approximately 25% (nitrogen content of sample grown with and without urea were 2.46 and 2.02%, respectively) and by 15% for U. lactuca (nitrogen content of sample grown with and without urea were 1.72 and 1.49%, respectively). In this study, both F/2 and urea could therefore be responsible for the higher level of TN observed in the nutrient cultured Cork and Galway origin samples (Table 1). On the other hand, it seems that seawater alone did not contain sufficient nitrogen to support P. palmata TN accumulation.
A similar explanation may be used to explain the increase in PN. Nutrient enrichment of seawater with F/2 or urea during culture could have enhanced the accumulation of protein in the biomass. In contrast, the control without nutrient enrichment showed a significant decrease in PN probably due to insufficient nutrients which led to a reduction in the accumulation of protein in the biomass (Fig. 1). Phycobiliproteins are protein pigments found in red algae and can represent up to 50% of the total protein content of algal cells (Stack et al. 2017). On converting the PN to protein using a nitrogen to protein conversion factor of 4.7 (Bjarnadottir et al. 2018), the protein content of the sample at day 0 was 9.96% whereas the maximum protein content observed using culture medium F/2 was 14.90% (day 12) and with the combination of culture medium F/2 and urea it was 15.05% with 0.05 g L−1 urea and 15.23% with 0.10 g L−1 urea. These findings show that it was possible to increase the protein content of P. palmata by ~ 50% on culturing with culture medium (F/2) alone or in combination with urea. Petridou (2020) reported an increase in protein content of 40% in a P. palmata sample grown with F/2 in comparison to P. palmata grown without culture medium (protein content of 34.83% vs 24.76% (dw)) without accounting for the NPN. In the case of the control sample, nutrient starvation may require the red algae to adjust its metabolic activities which in turn could lead to a decrease in the biosynthesis of phycobiliproteins in this seaweed (Zhou et al. 2020). It is well documented that photosynthetic organisms can alter their metabolism under nutrient-limited conditions (Fernandez et al. 1992; Taiz and Zeiger 2010). Similarly, macroalgae have been found to accumulate storage polysaccharides under nitrogen-limited conditions, and then resume protein and pigment synthesis under nitrogen-sufficient conditions (Chopin et al. 1995; Smit et al. 1996). This therefore may account for the observed decrease in PN and TN of the control sample during the course of incubation herein.
The nitrate present in the nutrient (F/2 and/or urea) medium could contribute to the NPN, therefore, the samples were washed with seawater prior to freeze-drying in order to avoid interference with N fraction analysis.
The differences in TN between the Cork and Galway samples (Table 1) may be due to the impact of different locations and time of harvest, as has been described previously for P. palmata proteins (Harnedy et al. 2014). When the relative increase in TN was calculated in relation to their respective initial contents as a function of the culture medium at day 18, the sample originating from Cork showed increases of 118, 140 and 138% while the sample originating from Galway showed increases of 31, 50 and 53% for the biomass cultured with F/2 alone, F/2 + 0.05 g L−1 urea and F/2 + 0.10 g L−1 urea, respectively. These data indicate that the initial samples have the phenotypic morphology (Nauer et al. 2018) to increase TN and protein content as a function of the culture medium composition. Therefore, overall, it may be concluded that nutrient enrichment increased the nitrogen and protein content of the P. palmata biomass.
