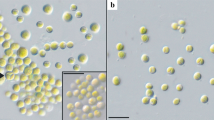
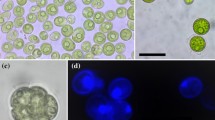
Abstract
The taxonomic diversity of the algal genus Meyerella is difficult to study because of its very simple morphology. Within the Chlorella-clade Meyerella members are distinguished from the others by the absence of the pyrenoid. However, it is not possible to identify them only on the basis of light microscopy data without the involvement of molecular genetic analysis methods. Some Meyerella representatives have high biotechnological potential, because they are able to accumulate valuable metabolites, including polyunsaturated fatty acids. As a rule, water bodies are the main habitats for these green microalgae. However, strains ACSSI 428 and ACSSI 429, which described in detail in this study, were isolated from peat cryozems (Sakha Republic, Russia). In this study, a detailed comparative analysis of the morphology, phylogeny and fatty acid profiles of these strains isolated from soil and representatives of other planktonic species, primarily Myerella similis, was carried out. Based on the results obtained, it was found that the studied strains are representatives of a new species with high biotechnological potential – Myerella krienitzii sp. nov.






Similar content being viewed by others
Data availability
All authors confirm that the data supporting the findings of this study are available within the article and its supplementary material. In addition, raw data supporting this study's findings are available from the corresponding author upon a reasonable request.
References
Abou-Shanab RAI, El-Dalatony MM, EL-Sheekh MM, Ji MK, Salama ES, Kabra AN, Jeon BH (2014) Cultivation of a new microalga, Micractinium reisseri, in municipal wastewater for nutrient removal, biomass, lipid, and fatty acid production. Biotechnol Bioproc Eng 19:510–518
Ando H, Ryu A, Hashimoto A, Oka M, Ichihashi M (1998) Linoleic acid and α-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin. Arch Dermatol Res 290:375–381
Andreeva VM (1998) Terrestrial and aerophytic green algae (Chlorophyta: Tetrasporales, Chlorococcales, Chlorosarcinales). Nauka, St. Petersburg, 352 p
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. J Biochem Physiol 37:911–917
Bock C, Proschold T, Krienitz L (2010) Two new Dictyosphaerium-morphotype lineages of the Chlorellaceae (Trebouxiophyceae): Heynigia gen. nov. and Hindakia gen. nov. Eur J Phycol 45:267–277
Bock C, Krienitz L, Pröschold T (2011) Taxonomic reassessment of the genus Chlorella (Trebouxiophyceae) using molecular signatures (barcodes), including description of seven new species. Fottea 11:293–312
Byun Y, Han K (2009) PseudoViewer3: generating planar drawings of large-scale RNA structures with pseudoknots. Bioinformatics 25:1435–1437
Caisová L, Marin B, Melkonian M (2013) A consensus secondary structure of ITS2 in the Chlorophyta identified by phylogenetic reconstruction. Protist 164:482–496
Chae HJ, Seo JB, Kim SH, Youn EJ, Kim S, Suh SS (2021) Antarctic freshwater microalga, Micractinium simplicissimum, suppresses inflammation. J Nanosci Nanotechnol 21:4098–4103
Coleman AW (2000) The significance of a coincidence between evolutionary landmarks found in mating affinity and a DNA sequence. Protist 151:1–9
Coleman AW (2007) Pan-eukaryote ITS2 homologies revealed by RNA secondary structure. Nucleic Acids Res 35:3322–3329
Coleman AW (2009) Is there a molecular key to the level of ‘biological species’ in eukaryotes? A DNA guide. Mol Phylogenet Evol 50:197–203
Coleman AW (2015) Nuclear rRNA transcript processing versus internal transcribed spacer secondary structure. Trends Genet 31:157–163
Dąbrowski G, Konopka I (2022) Update on food sources and biological activity of odd-chain, branched and cyclic fatty acids –– A review. Trends Food Sci Technol 119:514–529
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Meth 9:772
Decelle J, Romac S, Sasaki E, Not F, Mahé F (2014) Intracellular diversity of the V4 and V9 regions of the 18S rRNA in marine protists (Radiolarians) assessed by high-throughput sequencing. PLoS One 9:e104297
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973
Fawley MW, Fawley KP, Owen HA (2005) Diversity and ecology of small coccoid green algae from Lake Itasca, Minnesota, USA, including Meyerella planktonica, gen. et sp. nov. Phycologia 44:35–48
Fučíková K, Lewis PO, Lewis LA (2014) Widespread desert affiliation of trebouxiophycean algae (Trebouxiophyceae, Chlorophyta) including discovery of three new desert genera. Phycol Res 62:294–305
