Abstract
Laminarin is a source of immunostimulants and antioxidative biomolecules involved in supporting the performance and health of aquatic animals. Hence, this study investigated the growth performance, intestinal morphology, blood biomarkers, and immune response of Thinlip Grey Mullet (Liza ramada) fed dietary laminarin. For 60 days, mullets were fed diets supplemented with laminarin at 0, 200, 400, 600, and 800 mg kg−1, then the growth performance was evaluated, and samples were collected. The FBW, WG, SGR, PER, and carcass lipid content were markedly increased, while the FCR was significantly lowered by dietary 600 and 800 mg kg−1. Further, the lipase and protease activities were significantly higher in mullets fed laminarin at 600 mg kg−1 than those fed 0, 200, 400, and 800 mg kg−1. The intestinal histopathological evaluation revealed that all layers of the intestinal villi and the intestinal wall appeared intact without any deteriorating changes. The intestinal mucosal lining of anterior and middle segments showed improved morphological appearance with increased goblet cells in the intestinal villi associated with increased supplemented laminarin level. The total protein, globulin, and total cholesterol were markedly higher in fish fed 400 and 600 mg kg−1 laminarin than those fed 0, 200, and 800 mg kg−1. Furthermore, the lysozyme, catalase, and glutathione peroxidase activities were higher in mullets fed laminarin at 600 mg kg−1 than those fed a laminarin-free diet. The superoxide dismutase was higher in fish fed 200, 400, and 600 mg kg−1than those fed 0 and 800 mg kg−1. On the other hand, the malondialdehyde activity was markedly decreased by 400 and 600 mg kg−1 of dietary laminarin. Overall, dietary laminarin is required at 338–761 mg kg−1 to reveal the best growth performance, intestinal morphology, blood biomarkers, antioxidative, and immune response in mullets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture sustainability considers the necessity of diversification of finfish species to overcome the high need for valuable animal protein sources for humanity (FAO 2020). Mullet (Family Mugilidae) are suitable finfish species for farming associated with their eurythermal, euryhaline, and diversified feeding habits (Crosetti 2016). Several countries are growing mullets on a commercial scale depending on the wild fry capture (Quirós-Pozo et al. 2023). Grey mullet (Liza ramada) are farmed in several Mediterranean countries and are explored as a potential candidate for fish farming (Cardona 2016; Sadek 2016). Mullet are known for their high value as food and a source of income; therefore, more suitable approaches are needed for their high-quality production (Durand and Whitfield 2016). Feeding strategies are recently focused on using friendly additives to regulate feed digestion, productivity, and well-being of finfish species (Tacon et al. 2022). In this regard, a wide range of functional supplements was included in aquafeed and validated for the blue aquaculture industry (Dawood et al. 2018).
Seaweeds and their extracts have been included in aquafeed and approved as growth promotors and immunostimulants (Thépot et al. 2021). Seaweed contains necessary nutrients (e.g., vitamins and minerals), polysaccharides, and antioxidants (Liu et al. 2020; Chopin and Tacon 2021). Fucoidan, alginic acid, and laminarin are abundantly present in seaweeds and are known for their pharmacological effects (Abdel-Latif et al. 2022). More specifically, laminarin is among the polysaccharides present in brown algae (Zargarzadeh et al. 2020). Laminarin is a carbohydrate that contains β-1,3-glucans which can be formed by the conjunction between β-1,3-glucosidic and β-1,6-glucosidic bonds (Chen et al. 2021). In different studies, laminarin exhibited antibacterial (Noorjahan et al. 2022), anti-inflammatory and antioxidative roles (Rajauria et al. 2021). In addition, laminarin regulates intestinal health by modulating intestinal microorganisms' activity and metabolism (Huang et al. 2021). Markedly, laminarin regulates the adhesion of intestinal bacteria on the epithelial walls by affecting the pH and secretion of fatty acids and mucus (Vidhya Hindu et al. 2019). In aquaculture, dietary laminarin enhanced the growth performance and immune response in grouper (Epinephelus coioides) (Yin et al. 2014) and rainbow trout (Oncorhynchus mykiss) (Morales-Lange et al. 2015). Further, dietary laminarin regulated the digestive and antioxidative enzyme activities in Ictalurus punctatus (Jiang et al. 2021). Hence, this study aimed to evaluate the beneficial roles of laminarin on growth performance, digestive enzymes, blood biomarkers, intestinal health, antioxidative capacity, and immune response of grey mullets.
Materials and methods
Ethical approval
Experimental procedures were performed following the local Animal Care and Ethics Committee of Kafrelsheikh University, Egypt (Approval No. KFS-IACUC/111/2023).