Colour parameters of cultured P. palmata
Phycobiliproteins are present in red algae, which contain covalently attached pigments, their main roles are in relation to photosynthesis and nitrogen reserve (Xu et al. 2020a, b). Phycobiliproteins are classified into phycoerythrins and phycocyanins which display red and blue colours, respectively (Glazer 1994). According to Lalegerie et al. (2020), nutrients and the extent of irradiance contribute to the accumulation of the phycobiliproteins in P. palmata. This was evident herein, i.e., when the culture medium was rich in nutrients (addition of F/2), there was an increase in red and blue colour intensity associated with an increase in the synthesis of phycobiliproteins. On the other hand, in the absence of added nutrient in the culture medium (control sample), there was an increase in green and yellow colour due to the presence of chlorophyll and a limited accumulation of phycobiliprotein pigments. Moreover, the decrease in L* values in the F/2 cultured samples with or without the addition of urea indicates a darkening in the colour which implies the accumulation of pigments due to nutrient supplementation of the seawater (Fig. 2 and Table 2). The results obtained in this study were different from those reported by Vasconcelos et al. (2022) who studied the colour parameters of P. palmata fronds cultured in tanks with nutrient medium (Guillard’s nutrient supplement), at a temperature of 3˚C and illumination with white fluorescent light (250 μmol photons m−2 s−1); 16:8 L:D for 8 h. The values for the L*, a* and b* colour parameters of the freeze-dried P. palmata biomass obtained by these authors were 52.19 ± 1.16, 3.58 ± 0.82 and 2.91 ± 0.40, respectively. In the study herein, P. palmata was cultured for longer periods, i.e., 6, 12 and 18 days, illumination was at 100 µmol photons m−2 s−1 PAR, the temperature was maintained at 10˚C while the photoperiod (16:8) was similar. These growth condition differences between the two studies may have accounted for the variation observed.
While the initial samples (from Cork and Galway, Table 2 and Fig. 2) presented different initial colour values, the trends in the changes in colour as a function of the culture media were similar at day 18, i.e., the L*,a*, b* and ΔE values decreased when F/2 alone or F/2 + urea was included in the culture media. On the other hand, the a* value increased when P. palmata was cultured with F/2 alone or with F/2 and urea.
Correlation between TN, PN, NPN and ΔE
The positive correlation observed between TN and ΔE for P. palmata samples that originated from Cork indicated that nutrient enrichment of the growth medium led to the accumulation of colour pigments which was associated with an increase in TN (Tables 1 and 2). However, the negative correlation between TN and ΔE for P. palmata samples that originated from Galway (Table 3) could be attributed to differences in the TN and the colour parameters of the original vs the cultured sample, the extent of nitrogen accumulation during culture and the morphology of the samples. From these results, there seems to be no consistent pattern between colour parameters and the TN, since the correlation observed herein was sample origin specific. This is evidenced by the differences in the Pearson’s correlation between TN and ΔE (r = 0.945, p = 0.016) for the Cork sourced sample and between TN and ΔE (r =-0.896, p = 0.006) for Galway sourced sample despite nutrient enrichment during culture under similar laboratory conditions.
The Pearson’s correlation test has been previously used to assess the relationship between the crude protein, total soluble protein and total phenolic content of 15 varieties of seaweed. A positive correlation existed between crude protein and total soluble protein (r = 0.923, p = 0.001) and between total soluble protein and total phenolics (r = 0.431, p = 0.014), respectively. It was concluded that the higher the crude protein content of the seaweed the higher the possibility of obtaining more soluble protein and also the higher the phenolic content in the soluble protein fraction (Mohd Rosni et al. 2015). Similarly, Smit et al. (1996) reported a significant positive correlation between soluble protein and R-phycoerythrin content in Gracilaria gracilis cultured in nitrogen (ammonium sulphate) enriched seawater.
However, no information appears to exist specifically to assess the relationship between TN, PN, NPN and ΔE following growth of P. palmata at laboratory scale with culture medium (F/2) and urea (0.05 or 0.10 g L−1).
Protein profiles of the cultured P. palmata samples
The differences in the electrophoretic profile indicated that culture medium supplementation with F/2 with and without urea modulated the expression of P. palmata proteins (Supplementary Figs. 1 and 2, and Fig. 3). When purified R-phycoerythrin from P. palmata was analysed by SDS-PAGE it showed three bands with molecular masses between 29 and 14 kDa which correspond to the α, β and γ phycoerythrin subunits (Xu et al. 2020b). Moreover, Furuta et al. (2016) reported that the most intense band with a MW around 15 kDa corresponded to the α and β subunits of the phycobiliproteins in P. palmata. Other authors have reported that the subunit molecular weight of the phycobiliproteins range between 16–18 kDa (Dagnino-Leone et al. 2022) and 30–33 kDa (Sun et al. 2004), which corresponded to some of the bands obtained in this study. The protein profile obtained for P. palmata herein agrees with previous studies on aqueous protein extracts from P. palmata i.e., common bands at 55 and 16–18 kDa were observed (Harnedy and FitzGerald 2013a, b; Harnedy et al. 2014; Stévant et al. 2020).