Gaonkar CC, Piredda R, Minucci C, Mann DG, Montresor M, Sarno D, Kooistra WHCF (2018) Annotated 18S and 28S rDNA reference sequences of taxa in the planktonic diatom family Chaetocerotaceae. PLoS One 13:e0208929
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321
Guiry MD, Guiry GM (2023) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org. (16 March 2023, date last accessed)
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Heeg JS, Wolf M (2015) ITS2 and 18S rDNA sequence-structure phylogeny of Chlorella and allies (Chlorophyta, Trebouxiophyceae, Chlorellaceae). Plant Gene 4:20–28
Hoshina R, FujiwaraY (2013) Molecular characterization of Chlorella cultures of the National Institute for Environment Studies culture collection with description of Micractinium inermum sp. nov., Didymogenes sphaerica sp. nov. and Didymogenes soliella sp. nov. (Chlorellaceae, Trebouxiophyceae). Phycol Res 61:124–132
Hoshina R, Nakada T (2018) Carolibrandtia nom. nov. as a replacement name for Brandtia Hoshina (Chlorellaceae, Trebouxiophyceae). Phycol Res 66:82–83
Hoshina R, Iwataki M, Imamura N (2010) Chlorella variabilis and Micractinium reisseri sp. nov. (Chlorellaceae, Trebouxiophyceae): Redescription of the endosymbiotic green algae of Paramecium bursaria (Peniculia, Oligohymenophorea) in the 120th year. Phycol Res 58:188–210
Hoshina R, Kobayashi M, Suzaki T, Kusuoka, Y (2017) Brandtia ciliaticola gen. et sp. nov. (Chorellaceae, Trebouxiophyceae) a commom symbiotic green coccoid of various ciliate species. Phycol Res 66:76–81
Hoshina R, Tsukii Y, Harumoto T, Suzaki T (2021) Characterization of a green Stentor with symbiotic algae growing in an extremely oligotrophic environment and storing large amounts of starch granules in its cytoplasm. Sci Rep 11:2865
Jahromi KG, Koochi ZH, Kavoosi G, Shahsavar A (2022) Manipulation of fatty acid profile and nutritional quality of Chlorella vulgaris by supplementing with citrus peel fatty acid. Sci Rep 12:8151
Johnson JL, Fawley MW, Fawley KP (2007) The diversity of Scenedesmus and Desmodesmus (Chlorophyceae) in Itasca State Park, Minnesota, USA. Phycologia 46:214–229
Karpagam R, Preeti R, Jawahar RK, Saranya S, Ashokkumar B, Varalakshmi P (2015) Fatty acid biosynthesis from a new isolate Meyerella sp. N4: molecular characterization, nutrient starvation, and fatty acid profiling for lipid enhancement. Energ Fuel 29:143–149
Katana A, Kwiatowski J, Spalik K, Zakryś B, Szalacha E, Szymańska H (2001) Phylogenetic position of Koliella (Chlorophyta) as inferred from nuclear and chloroplast small subunit rDNA. J Phycol 37:443–451
Krienitz L, Hegewald EH, Hepperle D, Huss V, Rohr T, Wolf M (2004) Phylogenetic relationship of Chlorella and Parachlorella gen. nov. (Chlorophyta, Trebouxiophyceae). Phycologia 43:529–542
Krienitz L, Bock C, Dadheech PK, Pröschold T (2011) Taxonomic reassessment of the genus Mychonastes (Chlorophyceae, Chlorophyta) including the description of eight new species. Phycologia 50:89–106
Krivina E, Temraleeva A (2020) The difficulty identifying and the cryptic diversity of Chlorella-clade microalgae (Chlorophyta). Microbiology 89:714–727
Krivina ES, Boldina ON, Bukin YS, Bykova SV, Temraleeva AD (2023) Species delimitation polyphasic approach reveals Meyerella similis sp. nov.: a new species of 'small green balls" within the Chlorella-clade (Trebouxiophyceae, Chlorophyta). Org Divers Evol 23:25
Lanzoni O, Fokin SI, Lebedeva N, Migunova A, Petroni G, Potekhin A (2016) Rare freshwater ciliate Paramecium chlorelligerum Kahl, 1935 and its macronuclear symbiotic bacterium “candidatus Holospora parva”. PLoS One 11:e0167928
Letawe C, Boone M, Pierard GE (1998) Digital image analysis of the effect of topically applied linoleic acid on acne microcomedones. Clin Exp Dermatol 23:56–58
Luo W, Pflugmacher S, Pröschold T, Walz N, Krienitz L (2006) Genotype versus phenotype variability in Chlorella and Micractinium (Chlorophyta, Trebouxiophyceae). Protist 157:315–333
Mahboob S, Rauf A, Ashraf M, Sultana T, Sultana S, Jabeen F, Rajoka MI, Al-Balawi HF, Al-Ghamin KA (2012) High-density growth and crude protein productivity of a thermotolerant Chlorella vulgaris: production kinetics and thermodynamics. Aquac Int 20:455–466
Musharraf SG, Ahmed MA, Zehra N, Kabir N, Choudhary MI, Rahman AU (2012) Biodiesel production from microalgal isolates of southern Pakistan and quantification of FAMEs by GC-MS/MS analysis. Chem Cent J 6:149
