Abstract
The lack of adequate vertical mixing is one of the factors limiting the productivity of open raceway microalgae reactors. The existence of large gradients of light involves the cells being mainly adapted to local irradiance instead of average irradiance, which would allow for maximizing the light utilization efficiency, thus maximizing the biomass productivity of microalgae cultures. To overcome this problem, different alternatives have been proposed, one of the more suitable being the utilization of airfoils to improve vertical mixing. In this work, numerical and experimental studies were performed to analyse the effect of the aerodynamic airfoils patented by the University of Seville (WO2020120818A1). The goal is to improve the photosynthetic efficiency, but also a better understanding of the light regime to which the microalgae cells are exposed in these systems and how to improve it. Computational Fluid Dynamics (CFD) was used to optimize the flow generated by the airfoils. A dynamic photosynthesis model of Rubio Camacho et al. (Biotechnol Bioeng 81:459–473, 2003) was used to estimate the photosynthesis rate as a function of the light regime to which the cells are exposed, including photo-adaptation and photo-inhibition phenomena, the results confirm that the use of airfoils improves the vertical mixing and the photosynthesis rate. The photosynthetic benefits were observed 10 m downstream of the airfoils, resulting in an increase in photosynthesis rate and productivity by up to 30%. These results confirm the benefits of an increase in mixing in microalgae cultures, especially when focusing on the movement of the cells between the different illuminated zones while maintaining low energy consumption and capital expenses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than 90% of microalgae production systems worldwide are based on raceway reactors (Spolaore et al. 2006; Chisti 2016). One reason is that raceways can be easily scaled, allowing for high productivity with low costs and energy requirements (Mendoza et al. 2013; Inostroza et al. 2021). Microalgae cultures require mixing to keep the cells in suspension aided by turbulence, to achieve an even distribution of nutrients, and to avoid some microalgae cells remaining in the deep, dark regions of the ponds for prolonged periods (Sompech et al. 2012). The overall yield of any microalgae culture is always limited by the efficiency of light utilization; i.e. how the microalgae make use of the light that is shed on the reactor surface (Barceló-Villalobos et al. 2019a; Fernández del Olmo et al. 2021).
Conventional raceway reactors have limited vertical mixing and strategies must be sought to favour the transit of particles between the different illuminated areas, from the darkest near the bottom of the reactor to the most illuminated located near the surface. In addition to vertical mixing in a raceway, intensity and frequency are also important, as the pattern of variation of irradiance on microalgae cells produces a different photosynthetic response, depending on the light regime to which they are exposed. As microalgae grow and the cultures become more concentrated, light penetrates less in the pond and the stratification of lighting increases. This results in zones with different illumination and, therefore, with a different photosynthetic response and poor productivity. This negative effect can be solved by allowing the microalgae cells to move between the different illuminated zones with a high frequency. Ideally, during the illuminated period, the cells obtain enough light without reaching supersaturation and they remain in the dark zone for a time short enough to prevent them from performing cellular respiration.
Among the first experiments using wing-shaped obstacles, Laws et al. (1983) stands out for generating vortices in the culture due to the pressure differential created when water flows over and under the foils. In a channel with a flow rate of 0.3 m s−1, the foils produced vortices with a rotation speed of approximately 0.5–1.0 Hz, where solar energy conversion efficiency reached up to 10%. In summary, it is generally accepted that to allow complete integration of light as described by Terry (1986), light/dark cycles are required at frequencies above 1 Hz (Brindley et al. 2011). However, to date, it has not been clearly described how to achieve this increase in frequency in industrial facilities.
The most common method used to establish light regimes is by setting illuminated and dark culture volumes to determine the duty cycle and frequency of light exposure (Chen et al. 2016). Currently, based on the use of Computational Fluid Dynamics (CFD), a more accurate analysis has been achieved by coupling Lagrangian particle tracking with the use of dynamic photosynthetic models, such as those proposed by Eilers and Peeters (1988) and Rubio Camacho et al. (2003), where it is required to know the complete history of a large population of cells concerning time (Fernández-Del Olmo et al. 2017) and its position, especially in vertical resolution. To increase the vertical mixing along culture channels to provide more uniform exposure to sunlight, which generates long stable vortices which can transport the cells between the illuminated and dark zone (Cheng et al. 2015), thus generating improved conditions for the metabolism of photosynthesis. However, even with the use of airfoils, it is difficult to generate significant vertical mixing inside a raceway with a 15 cm depth to obtain the benefits of intermittent light that leads to higher growth rates (Nedbal et al. 1996).