Densitometry analysis indicated that protein bands 2, 4, 5 and 7 represent constitutive proteins whose expression was independent of culture medium composition. On the other hand, protein bands 1, 3 and 6 would appear to correspond with inducible proteins, whose expression increases or decreases as a function of nutrient availability. R-phycoerythrin is considered a storage protein in red algae which is metabolised during nitrogen deprivation to synthesized new proteins (Hsieh-Lo et al. 2019). Band 6 corresponding to R-phycoerythrin (Table 3) significantly increased (Fig. 3) when there was nitrogen in the culture media (F/2 alone and F/2 + urea) indicating and accumulation of stored nitrogen. However, this band (band 3 (lane d)) could not be identified in the case of the sample grown with F/2 + urea (0.05 g L−1, Supplementary Fig. 1). The reason for the lack of visualization of this band in the stain-free gel could potentially be due to modification of tryptophan residues when covalently bonded with trihalo compounds contained in the gel (Raynal et al. 2021). This appears to be the first time that P. palmata proteins have been quantified using densitometry analysis on stain free SDS-PAGE gels.
The in-gel digestion results herein (Table 4) are in agreement with a previous study that identified ribulose bisphosphate carboxylase large chain in P. palmata (Lee et al. 2017) using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. To the best of our knowledge, this is the first time that the proteins from P. palmata have been identified using in-gel digestion followed by LC–MS analysis using Peaks Studio 8.5 (Bioinformatics Solutions Inc., Canada). In general, the identified proteins (phycoerythrin, phycocyanin and allophycocyanin) are widely reported in the literature to be associated with the phylum Rhodophyta and specifically with P. palmata (Harnedy et al. 2015).
Amino acid profile of the cultured P. palmata samples
The predominant amino acids were Asx (combined aspartic acid and asparagine) and Glx (combined glutamic acid and glutamine) and they both accounted for between 30.9–35.6% of the TAA. Glx and Asx represented 39.6% of the TAA in P. palmata obtained from wild harvesting as reported by Galland-Irmouli et al. (1999). These residues were also predominant in other red seaweeds such as in Hypnea charoides and Hypnea japonica which comprised 20.8–22.9% of the TAA (Wong and Cheung 2000). In Gracilaria changii, these residues consisted of 18.69% of the TAA (Chan and Matanjun 2017).
Limited information appears to exist on the EAA, NEAA and TAA profiles of intact P. palmata biomass. De Bhowmick and Hayes (2022) reported the EAA content in a wild harvested P. palmata from the west coast of Ireland to be 0.31 g (100 g)−1 dw (42.9% of the total amino acids), the NEAA values of 0.41 g (100 g)−1 dw (57.09% of the total amino acid) and the TAA to be 0.72 g (100 g)−1 dw of biomass. In the study herein, the culture conditions were controlled and nutrient supplementation with urea and culture medium was included to induce accumulation of proteins, what may explain the differences in amino acids obtained by others in wild collected samples (De Bhowmick and Hayes 2022) which rely solely on seawater nutrients for growth. Variation due to season of harvest (winter vs summer), origin of the biomass (wild vs culture) and the methodology employed for determination of amino composition have been reported elsewhere to influence the amino acid composition of seaweeds (Cerna 2011).
The BCAAs are important in nutrition due to the vital role they play in metabolism, energy homeostasis and gut health (Nie et al. 2018). EAAs cannot be synthesized in the body and therefore must be provided in the human diet as recommended by the FAO and the World Health Organization (WHO) (Wu et al. 2013). The results presented herein demonstrate that nutrient addition can be employed to enrich the EAA content of cultured P. palmata.