Test diets and experimental design
The test diets were prepared as shown in Table 1. The diets were formulated using fish meal, soybean meal, and gluten as protein sources. Further, yellow corn, wheat bran, rice bran, and wheat flour were used as carbohydrates source besides their content of protein. After mixing ingredients with minerals and vitamins, fish oil was added as a lipid source then water was added to have a dough. Laminarin powder (purity of 95% sourced from Laminaria digitata, no. L9634, Sigma, USA) was added to the diets at 0, 200, 400, 600, and 800 mg kg−1. Wheat flour was mixed with laminarin to adjust the test diets to 100%. The pelletizing machine was then used to produce pellets of 2 mm size. Subsequently, the prepared pellets were dried at room temperature and kept in plastic bags until used. The chemical composition was confirmed by following AOAC (2012).
Thinlip Mullet (Liza ramada) juveniles were obtained from Bughaz El-Burullus (Baltim city, Kafr El-Sheikh governorate, Egypt) and gently moved to the Fish Nutrition Laboratory, Baltim Unit, National Institute of Oceanography and Fisheries (Baltim city, Kafr El-Sheikh governorate, Egypt). To acclimatize the fish to the trial conditions, all fish were stocked in a concrete tank (3 × 2 × 1.7 m) for two weeks before the trial. The tank was provided running water in a flow-through system (1 L min−1), and fish were fed the basal diet at 3% of the body weight (BW) twice daily (08:00 and 15:00). Then, five groups of 15 fish with an average weight of 6.49 ± 0.32 g fish−1 were distributed by 15 hapas (0.5 × 0.5 × 1 m) in order to have triplicated for each treatment. Fish were offered test diets at 3% of the BW during the trial for 60 days. The photoperiod was kept at a 12:12 light: dark cycle, and the water quality indices were regularly measured throughout the trial: temperature (25.31 ± 0.28 °C), pH (7.28 ± 0.19), oxygen (5.42 ± 0.34 mg L−1), salinity (11.26 ppt), and total ammonia (0.14 ± 0.01 mg L−1).
Final sampling
The trial was terminated after 60 days, and all fish were starved for 24 h before the sampling day. All fish were weighed individually and counted to calculate the growth performance and survival rate. The following equations were used to calculate the weight gain (WG) and specific growth rate (SGR). Besides, the feed conversion ratio (FCR), protein efficiency ratio (PER), and feed intake were also determined.
Then fish were anesthetized with 100 mg L−1 tricaine methane sulfonate (Sigma-Aldrich) (Führ et al. 2012), and blood was collected from 3 fish/hapa using 5 mL gauge syringes from the caudal vein. The blood was kept in non-heparinized tubes for serum collection. After 2 h, blood samples were centrifuged at 1008 × g for 15 min at 4 ºC; then, serum was separated and kept at − 20 ºC for further analysis. Then three fish per hapa were dissected and the intestines were collected and used to analyze digestive enzyme activity. Furthermore, three fish per hapa were dissected and their intestines and livers were extracted for the histological study.
Carcass composition and digestive enzyme activity
Three fish per hapa were collected, washed with fresh water and kept in the freezer for the proximate composition analysis at -20 °C. A standard method was used for the chemical composition (moisture, ash, lipids, and crude protein) of the whole fish body and the nutritional profile of test diets (AOAC 2012). The moisture content was measured using oven-drying at 110 °C to reach the constant weight while the ash samples were burnt in a muffle furnace at 550 °C for 6 h. The Kjeldahl method was used to find crude protein, while the Soxhlet extraction method was used to determine crude lipids. After being digested for 15 min in a solution of 5% sulfuric acid and 5% sodium hydroxide, the amount of crude fiber in the diets was estimated.
The collected intestines were frozen in liquid nitrogen and stored at -80 °C until used. The homogenate was prepared by rinsing the intestines in ice-cold Phosphate-Buffered Saline (PBS) (pH 7.5; 1 g per 10 mL). It was then homogenized and centrifuged at 8000 rpm for 5 min and the supernatant was collected and stored at 4 °C for further analysis. Briefly, the total amount of protein was determined using the Lowry et al. (1951) method and bovine serum albumin (BSA) as a standard. The Folin-Ciocalteu phenol reagent was then used to detect the protease activity. The amylase activity was tested using an iodine solution to identify non-hydrolyzed starch, as per Jiang (1982) and Worthington (1993). The specific activity of lipase was evaluated using olive oil as the substrate in accordance with the Borlongan (1990) and Jin (1995) procedure. Amylase and lipase activities were assessed in units per mg of protein.
Blood analysis
Serum total proteins and albumins were determined according to Doumas et al. (1981) and Dumas and Biggs (1972), while globulins content was calculated mathematically by deducting the albumins from the total proteins. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol, triglycerides, creatinine, and urea were detected by RA-50 chemistry analyzer (Bayer Diagnostics, Ireland) using readymade chemicals (kits) supplied by Spinreact Co., Girona, Spain, following the manufacturer's instructions.