Methods
Raceway reactor and microalga strain
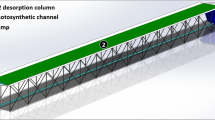
The raceway reactor used is located at the Research Center "IFAPA", (Almería, Spain). The reactor consists of two 40 m long channels (0.35 m high × 1.25 m wide), both connected by 180° bends at each end, an area exposed to solar radiation of 100 m2, a production volume of 15 m3 (depth of 15 cm), a paddlewheel system being used to recirculate the culture through the reactor at a linear velocity of 0.2 m s−1. As shown in Fig. 1 the study was performed in two cases: (1) called "Free flow" as it is used traditionally, with the channels free of obstacles, (2) called "with airfoils" where obstacles were installed in the form of airfoils to generate wingtip vortices to increase the vertical mixing of the system. A total of six packs with four airfoils each were installed throughout the channels. Five experimental checkpoints (CP) were located to carry out the experimental study. The dissolved oxygen saturation measure (DO) was used to determine the variation of photosynthesis using a HANNA brand optical meter, model HI98198.
To perform the Computational Fluid Dynamics (CFD) analysis several subdomains of the channels were defined to increase the accuracy of the results. The inlet has assumed that the flow velocity is uniform in both cases to compare the results under similar conditions. Additionally, in the open channel without the presence of an agitation mechanism, the flow tends to be laminar, a uniform velocity allows the results obtained at different points of the flow to be comparable.
The experimental implementation can be seen in Fig. 2 and Scenedesmus almeriensis strain used was obtained from the culture collection of the Department of Chemical Engineering of the University of Almeria. This microalga is a fast-growing and highly productive strain that is particularly adapted to stressful conditions. A culture medium that consisted of 0.90 g L−1 NaNO3, 0.18 g L−1 MgSO4, 0.14 g L−1 K2PO4, and 0.03 g L−1 of karentol® (Kenogard, Spain) was used. This is a commercial solid mixture of micronutrients that includes boron, copper, iron, manganese, molybdenum, and zinc (Morillas-España et al. 2020).
Numerical simulation
Two subdomains have been discretized by ANSYS Meshing 2020R2, both 10.3 m long × 0.3 m wide × 0.2 m high (CFD Analysis Domain, as shown in Fig. 1). Both meshes were designed using hexahedral-structured linear elements, with cell size of 5 mm and a total of 1.7 million elements for the total more than 10 m of computational domain analyzed with orthogonal quality of 100%.
The software ANSYS Fluent 2020R2 was utilized to solve the Reynolds-averaged Navier–stokes equation in the transient time method, using a time step of 0.001 s and resolving up to 130 s to achieve a steady state where the vertical velocities do not have significant variations. An implicit scheme of the Volume Of Fluid model (VOF) is used to track the free surface of the water (the average depth of the water phase is 0.15 m), with a surface tension coefficient of 0.072 N m−1 at 25 °C (Nikolaou et al. 2016a). The turbulence model used was κ-ω SST, one of the best models to capture the effect of turbulent flow conditions with Low-Re Corrections. The solution logarithms used were based on the Pressure–Velocity Coupling by the scheme SIMPLE; least squares cell-based for gradient, PRESTO! for pressure; second-order upwind for momentum; compressive for volume fraction and first-order upwind for turbulent kinetic energy and turbulent dissipation rate.
The following boundary conditions were used: a velocity inlet of U = 0.22 m s−1, a pressure outlet as an output, a symmetry condition on the lateral and top surfaces of the subdomain, a wall (no-slip condition) with a roughness of 0.0015 mm for the bottom and airfoil surface, and an atmospheric pressure located over the free surface of the water.
Particle tracking
The trajectories of Lagrangian particles were integrated using the balance of forces on the particles. This balance of forces equals the inertia of the particles with the forces acting on them, and it can be written as: \(\frac{d{u}_{p}}{dt}={F}_{D}\left(u-{u}_{p}\right)+\frac{g\left({\rho }_{p}-\rho \right)}{{\rho }_{p}}+{F}_{p}\), \({F}_{D}=\frac{18\mu }{{\rho }_{p}{d}_{p}^{2}}\frac{{C}_{D}Re}{24}\), where \(u\) is the velocity of the fluid or culture in m s−1, \({u}_{p}\) is the velocity of the particle in m s−1, \(\mu\) = 0.001 Pa s is the fluid viscosity, \(\rho\) = 1000 kg m−3 is the water density, the particle density has been considered \({\rho }_{p}\) = 864 kg m−3 and a particle diameter \({d}_{p}=7 \mu m\) (Ali et al. 2015; Akhtar et al. 2020; Chiarini and Quadrio 2021).\({F}_{D}\) is the drag force per unit mass, \({C}_{D}\) is the spherical drag coefficient, and \({F}_{p}\) represents additional acceleration terms (force/unit mass of the particle). A number of particles N = 60 were used, with the initial conditions shown in Fig. 3.