Different population cohorts require varying amounts of EAAs. Therefore, it is recommended that the quality of a food protein should be quantified based on reference amino acid scoring patterns (FAO 2013). Meanwhile, according to the FAO (2013), the AAS of a test food sample should be above 1 in all population cohorts in order to meet their dietary requirements. The study herein showed that the SAA content of cultured P. palmata meets the levels recommended for the children (6 months to 3 years) and older adult population cohorts (Fig. 4). The level of valine in P. palmata cultured with F/2 only and with urea (0.10 g L−1) obtained at day 12 did not meet the EAA requirement for children and for the older child, adolescent and adult. The level of isoleucine in P. palmata cultured with F/2 only and with urea (0.05 or 0.10 g L−1) obtained at day 12 did not meet the EAA requirement for both population cohorts. On the other hand, the level of valine and isoleucine in P. palmata cultured with F/2 only and with urea (0.05 or 0.10 g L−1) obtained at day 18 meet the EAA requirement for both population cohorts. In general, proteinaceous foods from other sources or amino acid supplementation would be a requirement in order to augment the limiting amino acids observed in both population groups. The aromatic amino acid (AAA) content of all cultured P. palmata was above 1 for both population cohorts.
Overall, based on their AAS, culture of P. palmata with nutrient supplementation using culture medium (F/2) and urea (0.05 or 0.10 g L−1) for 18 days appear to be most suitable for both population cohorts when compared to those obtained at day 12 and the initial sample (day 0). This correlated well with the higher proportion of the EAAs at day 18, as shown in Table 5. These results demonstrate the importance of nutrient addition during the culture of P. palmata in order to enhance overall protein quality. No previous reports appear to specifically exist investigating the amino acid profile and score of P. palmata cultured with and without nutrient supplementation.
Conclusion
From the results obtained for TN, PN, NPN, colour, protein and AA profiles herein, it is evident that the growth conditions impact the composition of P. palmata. Furthermore, from a nutritional perspective, nutrient supplementation specifically impacted the overall protein profile, and the AA composition and score. Overall, the addition of F/2 alone or F/2 in combination with urea increased the protein content and the amount of phycobiliproteins present in cultured P. palmata that originated from longlines located at two sites on the west coast of Ireland. The results obtained herein provide new information on the nutrient supplementation conditions required during the culture of P. palmata in order to enhance its protein content and quality. This information may be relevant when considering the large-scale cultivation of P. palmata as a protein source for human consumption.
Data availability
The authors confirm that all relevant data supporting the findings of this study are included in the article and its supplementary information files.
References
Ashokkumar S, Manimaran K, Kim K (2015) Cultivation and identification of microalgae (Diatom). In: Kim S-K, Cojnacka K (eds) Marine Algae Extracts: Processes, Products, and Applications, vol 2. Wiley, Amsterdam, pp 59–78
Bjarnadottir M, Aoalbjornsson BV, Nilsson A, Slizyte R, Roleda MY, Hreggviosson GO, Friojónsson OH, Jónsdóttir R (2018) Palmaria palmata as an alternative protein source: enzymatic protein extraction, amino acid composition, and nitrogen-to-protein conversion factor. J Appl Phycol 30:2061–2070
Bleakley S, Hayes M (2017) Algal proteins: extraction, application, and challenges concerning production. Foods 6:33–39
Cermeno M, Dermiki M, Kleekayai T, Cope L, Mcmanus R, Ryan C, Felix M, Flynn C, FitzGerald RJ (2021) Effect of enzymatically hydrolysed brewers’ spent grain supplementation on the rheological, textural and sensory properties of muffins. Future Foods 4:100085