Serum malondialdehyde levels were determined using the thiobarbituric acid method and calorimetrically determined using a commercial kit (LPO-586 Kit, OXIS International Inc., PDX, USA) as described by Ohkawa et al. (1979). The serum samples were used to detect superoxide dismutase (SOD) (McCord and Fridovich 1969), glutathione peroxidase, and catalase (Aebi 1984) activities using colorimetric methods by commercial fish-specific kits (Biodiagnostic Co., Egypt).
Serum lysozyme activity was determined using turbidimetric assay, according to the method described by Ellis (1990) based on the lysis of Micrococcus lysodeikticus (Sigma, USA). Phagocytic activity was determined according to Kawahara et al. (1991). The number of phagocytized and phagocytic cells was determined to calculate the phagocytic index according to the following equations: Phagocytic activity = macrophages containing yeast/total number of macrophages × 100; phagocytic index = number of cells phagocytized/number of phagocytic cells.
Histomorphology
At the end of the experiment, samples of anterior, middle, and posterior segments in the experimental groups were dissected and fixed in 10% neutral buffered formalin. After 24 h the samples were transferred to 70% alcohol. The tissue samples were then dehydrated in ascending graded series of ethanol, cleared in xylene, and impregnated and embedded in paraffin wax. Sections of 5 µm were cut using Leica rotatory microtome and mounted on glass slides. The prepared tissue sections were subjected to conventional staining of hematoxylin and eosin (H&E), according to Gewaily and Abumandour (2021). The stained sections were examined under a light microscope.
Statistical analysis
Shapiro–Wilk and Levene tests confirmed normal distribution and homogeneity of variance. The obtained data were subjected to one-way ANOVA. Differences between means were tested at P < 0.05 level using the Duncan test as a post-hoc test. All the statistical analyses were done via SPSS version 22 (SPSS Inc., USA). Moreover, orthogonal polynomial contrasts were performed to determine whether the observed trends were linear or quadratic (Davis 2010). The optimum laminarin dose was evaluated by quadratic polynomial regression analysis using the results of the FW, FCR, protease activity, lysozyme activity, and SOD (Yossa and Verdegem 2015).
Results
Growth performance, digestive enzyme activity, and carcass composition
Table 2 shows the final weight (FBW), weight gain (WG), specific growth rate (SGR), feed intake (FI), feed conversion ratio (FCR), and protein efficiency ratio (PER) in mullets fed dietary laminarin for 60 days. The FBW, WG, SGR, and PER were markedly increased in mullets fed laminarin at 600 and 800 mg kg−1 without significant differences with those fed 200 and 400 mg kg−1. At the same time, the FCR was significantly lowered in fish-fed diets supplemented with 600 and 800 mg kg−1compared to those fed a laminarin-free diet. The supplementation of laminarin did not significantly affect the FI (P>0.05). The survival showed no marked differences among dietary treatments and ranged between 93.33 ± 3.85 and 97.78 ± 2.22%.
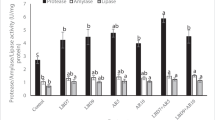
Table 3 shows the digestive enzyme activities in mullets fed dietary laminarin for 60 days. No marked effects were seen on the amylase activity after 60 days. However, the lipase and protease activities were significantly higher in mullets fed laminarin at 600 mg kg−1 than in those fed 0, 400 or 800 mg kg−1.
No marked effects of dietary laminarin were seen on the carcass composition except for the lipid content, which was significantly higher in mullets fed 400 and 600 mg kg−1 than in fish fed on the other diets (Table 4).
Intestinal histology
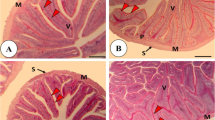
The histopathological evaluation demonstrated normal construction of the intestine's anterior, middle, and posterior segments in the control and laminarin-supplemented fish (Fig. 1), where all layers of the intestinal villi and the intestinal wall appeared intact without any deteriorating changes. The intestinal mucosal lining displayed an improved morphological appearance in the form of an increased number of intestinal villi and augmented villous surface area (in the anterior and middle segments), as well as an abundant number of goblet cells (particularly in the middle and posterior segments) accompanying the increased level of supplemented laminarin (Fig. 1).
Histomicrograph of anterior (upper panel), middle (middle panel), and posterior (lower panel) segments of mullet intestine in control (A1, B1, C1) and laminarin-treated groups at ascending levels; 200, 400, 600, and 800 mg kg-1 (A, B, C; 2–5). The intestinal morphology of groups supplemented with laminarin revealed improved, branched intestinal villi in the anterior and middle segments with increased goblet cells in the middle and posterior segments. Stain H&E. Scale bar = 100 µm
Blood biomarkers
The results of the biochemical blood indices are shown in Table 5. The results showed no marked effects on the ALT, AST, albumin, creatinine, urea, and triglycerides in mullets fed dietary laminarin for 60 days. However, the total protein, globulin, and total cholesterol were markedly higher in fish fed 400 and 600 mg kg−1 laminarin than those fed 0, 200, and 800 mg kg−1.