Photosynthetic efficiency of the cells
Particle motion prediction is used to determine the evolution of irradiance with time by tracking their evolution over time in the illuminated and dark zones (Chiarini and Quadrio 2021). Once the irradiance is obtained, the photosynthetic effect on microalgae cells in suspension can be analyzed using the dynamic models proposed by Rubio Camacho et al., (2003), who claim that during photosynthesis, a microalgae Photosynthetic Unit (PSU) that is in a non-activated state is first activated by absorbing photons. In subsequent steps, the activated photosynthetic unit is consumed in enzyme-mediated reactions to return to its non-activated state, providing energy for maintenance and producing biomass. Thus, microalgae respond to the amount of light available by varying photosynthetic activity. The available light (\(I(t)\) in µmol photons m−2 s−1) can be calculated using the Lambert–Beer Law:
where \({I}_{0}\) is the incident irradiance in µmol photons m−2 s−1, \({k}_{a}\) is the extinction coefficient in m2 g−1, \({C}_{b}\) is the biomass concentration in g m−3 and \(L(t)\) is the depth of the particle, i.e. its distance to the free surface at the time.
The vertical movement allows time steps between the different illuminated zones (see Fig. 3). The light/darkness frequency (ν) and the illuminated fraction or duty cycle (Ф) can be measured. They depend on: (i) the turbulent intensity of the flow, (ii) the intensity of the light source, and (iii) the concentration of the culture (Chiarini and Quadrio 2021). Using CFD, it is possible to obtain the particles trajectories and determine their time of permanence in each illuminated zone. The light availability on the vertical axis will determine the production (\(P\)) of the cells individually. Using Eq. 1, the specific location that separates the different illuminated zones in a culture can be determined, which are: the photoinhibition zone by the photoinhibition coefficient (\({K}_{I}\) = 800 µmol photons m−2 s−1) and dark zone by the compensation irradiance (\({I}_{c}\)= 4 µmol photons m−2 s−1), both of (Chisti 2016), the saturated zone by the saturation coefficient (α = 221.8 µmol photons m−2 s−1) obtained from Brindley et al., (2016) and the limited zone is located between α and \({I}_{c}\). The production of the cells in the photoinhibition zone depends on \(\delta \sqrt{I}\), where \(\delta\) is the photoinhibition parameter of the Rubio Camacho et al. (2003); in the saturated zone, the production P reaches its maximum value; while in the limited zone, \(P\) depends on \(I\); and in the dark zone, \(P\) = 0 or minimum. The different zones can be seen in detail in Fig. 4.
Results and discussion
Fluid dynamics of particles
A conventional raceway (“Free flow”) has a velocity field parallel to the direction of the current, which means that the particles follow very stable trajectories over time, easily represented in a 2D space. On the other hand, in a channel with vortices generators ("with airfoil"), the flow is highly three-dimensional and difficult to predict. The airfoil design has a great impact on the velocity field, and it must be carefully studied.
A detailed description of particle tracking using CFD was reported by Fernández del Olmo et al. (2021), Gao et al. (2017), Nikolaou et al. (2016b) and Prussi et al. (2014). In the present work, the benefits of implementing vortex generators in a raceway are demonstrated, that is, increasing the vertical mixing to allow microalgae cells to absorb photons and consume them before starting a new cycle. The only drawback is a slight decrease in horizontal velocity (Cheng et al. 2015), which is not important for this analysis. The vertical velocity difference of 30 particles between the 2 cases studied is shown in Fig. 5.
With the installation of airfoils, the vertical velocity has a significant increase with a maximum of ± 0.065 m s−1, compared to ± 0 m s−1 of the free channel. The vertical velocity is much higher in the first 4 m and acquires higher values above the vortex axis, which makes it easier for the cells to return to a highly illuminated area near the surface. The fact that the cells continuously move between the different illuminated zones entails an improvement in the photosynthetic behaviour (Ali et al. 2015). An elevated level of mixing is related to light integration (Γ), which in turn is directly related to the frequency (ν) of Light/Darkness (L/D) cycles, ultimately impacting the concentration of biomass(\({C}_{b}\)).
Light regime
To determine the evolution of the irradiance of a particle with time, \({I}_{p}=I\left(t\right)\), the Lambert–Beer Law has been used (Eq. 1). Typical values of the incident irradiance, \({I}_{0}\) = 1500 µmol photons m−2 s−1, and the extinction coefficient, \({k}_{a}\)= 0.10 m2 g−1, where chosen. Different values of \({C}_{b}\) = 0.4, 0.8, 1.2, 1.6, 2.0, 2.4, 2.8, and 3.2 g L−1 were used to determine the ideal operating concentration. After the integration of N particles, \({\nu }_{av}\) has been calculated using the following formula:
where, \(n\) is the number of total L/D cycles in the 10 m of the subdomain of each particle. \({t}_{f}\) is the time (in seconds) during which the particles are in light zones, i.e., when \(I(t)>{I}_{c}\), and \({t}_{d}\) is the time during which the particles are in the darkness, i.e., \(I(t){\le I}_{c}\). One L/D cycle is considered when there is \({t}_{f}\) and \({t}_{d}\).
Since it is important that the simulation and sampling properly capture the photosynthetic response of each particle, at least 50 position points of each particle have been recorded per second for a maximum of 50 s, which allows detecting frequencies between 0. 2 and 50 Hz (Fernández-Del Olmo et al. 2017).