Cermeno M, Felix M, Connolly A, Brennan E, Coffey B, Ryan E, FitzGerald RJ (2019) Role of carbohydrate conjugation on the emulsification and antioxidant properties of intact and hydrolysed whey protein concentrate. Food Hydrocoll 88:170–179
Cerna M (2011) Seaweed proteins and amino acids as nutraceuticals. Adv Food Nutr Res 64:297–312
Chan PT, Matanjun P (2017) Chemical composition and physicochemical properties of tropical red seaweed, Gracilaria changii. Food Chem 221:302–310
Chopin T, Gallant T, Davison I (1995) Phosphorus and nitrogen nutrition in Chondrus crispus (Rhodophyta): effects on total phosphorus and nitrogen content, carrageenan production, and photosynthetic pigments and metabolism. J Phycol 31:283–293
Connolly A, Piggott CO, FitzGerald RJ (2013) Characterisation of protein-rich isolates and antioxidative phenolic extracts from pale and black brewers’ spent grain. Int J Food Sci Technol 48:1670–1681
Dagnino-Leone J, Figueroa CP, Castañeda ML, Youlton AD, Vallejos-Almirall A, Agurto-Muñoz A, Pérez JP, Agurto-Muñoz C (2022) Phycobiliproteins: structural aspects, functional characteristics, and biotechnological perspectives. Comput Struct Biotech J 20:1506–1527
De Bhowmick G, Hayes M (2022) In vitro protein digestibility of selected seaweeds. Foods 11:289–295
Edwards MD, Dring MJ (2011) Open-sea cultivation trial of the red alga, Palmaria palmata from seeded tetraspores in Strangford Lough, Northern Ireland. Aquaculture 317:203–209
FAO (2013) Report of an FAO Expert Consultation. Dietary protein quality evaluation in human nutrition. FAO Food Nutr Pap 92:1–66
Fernandez E, Serret P, De Madariaga I, Harbour DS, Davies AG (1992) Photosynthetic carbon metabolism and biochemical composition of spring phytoplankton assemblages enclosed in microcosms: the diatom-Phaeocystis sp. succession. Mar Ecol Prog Ser 90:89–102
Friedman M (2004) Applications of the ninhydrin reaction for analysis of amino acids, peptides, and proteins to agricultural and biomedical sciences. J Agric Food Chem 52:385–406
Furuta T, Miyabe Y, Yasui H, Kinoshita Y, Kishimura H (2016) Angiotensin I converting enzyme inhibitory peptides derived from phycobiliproteins of dulse Palmaria palmata. Mar Drugs 14:32
Galland-Irmouli A-V, Fleurence J, Lamghari R, Luçon M, Rouxel C, Barbaroux O, Bronowicki J-P, Villaume C, Guéant J-L (1999) Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse). J Nutr Biochem 10:353–359
Glazer AN (1994) Phycobiliproteins - a family of valuable, widely used fluorophores. J Appl Phycol 6:105–112
Grote B (2019) Recent developments in aquaculture of Palmaria palmata (Linnaeus)(Weber & Mohr 1805): cultivation and uses. Rev Aquacult 11:25–41
Harnedy-Rothwell PA, Khatib N, Sharkey S, Lafferty RA, Gite S, Whooley J, O’Harte FP, FitzGerald RJ (2021) Physicochemical, nutritional and in vitro antidiabetic characterisation of Blue Whiting (Micromesistius poutassou) protein hydrolysates. Mar Drugs 19:383–390
Harnedy PA, FitzGerald RJ (2011) Bioactive proteins, peptides, and amino acids from macroalgae. J Phycol 47:218–232
Harnedy PA, FitzGerald RJ (2013a) Extraction of protein from the macroalga Palmaria palmata. LWT-Food Sci Technol 51:375–382
Harnedy PA, FitzGerald RJ (2013b) In vitro assessment of the cardioprotective, anti-diabetic and antioxidant potential of Palmaria palmata protein hydrolysates. J Appl Phycol 25:1793–1803
Harnedy PA, O’Keeffe MB, FitzGerald RJ (2015) Purification and identification of dipeptidyl peptidase (DPP) IV inhibitory peptides from the macroalga Palmaria palmata. Food Chem 172:400–406
Harnedy PA, Soler-Vila A, Edwards MD, FitzGerald RJ (2014) The effect of time and origin of harvest on the in vitro biological activity of Palmaria palmata protein hydrolysates. Food Res Int 62:746–752
Hsieh-Lo M, Castillo G, Ochoa-Becerra MA, Mojica L (2019) Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res 42:101600