Blood immunity and antioxidative responses
The lysozyme, catalase, and glutathione peroxidase activities were higher in mullets fed laminarin at 600 mg kg−1 than in those fed a laminarin-free diet (Table 6). The phagocytic activity was higher in fish fed 600 mg kg−1 than in those fed 0, 200, 400, and 800 mg kg−1, while the phagocytic index was higher in fish fed 400 and 600 mg kg−1 than in those fed 0, 200, and 800 mg kg−1 (Table 6). The superoxide dismutase was higher in fish fed 200, 400, and 600 mg kg−1 than in those fed 0 and 800 mg kg−1. On the other hand, the malondialdehyde level was markedly decreased in fish fed either 400 or 600 mg kg−1 of dietary laminarin compared to those fed the laminarin-free diet (Table 6).
Quantification of optimum laminarin dose
Figure 2 shows the regression analysis of the FBW, FCR, protease activity, lysozyme activity, and SOD. The regression analysis shows that the best FBW, FCR, protease activity, lysozyme activity, and SOD in mullets require dietary laminarin 558.33, 761, 595, 645, and 338 mg kg−1, respectively. Overall, dietary laminarin is required at 338–761 mg kg−1 to reveal the best performances in mullets.
Quantification of the optimum laminarin dose using regression analysis. The optimum laminarin dose was evaluated by quadratic polynomial regression analysis using the results of the final body weight (A), feed conversion ratio (B), protease activity (C), lysozyme activity (D), and superoxide dismutase (E)
Discussion
Seaweeds and their extracts have an effective immunostimulant activity involved in regulating local intestinal immunity (Thépot et al. 2021) and thereby, the entire body’s performance (López Nadal et al. 2020). Laminarin is a functional polysaccharide with several antibacterial, antioxidation, and anti-inflammation benefits (Chen et al. 2021; Noorjahan et al. 2022). Therefore, the supplementation of laminarin at specific doses has been validated for several fish species with high potential as growth promotors, antioxidative, and immunostimulant agents (Yin et al. 2014; Morales-Lange et al. 2015; Jiang et al. 2021).
The quantitative assessment of proper doses of dietary supplementations can be determined by applying polynomial regression analysis (Yossa and Verdegem 2015). In the current study, we considered representative variables for the growth performance (e.g., the final weight), feed utilization (e.g., FCR), intestinal digestion (e.g., protease activity), immune response (e.g., lysozyme activity), and antioxidative capacity (e.g., SOD) of mullets fed on dietary laminarin. Subsequently, the estimated dose for laminarin could be determined based on the overall performances of mullets. Herein, the optimum dose of laminarin for the proper performances of mullets is 338–761 mg kg−1. The results are comparable with Morales-Lange et al. (2015), who stated that laminarin is needed at 200 mg kg−1 to enhance the immunity of rainbow trout. Further, Jiang et al. (2021) indicated that laminarin can be added up to 8000 mg kg−1 in the diets of Ictalurus punctatus to enhance the growth performance, digestion, and immune response. Yin et al. (2014) also reported that laminarin could be added at 500–1000 mg kg−1 to enhance the growth performance and immunity of grouper. In light of the earlier efforts, laminarin addition differs among fish species; thus, more future studies are needed since the differences in feeding habits, fish species and sizes, and feeding duration can substantially affect the recommended doses.
In this study, mullets treated with laminarin revealed improved growth performance and digestive enzyme activity. Further, activated antioxidative and immunity were also seen in mullets treated with laminarin. The results showed marked improvements in the growth performance of L. ramada-fed laminarin in a dose-dependent manner. The results are in line with Yin et al. (2014) and Jiang et al. (2021), who reported enhanced growth performance in grouper and Ictalurus punctatus-fed dietary laminarin at 500–1000 mg kg−1. The study also indicated enhanced feed utilization indices (FCR and PER), which may explain the improved growth performance in mullets by dietary laminarin. Indeed, laminarin can regulate the absorption of digested nutrients in fish intestines leading to high absorption capacity (Huang et al. 2021). Although the results showed that dietary laminarin did not significantly affect feed intake, mullets fed laminarin had improved growth performance. These results indicate enhanced feed utilization by dietary laminarin in mullets resulting in improved FCR and PER. The study indicated high digestive enzyme activity in mullets treated with laminarin to support this hypothesis. The improved digestion in the mullet's intestines can be linked to the potential antibacterial role of laminarin in eliminating harmful microorganisms (Noorjahan et al. 2022). Hence, the local intestinal digestion and immunity enhance and result in high feed utilization and the health status of the fish's entire body (Dawood 2021). Enhanced local intestinal immunity by dietary laminarin would result in regulated metabolic and physiological capacity (Li et al. 2021) and, thereby the entire body's growth performance.