Determining the frequency of L/D cycles is a first step to predict the photosynthetic behaviour in dense cultures (Brindley et al. 2016). This has been a widely discussed topic because not all particles move between the illuminated zone and the dark zone. In that sense, it is important to consider two important thresholds (Brindley et al. 2011). The parameters that define the limit of the dark zone are the compensation irradiance (\({I}_{c}\)) and the saturation coefficient (α), (limits shown in Fig. 4). As both approaches are incompatible, this analysis makes use of the limit \({I}_{c}\), therefore, assuming that productivity is equal to 0 where the particles have no photosynthetic activity. The comparative results can be seen in Fig. 6.
In the case of Free flow \({\nu }_{av}\) is very low, presenting a maximum of 0.1 Hz at the minimum concentration (\({C}_{b}\) = 0.4 g L−1) and at the minimum linear displacement of 1 m. \({\nu }_{av}\) decreases exponentially due to the increase in concentration (\({C}_{b}\)) and also due to the linear displacement length of the particles, which results in a limitation of the operation and explains why raceways reactors are constantly treated with limited productivity (Inostroza et al. 2021), This is explained by the fact that a large number of particles have slight changes in their vertical position, according to the literature (Amini et al. 2016; Leman et al. 2018; Barceló-Villalobos et al. 2019a). The analysis has been carried out to a velocity of 0.22 m s−1, even though authors such as Barceló-Villalobos et al. (2019a, b) and Brindley et al. (2016) recommend increasing the velocity of the liquid to increase the frequency. The decision was made because in real cases, it is not possible to increase velocity without causing high energy consumption (Mendoza et al. 2013).
On the other hand, in the case With airfoils, the particles achieve a greater vertical displacement, which means that they enter and leave the dark zone more often. Even at low concentrations (\({C}_{b}\) between 0.4 and 1.2 g L−1), the \({\nu }_{av}\) is smaller than 0.8, because the thickness of the dark zone is low and a large number of cells fail to pass through a period of darkness. At concentrations higher than 1.2 g L−1, a decrease in frequency is observed, which occurs because the thickness of the dark zone has increased and a large population of particles never enter the illuminated zone, travelling during the 10 m of the subdomain in the darkness.
The use of airfoils allows working with a much denser culture than in a conventional raceway, being the optimal concentration \({C}_{b}\) = 0.8 g L−1, a value that has been set for the following comparative analyses. In addition, it could be observed that the use of airfoils involves a higher influence on the photosynthetic response along the channel. The comparison of the vertical position followed by the particles along both subdomains can be seen in more detail in Fig. 7.
The vertical movement of the cells is one of the main factors that affect the performance of a raceway photobioreactor. Thus, the photosynthetic response of each cell will depend on the light intensity of each zone through which it moves, especially the saturated zone and the dark zone, where the transition between the minimum and the maximum occurs. After the integration of N particles, the numerical parameter representing the illuminated fraction or duty cycle (ϕ) must be calculated. The average value \({\phi }_{av}\) has been calculated using the following formula:
Another benefit of using vortex generators is that it increases the frequency to change the position of the particles travelling through the photoinhibition zone, reducing the damage produced due to excess light. The photoinhibited fraction (\({\phi }_{KI}\)) has been calculated using the following formula:
where \({t}_{{K}_{I}}\) is the time during which a particle receives an irradiance \({I}_{p}\left(t\right)\) ≥\({K}_{I}\) and \({t}_{c}\) is the total time required for each particle to move along the 10 m of the subdomain.
In the case without airfoils or Free flow, there is no complete movement of cells between the surface to the bottom area. Thus, considering that there are no complete saturation conditions (Ali Kubar et al. 2020), some cells remain for long periods in the continuous radiation zone, as can be easily observed in Figs. 7 and 8. The illuminated fraction corresponds to 0.485, indicating that about half of the cells are in the non-productive phase ( \({I}_{p}\left(t\right)\le {I}_{c}\to P=0\)). According to Barceló-Villalobos et al. (2019a, b) and Brindley et al. (2016) the \({\phi }_{av}\) obtained corresponds to diluted cultures which may favor the integration of light (Γ) depending on irradiance and frequency.
On the other hand, the case with airfoils has different behaviour. The vortices generated transport particles to a shallow depth of the channel. In that way, the vortex generator constitutes a hybrid method between continuous lighting and intermittent lighting, producing light fluctuations between the illuminated and dark zone with a high frequency. The \({\phi }_{av}\) increases to a maximum of 0.595 at 5 s (1 m) and decreases until 35 s (4 m where the photosynthetic influence is equal to the case without airfoils), allowing a rapid activation of PSUs, as well as better integration of light and a higher stability of productivity over time.