Kambey CS, Campbell I, Cottier-Cook EJ, Nor AR, Kassim A, Sade A, Lim PE (2021) Seaweed aquaculture: a preliminary assessment of biosecurity measures for controlling the ice-ice syndrome and pest outbreaks of a Kappaphycus farm. J Appl Phycol 33:3179–3197
Kim M-K, Jeune K-H (2009) Use of FT-IR to identify enhanced biomass production and biochemical pool shifts in the marine microalgae, Chlorella ovalis, cultured in media composed of different ratios of deep seawater and fermented animal wastewater. J Microbiol Biotechnol 19:1206–1212
Lalegerie F, Stiger-Pouvreau V, Connan S (2020) Temporal variation in pigment and mycosporine-like amino acid composition of the red macroalga Palmaria palmata from Brittany (France): Hypothesis on the MAA biosynthesis pathway under high irradiance. J Appl Phycol 32:2641–2656
Lee D, Nishizawa M, Shimizu Y, Saeki H (2017) Anti-inflammatory effects of dulse (Palmaria palmata) resulting from the simultaneous water-extraction of phycobiliproteins and chlorophyll a. Food Res Int 100:514–521
Mac Monagail M, Morrison L (2020) The seaweed resources of Ireland: a twenty-first century perspective. J Appl Phycol 32:1287–1300
Mohd Rosni S, Fisal A, Azwan A, Chye F, Matanjun P (2015) Crude proteins, total soluble proteins, total phenolic contents and SDS-PAGE profile of fifteen varieties of seaweed from Semporna, Sabah, Malaysia. Int Food Res J 22:1483–1493
Mouritsen OG, Dawczynski C, Duelund L, Jahreis G, Vetter W, Schroder M (2013) On the human consumption of the red seaweed dulse (Palmaria palmata (L.) Weber & Mohr). J Appl Phycol 25:1777–1791
Nadeeshani H, Hassouna A, Lu J (2021) Proteins extracted from seaweed Undaria pinnatifida and their potential uses as foods and nutraceuticals. Crit Rev Food Sci Nutr 3:1–17
Nauer F, Amorim AM, Santos JP, Chow F, Oliveira MC (2018) Physiological plasticity in morphological variations of red seaweed Hypnea pseudomusciformis (Gigartinales, Rhodophyta) uncovered by molecular, antioxidant capacity and pigments content data. Braz J Bot 41:567–577
Nie C, He T, Zhang W, Zhang G, Ma X (2018) Branched chain amino acids: beyond nutrition metabolism. Int J Mol Sci 19:954–966
Petridou K (2020) Growth of two red algae species (Palmaria palmata and Vertebrata lanosa) in lab culture. University of Oslo, Norway. Masters Thesis, University of Oslo
Raynal B, Brûlé S, Uebel S, Knauer SH (2021) Assessing and improving protein sample quality. Meth Mol Biol 2263:3–46
Sanderson J, Dring M, Davidson K, Kelly M (2012) Culture, yield and bioremediation potential of Palmaria palmata (Linnaeus) Weber & Mohr and Saccharina latissima (Linnaeus) CE Lane, C. Mayes, Druehl & GW Saunders adjacent to fish farm cages in northwest Scotland. Aquaculture 354:128–135
Santos-Hernandez M, Cermeno M, Recio I, FitzGerald RJ (2021) In vitro dipeptidyl peptidase IV inhibitory activity and in situ insulinotropic activity of milk and egg white protein digests. Food Funct 12:12372–12380
Schmedes PS, Nielsen MM (2020) Productivity and growth rate in Palmaria palmata affected by salinity, irradiance, and nutrient availability—the use of nutrient pulses and interventional cultivation. J Appl Phycol 32:4099–4111
Schmedes PS, Nielsen MM, Petersen JK (2019) Improved Palmaria palmata hatchery methods for tetraspore release, even settlement and high seedling survival using strong water agitation and macerated propagules. Algal Res 40:101494
Shevchenko A, Tomas H, Havli J, Olsen JV, Mann M (2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1:2856–2860
Smit AJ, Robertson BL, Du Preez DR (1996) Influence of ammonium-N pulse concentrations and frequency, tank condition and nitrogen starvation on growth rate and biochemical composition of Gracilaria gracilis. J Appl Phycol 8:473–481
Smith JM, Brzezinski MA, Melack JM, Miller RJ, Reed DC (2018) Urea as a source of nitrogen to giant kelp (Macrocystis pyrifera). Limnol Oceanogr Lett 3:365–373