The intestinal morphological features of mullets indicated the absence of undesirable effects of dietary laminarin. The intestinal and mucosal layers were intact and showed no degenerative features by dietary laminarin in mullets. In the same line, incorporating polysaccharides-rich additives enhanced the intestinal morphological features in hybrid red tilapia (Abdelrhman et al. 2022). Accordingly, fish show high digestibility and absorption capacity for lipids in the intestines, which can enhance local intestinal immunity and digestibility (Dawood 2021; Abdelrhman et al. 2022). The results also indicated enhanced lipase and protease activities in mullets by laminarin (600 mg kg−1) to support the digestion capacity. The results agree with Jiang et al. (2021), who stated that improved trypsin, amylase, and lipase in I. punctatus-fed dietary laminarin. Improved digestion of carbohydrates, lipids, and proteins is the primary function of activated amylase, lipase, and protease activities in fish intestines (Zheng et al. 2020). Proper feed digestion results in regulated metabolic function and thereby enhanced physiological and immunological responses (Yukgehnaish et al. 2020). The current study showed a marked enhancement in feed utilization in mullets treated with laminarin through the improved PER and FCR. Concurrently, the absence of abnormal intestinal features and enhanced digestive enzyme activity may explain the improved growth performance and regulated blood biomarkers in mullets fed laminarin.
No marked effects were observed on the carcass composition except the lipid content, which showed increased levels in mullets fed dietary laminarin. Usually, the measurements of the carcass composition refer to the level of nutrient metabolism and accumulation in the entire fish body (Ahmed et al. 2022). Seaweed is involved in lipid metabolism and could reduce lipid accumulation (Ferreira et al. 2020). However, this mechanism varies among fish species and can be related to the diet composition, feeding behavior, and type of seaweed (Ragaza et al. 2021). Thus, the increased levels of lipids in mullets fed laminarin require further studies to explain the mechanism involved in lipid accumulation.
The study also tested the biochemical indices of serum samples from mullets fed with or without laminarin. Routinely, the biochemical markers can show the liver and kidney function and the metabolism of proteins and lipids in the fish's blood (Shahjahan et al. 2022). High protein biomarkers, globulin, and lipids were seen in mullets fed laminarin. The increased protein profile can be related to the enhanced feed utilization and efficient metabolic function involved in the high availability of proteins in the blood (Wang et al. 2023). Liver (ALT and AST) and kidney (urea and creatinine) function indices showed non-significant values in mullets fed with or without laminarin. The results indicate that laminarin did not harm the liver and kidney.
Oxidative stress may occur under stressful conditions and can induce health interruption, including fish's impaired physiological and immunological status (Chowdhury and Saikia 2020). Malnutritional strategies and farming stressors are the main reasons for oxidative stress in aquatic animals (Dawood 2021). Hence, incorporating suitable and functional additives in aquafeed can regulate the antioxidative responses and protect from impaired health status (Habotta et al. 2022; Vijayaram et al. 2022). The oxidative stress resulting from the overproduction of reactive oxygen species (ROS) causes inflammation via cellular lipid peroxidation, which malondialdehyde (MDA) levels can evaluate (Birnie-Gauvin et al. 2017; Ratn et al. 2018). Consequently, antioxidative responses attenuate the ROS by activating SOD, CAT, and GPx (Martínez-Álvarez et al. 2005). Therefore, the activated antioxidative capacity of fish indicates a healthy and immunized status, thereby enhancing feed digestion and growth performance. In this regard, the current trial illustrated that laminarin enhanced the antioxidative capacity (SOD, CAT, and GPx) while decreasing the level of MDA in mullet. The CAT and GPx were higher in mullets fed laminarin at 600 mg kg−1 than in those fed a laminarin-free diet, while SOD was higher in fish fed 200, 400, and 600 mg kg−1 than in those fed 0 and 800 mg kg−1. On the other hand, the MDA level was markedly decreased in fish fed either 400 or 600 mg kg−1 of dietary laminarin compared to those fed the laminarin-free diet. Similarly, Jiang et al. (2021) and Yin et al. (2014) reported activated antioxidative capacity in I. punctatus and grouper. The enhanced antioxidative capacity can be related to the role of polysaccharides, which reduce lipid peroxidation and, thereby, the possibility of forming lipid peroxides and ROS (Chen et al. 2021; Noorjahan et al. 2022). Further, the absence of stress in mullets due to laminarin supplementation explains the activation of antioxidative status.