Photosynthetic evolution
In the previous section, for a given value of the incident irradiance (\({I}_{0}\)), we studied the variations in the light regimes of the culture in terms of frequency (\(\upnu\)) and fraction illuminated (\(\phi\)), and how it, in turn, affects concentration (Cb). These parameters allow to know different photosynthetic responses or production (P) depending on the light that each particle receives dynamically \({(I}_{p}\left(t\right))\). P has been calculated as the rate of photosynthesis, which is represented by RO2 (mg O2 gb−1 h−1) and is obtained from the following formulas:
where, \({RO}_{2, max}\) = 88 mg O2 gb−1 h−1 (Costache et al. 2013), and the measurement is considered to be representative under optimal temperature conditions at 25 °C and pH of 8 (Barceló-Villalobos et al. 2019b), κ = 0.1 and \(\delta =0.05\) µmol photons m−0.5 s−0.5 (Rubio Camacho et al. 2003). RO2 is directly proportional to biomass production (Pb) in g m−2 day−1. Ten productive hours per day and 150 L m−2 have been considered. Productivity (Pb) is an effect that is observed for a longer time than photosynthesis, which is instantaneous. To perform the analysis, results have been projected on the same graph, in which a stoichiometric value of 1.33 g O2 per gram of biomass obtained from the basic photosynthesis equation has been considered (Barceló-Villalobos et al. 2018). The comparative results can be seen in Fig. 9.
By maintaining a constant light regime in the cells, the production does not show significant variations with conventional channels without airfoils. However, using vortex generators, oxygen production has increased up to 30% more, reaching a 21% better production in its stationary phase. Indeed, oxygen production grew from 16.2 to 22.8 mgO2 g−1 h−1 calculated using a dynamic model without considering the elimination of oxygen to the environment., and biomass production increased from 15.8 to 20.2 g m−2 day−1. These changes may not be relevant in small photobioreactors, but they imply large decreases in production costs at industrialized levels.
In terms of the production with the airfoils, a very fast acceleration is observed in the first 10 s, which is directly related to the fraction illuminated in that first period of the analysis. (See Fig. 8). On the other hand, after 20 s, the production is stable over the domain. The photosynthetic effect has been improved near to 10 m in length. This result has important implications as it allows placing the airfoils at a large distance from each other, implying a low inversion. Thus, its photosynthetic potential is similar to the thin-layer cascade (TLC) photobioreactors.
The use of airfoils allows a greater improvement than if the biomass concentration is changed, or if there is an increase in the fluid flow velocity. It was numerically demonstrated (Fernández del Olmo et al. 2021) that increasing the velocity from 0.2 to 0.8 m s−1 would only achieve 5% more productivity. This author also obtained a 10 to 15% improvement in productivity when the concentration changed from 0.8 to 1.6 g L−1. Morillas-España et al. 2020 worked at a raceway with an incident irradiance (\({I}_{0}\)) of 2000 µmol photons m−2 s−1, obtaining productivity of 20–25 g m−2 day−1, a slightly higher result. It should be noted that, despite using a lower value of \({I}_{0}\) than the one used in that previous study (1500 vs. 2000 µmol photons m−2 s−1), the productivity values are similar, thanks to the mixing produced in the raceway by the vortex generators.
Values above 30 g m−2 day−1 are targeted, with productivity similar to that obtained in TLC (Morillas-España et al. 2020; Villaró et al. 2022), which are photobioreactors with a much higher light regime. This improvement must be achieved by keeping a large part of the cells saturated and at much higher biomass concentrations. Masojídek et al. 2011 reported an improvement of up to 55 g m−2 day−1 in a TLC performing the function of cascades to increase turbulence so that the culture concentration increased to 12.5 g·L−1 due to a high frequency of the L/D cycles (here achieved by vortex generators). High productivity is manifested in high oxygen production and may lead to some inhibition of cell growth due to the oxygen saturation in the culture, which is a problem to be solved in the future when a greater integration of light is achieved, a phenomenon that occurs when maintaining a high degree of saturated microalgae at a high light regime.
From the data presented, insights can be gained to explore which light regime conditions improve the performance of existing cultivation systems or to design more efficient ones. The comparative analysis of the integration factor (Γ) is equal to 1 when the light distribution is sufficiently uniform or well-mixed for the cells to respond to the average irradiance \(({I}_{av})\). Meanwhile, it is equal to 0 in a sufficiently segregated environment to experience different local growth rates (Terry 1986; Brindley et al. 2011), as a function of the local irradiance (\({I}_{p}\left(t\right)\)). The integration factor can be calculated by
where, \(\widetilde{{RO}_{2}}\) is the oxygen productivity and is related to the photosynthesis proportional to the metabolic rate of energy consumption and is measured in mgO2 gb−1 h−1. This model does not deny the possibility that photosynthetic production occurs during periods of darkness, as proposed by Eilers and Peeters (1988), because part of the energy obtained during the illuminated period remains stored in the activated PSUs, so that they can support the metabolic demand as long as the period in darkness is not too long. (Rubio Camacho et al. 2003). \(\widetilde{{RO}_{2}}\) has been calculated using the following formula:
where \({x}^{*}\) is the fraction of photosynthetic units (PSUs) and \(\beta =5\) Hz is a characteristic frequency of the system and represents the specific photosynthetic maximum rate (Rubio Camacho et al. 2003).