Stack J, Tobin PR, Gietl A, Harnedy PA, Stengel DB, FitzGerald RJ (2017) Seasonal variation in nitrogenous components and bioactivity of protein hydrolysates from Porphyra dioica. J Appl Phycol 29:2439–2450
Stevant P, Olafsdottir A, Deleris P, Dumay J, Fleurence J, Ingadottir B, Jonsdottir R, Ragueneau E, Rebours C, Rustad T (2020) Semi-dry storage as a maturation process for improving the sensory characteristics of the edible red seaweed dulse (Palmaria palmata). Algal Res 51:102048
Sun L, Wang S, Gong X, Chen L (2004) A rod-linker-contained R-phycoerythrin complex from the intact phycobilisome of the marine red alga Polysiphonia urceolata. Photochem Photobiol B 76:1–11
Taiz L, Zeiger E (2010) A book on plant physiology (5th ed.). Sinauer Associates Publishing Group. Sunderland, United Kingdom
Tyler AC, Mcglathery KJ, Macko SA (2005) Uptake of urea and amino acids by the macroalgae Ulva lactuca (Chlorophyta) and Gracilaria vermiculophylla (Rhodophyta). Mar Ecol Progr Ser 294:161–172
Vasconcelos MMM, Marson GV, Turgeon SL, Tamigneaux E, Beaulieu L (2022) Environmental conditions influence on the physicochemical properties of wild and cultivated Palmaria palmata in the Canadian Atlantic shore. J Appl Phycol 34:2565–2578
Walsh M, Watson L (2011) A market analysis towards the further development of seaweed aquaculture in Ireland; Part 1. Irish Sea Fisheries Board. https://epub.sub.uni-hamburg.de/epub/volltexte/2013/18805/pdf/A_Market_Analysis_towards_the_Further_Development_of_Seaweed_Aquaculture_in_Ireland.pdf
Wang W, Vignani R, Scali M, Cresti M (2006) A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis 27:2782–2786
Wong KH, Cheung PC (2000) Nutritional evaluation of some subtropical red and green seaweeds: Part I—proximate composition, amino acid profiles and some physico-chemical properties. Food Chem 71:475–482
Wu G, Wu Z, Dai Z, Yang Y, Wang W, Liu C, Wang B, Wang J, Yin Y (2013) Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids 44:1107–1113
Xu N, Xu K, Wang W, Xu Y, Ji D, Chen C, Xie C (2020a) Nutrient enrichment improves growth and food quality of two strains of the economic seaweed Pyropia haitanensis. Front Mar Sci 7:544582
Xu Y, Wang Q, Hou Y (2020b) Efficient purification of R-phycoerythrin from marine algae (Porphyra yezoensis) based on a deep eutectic solvents aqueous two-phase system. Mar Drugs 18:618–622
Zhou Y, Li X, Xia Q, Dai R (2020) Transcriptomic survey on the microcystins production and growth of Microcystis aeruginosa under nitrogen starvation. Sci Total Environ 700:134501
Acknowledgements
Allihies Seafood/Pure Ocean Algae Ltd is acknowledged for the provision of P. palmata samples.
Funding
Open Access funding provided by the IReL Consortium This work was funded by Enterprise Ireland under Disruptive Technologies Innovation Fund (DTIF; grant no. DT20180088 “Optimised commercial-scale cultivation of protein-rich biomass from Palmaria palmata for the generation of health enhancing plant based proteinaceous ingredients (BioDulse project)”. RJF acknowledges funding from COST Action CA18238 (Ocean4Biotech), supported by the COST (European Cooperation in Science and Technology) program.
Author information
Authors and Affiliations
Contributions
ATI: Investigation, Formal analysis, Writing- original draft. MAB: Supervision, Writing- review & editing. MSH: Investigation, Formal analysis, Writing- review and editing. SW: Investigation. ME: Conceptualisation, Supervision, Funding acquisition, Writing- review and editing. RF: Conceptualisation, Overall Supervision, Funding acquisition, Writing- review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Idowu, A.T., Amigo-Benavent, M., Santos-Hernández, M. et al. Impact of growth conditions on the nitrogen, protein, colour and amino acid profiles of the cultured macroalga, Palmaria palmata. J Appl Phycol 35, 2397–2411 (2023). https://doi.org/10.1007/s10811-023-03057-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03057-1