Laminarin is a source of polysaccharides involved in fish immunomodulation and activation of antioxidative capacity (Mohan et al. 2019). Protecting host immunity is crucial since fish are suspected of biotic and abiotic stressors (Dawood et al. 2022). Instantly, phagocytosis and lysozyme activity protect fish from bacterial infection induced by immunity suppression under infection attack (Saurabh and Sahoo 2008). Through the activation of the antibacterial capacity, phagocytic and lysozyme activities defend fish by inhibiting pathogenic bacterial invasion (Magnadóttir 2006). It has been confirmed that polysaccharides extracted from seaweed can activate the immunity of fish (Chen et al. 2021). The results showed that lysozyme activity was higher in mullets fed laminarin at 600 mg kg−1 than in those fed a laminarin-free diet. The phagocytic activity was higher in fish fed 600 mg kg−1 than those fed 0, 200, 400, and 800 mg kg−1. In this regard, Jiang et al. (2021), Yin et al. (2014), and Morales-Lange et al. (2015) illustrated that I. punctatus, grouper, and rainbow trout-fed dietary laminarin showed an increased immune response (phagocytic and lysozyme activities). Seaweed-derived polysaccharides can enhance immunity due to their high content of β-glucans which have been confirmed to pass through the walls of immune cells via specific receptors (Petit and Wiegertjes 2016; Chen et al. 2021). Further, polysaccharides can enhance local intestinal immunity and, thereby, the entire body's immunity by reducing inflammation and inhibiting harmful bacterial effects (Vidhya Hindu et al. 2019).
Conclusion
Laminarin showed a potential effect on the diets of grey mullets. In this regard, dietary laminarin enhanced growth performance, feed utilization, and digestion capacity. Further, dietary laminarin did not affect the histomorphological features of the anterior, middle, and posterior intestines of grey mullets, indicating the safe use of laminarin. Accordingly, dietary laminin in mullets regulated the blood biomarkers and immune and antioxidative responses. Comprehensively, dietary laminarin is required at 338–761 mg kg−1 to reveal the best growth performance, intestinal morphology, blood biomarkers, antioxidative, and immune response in mullets.
Data availability
All other relevant data are available from the corresponding authors upon reasonable request.
References
Abdel-Latif HMR, Dawood MAO, Alagawany M, Faggio C, Nowosad J, Kucharczyk D (2022) Health benefits and potential applications of fucoidan (FCD) extracted from brown seaweeds in aquaculture: An updated review. Fish Shellfish Immunol 122:115–130
Abdelrhman AM, Ashour M, Al-Zahaby MA, Sharawy ZZ, Nazmi H, Zaki MAA, Ahmed NH, Ahmed SR, El-Haroun E, Van Doan H, Goda AMA (2022) Effect of polysaccharides derived from brown macroalgae Sargassum dentifolium on growth performance, serum biochemical, digestive histology and enzyme activity of hybrid red tilapia. Aquacult Rep 25:101212
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126
Ahmed I, Jan K, Fatma S, Dawood MAO (2022) Muscle proximate composition of various food fish species and their nutritional significance: A review. J Anim Physiol Animal Nutr 106:690–719
AOAC (2012) Official Methods of Analysis of AOAC international. 19th edition. AOAC International, Gaithersburg, Maryland, USA
Birnie-Gauvin K, Costantini D, Cooke SJ, Willmore WG (2017) A comparative and evolutionary approach to oxidative stress in fish: A review. Fish Fish 18:928–942
Borlongan IG (1990) Studies on the digestive lipases of milkfish, Chanos chanos. Aquaculture 89:315–325
Cardona L (2016) Food and feeding of Mugilidae. In: Croesetti D, Blaber SJM (eds) Biology, Ecology and Culture of Grey Mullets (Mugilidae). CRC Press, Boca Raton, pp 165–190
Chen J, Yang J, Du H, Aslam M, Wang W, Chen W, Li T, Liu Z, Liu X (2021) Laminarin, a major polysaccharide in stramenopiles. Mar Drugs 19:576
Chopin T, Tacon AGJ (2021) Importance of seaweeds and extractive species in global aquaculture production. Rev Fish Sci Aquacult 29:139–148
Chowdhury S, Saikia SK (2020) Oxidative stress in fish: A review. J Sci Res 12:145–160
Crosetti D (2016) Current state of grey mullet fisheries and culture. In: Croesetti D, Blaber SJM (eds) Biology, Ecology and Culture of Grey Mullets (Mugilidae). CRC Press, Boca Raton, pp 398–450
Davis MJ (2010) Contrast coding in multiple regression analysis: strengths, weaknesses, and utility of popular coding structures. J Data Sci 8:61–73
Dawood MAO (2021) Nutritional immunity of fish intestines: important insights for sustainable aquaculture. Rev Aquacult 13:642–663