\({RO}_{2}\left({I}_{av}\right)\) assumes that the entire volume of the reactor is mixed, independent of the particle location and time, assuming that all particles are exposed to an average irradiance (\({I}_{av}\)), Therefore, to determine this parameter the simplified equation proposed by Molina Grima et al. (1994) can be used. It can be calculated by the following equations.
The results can be seen in Fig. 10.
Since it is possible to obtain a reliable representation of the light conditions of each particle and its multiple trajectories, and the dynamic model of photosynthesis represents well the effects of the duty cycle and frequency, it is feasible to predict productivity reliably using a simple static model. This provides valuable information about the design and operation of a reactor, evaluation and redesign in case of improvements, or even choosing a method of agitation without wasting energy (Brindley et al. 2016) to move the cells between bright and dark zones very quickly, thereby increasing the efficiency in the use of light, thus reaching a state of integration of light.
Higher light exposure tends to level out the PSUs (\({x}^{*}\)), reducing the PSU consumption rate. In the case of a conventional channel without airfoils, it is observed that the light exposure rate is low or maintains long periods in darkness, completely depleting the energy stored in the excited PSUs. This same phenomenon is also possible in circumstances of light saturation slowing to a standstill, which may lead to a higher risk of photoinhibition because the active centers continue to absorb light but does not drive the formation of PSUs. On the other hand, the use of airfoils generates a photosynthetic situation very favourable for photosynthetic efficiency by promoting high frequencies of exposure to light and avoiding the three most important phenomena that weaken productivity in photobioreactors: photorespiration (due to prolonged time in the dark zone); supersaturation and photoinhibition for prolonged times in saturated zone, and photoinhibition respectively (view in Fig. 7). The representation of the integration light factor can be seen in Fig. 10, where 2 stages can be observed: 1) up to 25 s with 15% more light integration and 2) up to a steady state (from 45 s). On balance, in the case with airfoils, the integration light factor is 36% higher over the 10 m analysed, suggesting the possibility of achieving a higher increase (e.g., by placing the airfoils closer together). Airfoils allow light to be supplied gradually, being always closer to the integration of light than continuous exposure to light. When light changes are too slow or are supplied continuously, PSUs can be excessively excited, absorbing light that is not converted into photosynthetically available energy, which is probably dissipated as fluorescence or heat. This phenomenon is called dissipated flow and is a very important parameter when optimizing a production system.
In the following, the optimization parameter is defined, which represents the metabolic photosynthetic efficiency between the 2 case studies (\({\varepsilon }_{av,Airfoils}/{\varepsilon }_{av,Free})\):
The results can be seen in Fig. 11.
The use of airfoils optimizes the use of light or photosynthetic efficiency in comparison to a conventional channel, as has been shown. Up to the initial 7–8 s, it has a strong increase of 1.27 compared to the conventional configuration without airfoils, which is due to a high frequency of light exposure above its saturation point, allowing a rapid activation of the PSUs. However, operating close to saturation causes light leakage, decreased photosynthetic efficiency and risk of photoinhibition. The optimization decreases up to 1.25 comparatively in the 10 s of analysis, at 20 s the state of adaptation begins because it starts to operate below the saturation point and the illuminated fraction decreases, which is the most common and desirable situation (Fernández-Sevilla et al. 2018). The comparative optimization stabilizes after 45 s and equals the initial maximum load of 1.27. In the configuration without airfoils, the \({I}_{av}\) is less than \(\alpha\) and the cells have very little transition between the different illuminated areas, so it is not possible to take full advantage of the photosynthetic devices of the cells. However, with the use of airfoils, many more cells interact on the saturation point and leave it, generating a dynamic metabolism of generation and consumption, and causing metabolic adaptation to be much greater in time than the hydrodynamic influence of turbulence. that means that the vertical mixing has a higher influence during the first 4 m, but the photosynthetic influence can be higher than 10 m.
Experimental validation through dissolved oxygen saturation analysis
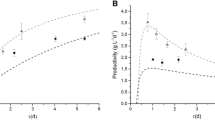
Figure 1 shows again the 5 experimental checkpoints, where sampling was carried out for 5 consecutive days from 9.00 to 13.00 h. The averaged results can be seen in Fig. 12.
In experimental terms, the use of airfoils has achieved a 30% improvement over a raceway without airfoils, which is consistent with the simulated numerical analyses. The improvement in oxygen production obtained by numerical simulation and mathematical modelling was 21% in steady state. Experimentally, Photosynthesis has been increased to the maximum of 250% DO Sat. recommended by Barceló-Villalobos et al. (2019b) and even more than the maximum of 225% DO Sat. recommended by Petera et al. (2021). The DO Sat. of the raceway photobioreactor without airfoils is over 200%, which are acceptable operability values. Therefore, it is estimated that improvements in experimental oxygen saturation can be up to a maximum of 30%.