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Pan A, Chen M, Chowdhury R, Wu JH, Sun Q, Campos H, Mozaffarian D, Hu FB (2012) α-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr 96:1262–1273
Poger D, Caron B, Mark AE (2014) Effect of methyl-branched fatty acids on the structure of lipid bilayers. J Phys Chem B 118:13838–13848
Pröschold T, Darienko T (2020) Choricystis and Lewiniosphaera gen. nov. (Trebouxiophyceae, Chlorophyta), two different green algal endosymbionts in freshwater sponges. Symbiosis 82:175–188
Pröschold T, Bock C, Luo W, Krienitz L (2010) Polyphyletic distribution of bristle formation in Chlorellaceae: Micractinium, Diacanthos, Didymogenes and Hegewaldia gen. nov. (Trebouxiophyceae, Chlorophyta). Phycol Res 58:1–8
Pröschold T, Darienko T, Silva PC, Reisser W, Krienitz L (2011) The systematics of Zoochlorella revisited employing an integrative approach. Environ Microbiol 13:350–364
Pröschold T, Pitsch G, Darienko T (2020) Micractinium tetrahymenae (Trebouxiophyceae, Chlorophyta), a new endosymbiont isolated from ciliates. Diversity 12:200
Pröschold T, Rieser D, Darienko T, Nachbaur L, Kammerlander B, Qian K, Pitsch G, Bruni EP, Qu Z, Forster D, Rad-Menendez C, Posch T, Stoeck T, Sonntag B (2021) An integrative approach sheds new light onto the systematics and ecology of the widespread ciliate genus Coleps (Ciliophora, Prostomatea). Sci Rep 11:5916
Seibel PN, Müller T, Dandekar T, Schultz J, Wolf M (2006) 4SALE - a tool for synchronous RNA sequence and secondary structure alignment and editing. BMC Bioinform 7:498
Sidorov RA, Zhukov AV, Pchelkin VP (2014) Dynamics of fatty acid composition of neutral acylglycerols in maturing euonymus fruits. Chem Biodivers 11:581–592
Simpson PD, Van Valkenburg SD (1978) The ultrastructure of Mychonastes ruminatus gen. et sp. nov., a new member of the Chlorophyceae isolated from brackish water. Br Phycol J 13:117–130
Sogin ML, Morrison HG, Huber JA, Mark Welch D, Huse SM, Neal PR, Arrieta JM, Herndl GJ (2006) Microbial diversity in the deep sea and the underexplored rare biosphere. Proc Nat Acad Sci 103:12115–12120
Somogyi B, Felföldi T, Solymosi K, Flieger K, Márialigeti K, Böddi B, Vörös L (2014) One step closer to eliminating the nomenclatural problems of minute coccoid green algae: Pseudochloris wilhelmii, gen. et sp. nov. (Trebouxiophyceae, Chlorophyta). Eur J Phycol 8:427–436
Spanner C, Darienko T, Biehler T, Sonntag B, Pröschold T (2020) Endosymbiotic green algae in Paramecium bursaria: A new isolation method and a simple diagnostic PCR approach for the identification. Diversity 12:240
Sverdrup AE, Frolova LL (2020) Saprobity identification of hydrobiont species of Verhniy Kaban lake of Kazan by 18S rRNA marker gene. Scientific Notes of V. Vernadsky Crimean Federal University. Biol Chem 4:127–142
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Boil Evol 30:2725–2729
Temraleeva A, Krivina E, Boldina O (2022) Edaphochloris gen. nov.: a new genus of soil green algae (Trebouxiophyceae, Chlorophyta) with simple morphology. Plant Syst Evol 308:4
Vorobyev K, Andronov E, Rautian M, Skoblo I, Migunova A, Kvitko K (2009) An atypical Chlorella symbiont from Paramecium bursaria. Protistology 6:39–44
Funding
The work was funded by Ministry of Science and Higher Education of the Russian Federation, state assignment of Federal Research Center “Pushchino Scientific Center for Biological Research of the Russian Academy of Sciences”, theme №122040500037–6 (sampling, isolation and cultivation, morphological observation, and phylogenetic analysis), by the Russian Science Foundation, grant No 22–16-00047 (analysis of fatty acids).
GC–MS analysis was carried out using the equipment of the Center for Collective Use of the "Pushchino Scientific Center for Biological Research of the Russian Academy of Sciences".
Author information
Authors and Affiliations
Contributions
Elena Krivina, Tatyana Savchenko, Elizaveta Tebina, Anastasia Shatilovich, and Anna Temraleeva contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krivina, E.S., Savchenko, T.V., Tebina, E.M. et al. Morphology, phylogeny and fatty acid profiles of Meyerella similis from freshwater ponds and Meyerella krienitzii sp. nov. from soil (Trebouxiophyceae, Chlorophyta). J Appl Phycol 35, 2295–2307 (2023). https://doi.org/10.1007/s10811-023-03038-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03038-4