Dawood MAO, El Basuini MF, Yilmaz S, Abdel-Latif HMR, Alagawany M, Kari ZA, Abdul Razab MK, Hamid NK, Moonmanee T, Van Doan H (2022) Exploring the roles of dietary herbal essential oils in aquaculture: a review. Animals 12(7):823
Dawood MAO, Koshio S, Esteban MÁ (2018) Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev Aquacult 10:950–974
Doumas BT, Bayse DD, Carter RJ, Peters T, Schaffer R (1981) A candidate reference method for determination of total protein in serum. I. Development and validation. Clin Chem 27:1642–1650
Dumas BT, Biggs HG (1972) Standard Methods of Clinical Chemistry. Academic Press, New York
Durand J-D, Whitfield AK (2016) Biogeography and distribution of Mugilidae in the western, central and southern regions of Africa. In: Croesetti D, Blaber SJM (eds) Biology, Ecology and Culture of Grey Mullets (Mugilidae). CRC Press, Boca Raton, pp 102–115
Ellis AE (1990) Lysozyme assays. Tech Fish Immunol 1:101–103
FAO (2020) The State of World Fisheries and Aquaculture. Sustainability in action. FAO, Rome
Ferreira M, Larsen BK, Granby K, Cunha SC, Monteiro C, Fernandes JO, Nunes ML, Marques A, Dias J, Cunha I, Castro LFC, Valente LMP (2020) Diets supplemented with Saccharina latissima influence the expression of genes related to lipid metabolism and oxidative stress modulating rainbow trout (Oncorhynchus mykiss) fillet composition. Food Chem Toxicol 140:111332
Führ F, Pereira Junior J, Romano LA, Almeida FdM (2012) Gill injury after treatment with mebendazole on mullets Mugil liza. Bull Eur Ass Fish Pathol. 32(5):151
Gewaily MS, Abumandour MM (2021) Gross morphological, histological and scanning electron specifications of the oropharyngeal cavity of the hooded crow (Corvus cornix pallescens). Anat Histol Embryol 50:72–83
Habotta OA, Dawood MAO, Kari ZA, Tapingkae W, Van Doan H (2022) Antioxidative and immunostimulant potential of fruit derived biomolecules in aquaculture. Fish Shellfish Immunol 130:317–322
Huang Y, Jiang H, Mao X, Ci F (2021) Laminarin and laminarin oligosaccharides originating from brown algae: Preparation, biological activities, and potential applications. J Ocean Univ China 20:641–653
Jiang C (1982) Activity measuring for implemental enzyme. Science and Technology Press, Shanghai
Jiang H, Wang M, Zheng Y, Chen F, Fu L, Zhong L, Chen X, Bian W (2021) Dietary laminarin administration to enhance the immune responses, promote growing and strengthen physique in Ictalurus punctatus. Aquacult Nutr 27:1181–1191
Jin Z (1995) The evaluation principle and method of functional food. Beijing Publishers, Beijing
Kawahara E, Ueda T, Nomura S (1991) In vitro phagocytic activity of white-spotted char blood cells after injection with Aeromonas salmonicida extracellular products. Fish Pathol 26:213–214
Khalafalla MM, Zayed NF, Amer AA, Soliman AA, Zaineldin AI, Gewaily MS, Hassan AM, Van Doan H, Tapingkae W, Dawood MA (2022) Dietary lactobacillus acidophilus ATCC 4356 relieves the impacts of aflatoxin B1 toxicity on the growth performance, hepatorenal functions, and antioxidative capacity of thinlip grey mullet (Liza ramada)(Risso 1826). Probiotics and Antimicrobial Proteins, 14(1):189–203
Li Y, Wang S, Hu Y, Cheng J, Cheng X, Cheng P, Cui Z (2021) Dietary bile acid supplementation reveals beneficial effects on intestinal healthy status of tongue sole (Cynoglossus semiliaevis). Fish Shellfish Immunol 116:52–60
Liu W-C, Zhou S-H, Balasubramanian B, Zeng F-Y, Sun C-B, Pang H-Y (2020) Dietary seaweed (Enteromorpha) polysaccharides improves growth performance involved in regulation of immune responses, intestinal morphology and microbial community in banana shrimp Fenneropenaeus merguiensis. Fish Shellfish Immunol 104:202–212
López Nadal A, Ikeda-Ohtsubo W, Sipkema D, Peggs D, McGurk C, Forlenza M, Wiegertjes GF, Brugman S (2020) Feed, microbiota, and gut immunity: Using the Zebrafish model to understand fish health. Front Immunol 11:114
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Magnadóttir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20:137–151
Martínez-Álvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: Biotic and abiotic factors. Rev Fish Biol Fish 15:75–88
McCord JM, Fridovich I (1969) Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Mohan K, Ravichandran S, Muralisankar T, Uthayakumar V, Chandirasekar R, Seedevi P, Abirami RG, Rajan DK (2019) Application of marine-derived polysaccharides as immunostimulants in aquaculture: A review of current knowledge and further perspectives. Fish Shellfish Immunol 86:1177–1193
Morales-Lange B, Bethke J, Schmitt P, Mercado L (2015) Phenotypical parameters as a tool to evaluate the immunostimulatory effects of laminarin in Oncorhynchus mykiss. Aquacult Res 46:2707–2715