Conclusion
It has been shown that the light regime is improved with the use of airfoils in raceway reactors, in addition, production and light integration has increased. The frequency of exposition improves three times concerning conventional systems which allows an increase in photosynthesis rate and productivity up to 30% higher, light integration is 36% higher using airfoils, optimization has been 27% higher comparatively, and experimentally, oxygen saturation has been 30% higher. These results confirm the benefits of optimizing the mixing in microalgae cultures, especially focusing on the movement of the cells between the different illuminated zones. This method has great expectations because the photosynthetic influence of the airfoils extends up to 10 m of the channel from the physical position of the airfoils and because it is also independent of out-of-control conditions such as irradiance from the sun and other conditions, such as increasing the velocity of circulation which directly affects production costs. Additional work is underway to increase the photosynthesis rate and final biomass productivity, it is expected to increase the microalgae production capacity of actual photobioreactors up to 50%, regardless of location, maintaining low energy consumption and CAPEX.
Data availability
The data used is confidential.
Change history
16 July 2023
Incorrect Open Access funding information has been corrected in the Funding Note.
References
Akhtar S, Ali H, Park CW (2020) Complete evaluation of cell mixing and hydrodynamic performance of thin-layer cascade reactor. Appl Sci 10:746
Ali H, Cheema TA, Yoon H-S, Do Y, Park CW (2015) Numerical prediction of algae cell mixing feature in raceway ponds using particle tracing methods. Biotechnol Bioeng 112:297–307
Ali Kubar A, Cheng J, Guo W, Kumar S, Song Y (2020) Development of a single helical baffle to increase CO2 gas and microalgal solution mixing and Chlorella PY-ZU1 biomass yield. Bioresour Technol 307:123253
Amini H, Hashemisohi A, Wang L, Shahbazi A, Bikdash M, KC D, Yuan W (2016) Numerical and experimental investigation of hydrodynamics and light transfer in open raceway ponds at various algal cell concentrations and medium depths. Chem Eng Sci 156:11–23
Barceló-Villalobos M, Fernández-del Olmo P, Guzmán JLL, Fernández-Sevilla JM, AciénFernández FG (2019a) Evaluation of photosynthetic light integration by microalgae in a pilot-scale raceway reactor. Bioresour Technol 280:404–411
Barceló-Villalobos M, Guzmán Sánchez JL, Martín Cara I, Sánchez Molina JA, AciénFernández FG (2018) Analysis of mass transfer capacity in raceway reactors. Algal Res 35:91–97
Barceló-Villalobos M, Serrano CG, Zurano AS, García LA, Maldonado SE, Peña J, Fernández FGA (2019b) Variations of culture parameters in a pilot-scale thin-layer reactor and their influence on the performance of Scenedesmus almeriensis culture. Bioresour Technol Rep 6:190–197
Brindley C, AciénFernández FG, Fernández-Sevilla JM (2011) Analysis of light regime in continuous light distributions in photobioreactors. Bioresour Technol 102:3138–3148
Brindley C, Jiménez-Ruíz N, Acién FG, Fernández-Sevilla JM (2016) Light regime optimization in photobioreactors using a dynamic photosynthesis model. Algal Res 16:399–408
Chen Z, Zhang X, Jiang Z, Chen X, He H, Zhang X (2016) Light/dark cycle of microalgae cells in raceway ponds: Effects of paddlewheel rotational speeds and baffles installation. Bioresour Technol 219:387–391
Cheng J, Yang Z, Ye Q, Zhou J, Cen K (2015) Enhanced flashing light effect with up-down chute baffles to improve microalgal growth in a raceway pond. Bioresour Technol 190:29–35
Chiarini A, Quadrio M (2021) The light/dark cycle of microalgae in a thin-layer photobioreactor. J Appl Phycol 33:183–195
Chisti Y (2016) Large-scale production of algal biomass: Photobioreactors. In: Bux F, Chisti Y (eds) Algae Biotechnology. Springer, Cham, pp 41–66
Costache TA, Gabriel Acien Fernandez F, Morales MM, Fernández-Sevilla JM, Stamatin I, Molina E (2013) Comprehensive model of microalgae photosynthesis rate as a function of culture conditions in photobioreactors. Appl Microbiol Biotechnol 97:7627–7637
Eilers PHC, Peeters JCH (1988) A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol Modell 42:199–215
Fernández-Del Olmo P, Fernández-Sevilla JM, Acién FG, González-Céspedes A, López-Hernández JC, Magán JJ (2017) Modeling of biomass productivity in dense microalgal culture using computational fluid dynamics. Acta Hortic 1170:111–118
Fernández-Sevilla JM, Brindley C, Jiménez-Ruíz N, Acién FG (2018) A simple equation to quantify the effect of frequency of light/dark cycles on the photosynthetic response of microalgae under intermittent light. Algal Res 35:479–487
Fernández del Olmo P, Acién FG, Fernández-Sevilla JM (2021) Analysis of productivity in raceway photobioreactor using computational fluid dynamics particle tracking coupled to a dynamic photosynthesis model. Bioresour Technol 334:125226
Gao X, Kong B, Vigil RD (2017) Comprehensive computational model for combining fluid hydrodynamics, light transport and biomass growth in a Taylor vortex algal photobioreactor: Lagrangian approach. Bioresour Technol 224:523–530
Inostroza C, Solimeno A, García J, Fernández-Sevilla JM, Acién FG (2021) Improvement of real-scale raceway bioreactors for microalgae production using Computational Fluid Dynamics (CFD). Algal Res 54: 102207.