Noorjahan A, Mahesh S, Anantharaman P, Aiyamperumal B (2022) Antimicrobial potential of seaweeds: Critical review. In: Ranga Rao A, Ravishankar GA (eds) Sustainable Global Resources Of Seaweeds Volume 1: Bioresources, cultivation, trade and multifarious applications. Springer, Cham pp 399-420
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Petit J, Wiegertjes GF (2016) Long-lived effects of administering β-glucans: Indications for trained immunity in fish. Dev Comp Immunol 64:93–102
Quirós-Pozo R, Robaina L, Calderón JA, Filgueira JR (2023) Reproductive management of the mugilid Liza aurata and characterization of proximate and fatty acid composition of broodstock tissues and spawnings. Aquaculture 564:739055
Ragaza JA, Hossain MS, Koshio S, Ishikawa M, Yokoyama S, Kotzamanis Y, Brezas A, Kumar V (2021) Brown seaweed (Sargassum fulvellum) inclusion in diets with fishmeal partially replaced with soy protein concentrate for Japanese flounder (Paralichthys olivaceus) juveniles. Aquacult Nutr 27:1052–1064
Rajauria G, Ravindran R, Garcia-Vaquero M, Rai DK, Sweeney T, O’Doherty J (2021) Molecular characteristics and antioxidant activity of laminarin extracted from the seaweed species Laminaria hyperborea, using hydrothermal-assisted extraction and a multi-step purification procedure. Food Hydrocoll 112:106332
Ratn A, Prasad R, Awasthi Y, Kumar M, Misra A, Trivedi SP (2018) Zn2+ induced molecular responses associated with oxidative stress, DNA damage and histopathological lesions in liver and kidney of the fish, Channa punctatus (Bloch, 1793). Ecotoxicol Environ Saf 151:10–20
Sadek S (2016) Culture of Mugilidae in Egypt. In: Croesetti D, Blaber SJM (eds) Biology, Ecology and Culture of Grey Mullets (Mugilidae). CRC Press, Boca Raton pp 501-513
Saurabh S, Sahoo PK (2008) Lysozyme: an important defence molecule of fish innate immune system. Aquacult Res 39:223–239
Shahjahan M, Islam MJ, Hossain MT, Mishu MA, Hasan J, Brown C (2022) Blood biomarkers as diagnostic tools: An overview of climate-driven stress responses in fish. Sci Total Environ 843:156910
Tacon AGJ, Metian M, McNevin AA (2022) Future feeds: Suggested guidelines for sustainable development. Rev Fish Sci Aquacult 30:135–142
Thépot V, Campbell AH, Rimmer MA, Paul NA (2021) Meta-analysis of the use of seaweeds and their extracts as immunostimulants for fish: a systematic review. Rev Aquacult 13:907–933
Vidhya Hindu S, Chandrasekaran N, Mukherjee A, Thomas J (2019) A review on the impact of seaweed polysaccharide on the growth of probiotic bacteria and its application in aquaculture. Aquacult Int 27:227–238
Vijayaram S, Sun Y-Z, Zuorro A, Ghafarifarsani H, Van Doan H, Hoseinifar SH (2022) Bioactive immunostimulants as health-promoting feed additives in aquaculture: A review. Fish Shellfish Immunol 130:294–308
Wang L, Sagada G, Wang C, Liu R, Li Q, Zhang C, Yan Y (2023) Exogenous bile acids regulate energy metabolism and improve the health condition of farmed fish. Aquaculture 562:738852
Worthington V (1993) Worthington enzyme manual: enzymes and related biochemicals. Worthingthon Chemical, New Jersey
Yin G, Li W, Lin Q, Lin X, Lin J, Zhu Q, Jiang H, Huang Z (2014) Dietary administration of laminarin improves the growth performance and immune responses in Epinephelus coioides. Fish Shellfish Immunol 41:402–406
Yossa R, Verdegem M (2015) Misuse of multiple comparison tests and underuse of contrast procedures in aquaculture publications. Aquaculture 437:344–350
Yukgehnaish K, Kumar P, Sivachandran P, Marimuthu K, Arshad A, Paray BA, Arockiaraj J (2020) Gut microbiota metagenomics in aquaculture: factors influencing gut microbiome and its physiological role in fish. Rev Aquacult 12:1903–1927
Zargarzadeh M, Amaral AJR, Custódio CA, Mano JF (2020) Biomedical applications of laminarin. Carbohydr Polym 232:115774
Zheng CC, Wu JW, Jin ZH, Ye ZF, Yang S, Sun YQ, Fei H (2020) Exogenous enzymes as functional additives in finfish aquaculture. Aquacult Nutr 26:213–224
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mona S. Abdel-Mawla, Fawzy I. Magouz, Malik M. Khalafalla, Asem A. Amer, Ali A. Soliman, Amr I. Zaineldin, Mahmoud S. Gewaily, Mahmoud A.O. Dawood: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing the original draft. All the authors contributed to the writing of the final version and approved the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Mawla, M.S., Magouz, F.I., Khalafalla, M.M. et al. Growth performance, intestinal morphology, blood biomarkers, and immune response of Thinlip Grey Mullet (Liza ramada) fed dietary laminarin supplement. J Appl Phycol 35, 1801–1811 (2023). https://doi.org/10.1007/s10811-023-02973-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-02973-6