Laws EA, Terry KL, Wickman J, Chalup MS (1983) A simple algal production system designed to utilize the flashing light effect. Biotechnol Bioeng 25:2319–2335
Leman A, Holland M, Tinoco RO (2018) Identifying the dominant physical processes for mixing in full-scale raceway tanks. Renew Energy 129:616–628
Masojídek J, Kopecký J, Giannelli L, Torzillo G (2011) Productivity correlated to photobiochemical performance of Chlorella mass cultures grown outdoors in thin-layer cascades. J Ind Microbiol Biotechnol 38:307–317
Mendoza JL, Granados MR, de Godos I, Acién FG, Molina E, Banks C, Heaven S (2013) Fluid-dynamic characterization of real-scale raceway reactors for microalgae production. Biomass Bioenergy 54:267–275
Molina Grima E, Garcia Camacho F, Sanchez Perez JA, Fernandez Sevilla JM, Acien Fernandez FG, Contreras Gomez A (1994) A mathematical model of microalgal growth in light-limited chemostat culture. J Chem Technol Biotechnol 61:167–173
Morillas-España A, Lafarga T, Gómez-Serrano C, Acién-Fernández FG, González-López CV (2020) Year-long production of Scenedesmus almeriensis in pilot-scale raceway and thin-layer cascade photobioreactors. Algal Res 51:102069
Nedbal L, Tichý V, Xiong F, Grobbelaar JU (1996) Microscopic green algae and cyanobacteria in high-frequency intermittent light. J Appl Phycol 8:325–333
Nikolaou A, Booth P, Gordon F, Yang J, Matar O, Chachuat B (2016a) Multi-physics modeling of light-limited microalgae growth in raceway ponds. IFAC-PapersOnLine 49:324–329
Nikolaou A, Hartmann P, Sciandra A, Chachuat B, Bernard O (2016b) Dynamic coupling of photoacclimation and photoinhibition in a model of microalgae growth. J Theor Biol 390:61–72
Petera K, Papáček Š, González CI, Fernández-Sevilla JM, AciénFernández FG (2021) Advanced computational fluid dynamics study of the dissolved oxygen concentration within a thin-layer cascade reactor for microalgae cultivation. Energies 14:7284
Prussi M, Buffi M, Casini D, Chiaramonti D, Martelli F, Carnevale M, Tredici M, Rodolfi L (2014) Experimental and numerical investigations of mixing in raceway ponds for algae cultivation. Biomass Bioenergy 67:390–400
Rubio Camacho F, García Camacho F, FernándezSevilla JM, Chisti Y, Molina Grima E (2003) A mechanistic model of photosynthesis in microalgae. Biotechnol Bioeng 81:459–473
Sompech K, Chisti Y, Srinophakun T (2012) Design of raceway ponds for producing microalgae. Biofuels 3:387–397
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Terry KL (1986) Photosynthesis in modulated light: Quantitative dependence of photosynthetic enhancement on flashing rate. Biotechnol Bioeng 28:988–995
Villaró S, Sánchez-Zurano A, Ciardi M, Alarcón FJ, Clagnan E, Adani F, Morillas-España A, Alvarez C, Lafarga T (2022) Production of microalgae using pilot-scale thin-layer cascade photobioreactors: Effect of water type on biomass composition. Biomass Bioenergy 163:106534
Funding
Funding for open access publishing: Universidad de Almería/CBUA. This work has been partially funded by the H2020 Research and Innovation Framework Programme (projects: PRODIGIO, 101007006; REALM, 101060991). Also, this work was supported by the Spanish Ministry of Science and Innovation (project: HYCO2BO, PID2020-112709RB-C21). All authors would like to thank the Institute for Agricultural and Fisheries Research and Training (IFAPA). The authors thank the company D&BTech (www.dbtech.tech) for optimizing the geometry of the airfoils and for manufacturing the airfoils used in the experimental work.
Author information
Authors and Affiliations
Contributions
Cristian Inostroza performed CFD simulations, data analysis and coupling of photosynthetic mechanisms, structuring of scientific articles and writing. Javier Dávila and Sergio Roman designed the airfoils used in this project and collaborated in the development of particle analysis tools. They also reviewed the article writing, data analysis and relevant effects of the airfoils fluid dynamics. José M. Fernández-Sevilla and F. Gabriel Acién carried out final reviews, data analysis and relevant effects regarding photosynthetic mechanisms.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflicts of interest
none.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Inostroza, C., Dávila, J., Román, S. et al. Use of airfoils for enhancement of photosynthesis rate of microalgae in raceways. J Appl Phycol 35, 2571–2581 (2023). https://doi.org/10.1007/s10811-023-02996-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-02996-z