Abstract
The green marine seaweed Ulva (Chlorophyta) is widely suggested as a biofilter for cost-effective bioremediation in integrated multitrophic aquaculture and wastewater management. Micropollutants (MPs), including antibiotics, endocrine disruptors, and herbicides, can severely affect humans and the environment. As these compounds may be accumulated or transformed by Ulva, its simultaneous function as an efficient biofilter and as a food and feed source might be affected. Therefore, we investigated the removal of ten MPs often found in wastewater effluents by Ulva and its associated bacteria, and characterized the effects of these MPs on the alga during two crucial lifecycle phases (germination and vegetative growth) using dose dependent tests. We monitored MP detoxification at elevated concentrations in a reductionistic tripartite Ulva mutabilis-Roseovarius-Maribacter model system to reduce interference from the fluctuating algal microbiome. Our results showed that the tripartite community was resistant to the MPs tested, although the gametes were between 2 to 140 times more susceptible based on the half-effective concentrations (EC50) than the growing vegetative alga. The herbicide atrazine and the endocrine disruptor bisphenol A proved the most toxic MPs for germinating gametes. U. mutabilis and its associated bacteria could not eliminate the tested antibiotics and herbicides but efficiently reduced the concentration of endocrine disruptors, including bisphenol A, estradiol, and ethinylestradiol, by over 98% to below the detection limit. We also confirmed that Ulva is not likely to become contaminated under the studied exposure conditions because no biotic processes are used to remove the other MPs, which emphasizes yet another benefit of its use in aquaculture. Compared to green microalgae, U. mutabilis appears to be more resistant to micropollutants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of algae to remediate nitrogen, phosphorus, heavy metals, and organic contaminants in wastewater and the environment has been suggested in several studies (Norvill et al. 2016; Mohsenpour et al. 2021; Viegas et al. 2021). Macroalgae, in particular, are regarded as an effective bioremediation technology with numerous advantages; notably, they are simple to separate from water, facilitating efficient harvesting and retention in a potential cleaning system (Qiu et al. 2017; Liu et al. 2020). In addition, seaweeds are considered a cost-effective, adaptable, and economical alternative tool for bioremediation (Arumugam et al. 2018). Among the seaweeds, the green macroalga Ulva (Chlorophyta) has various applications in integrated multitrophic aquaculture (IMTA) (Neori et al. 2003; Bolton et al. 2009; Guttman et al. 2018) as it incorporates nitrogen, phosphorus, and carbon dioxide to generate biomass for biofuel and commodity chemicals (Baghel et al. 2015; Zollmann et al. 2021; Simon et al. 2022). Ulva is often used to clean effluent from fish discharge and water for recirculation (Neori 1991; Shpigel et al. 1993; Lawton et al. 2013).
Ulva mutabilis, which is conspecific with the cosmopolitan Ulva compressa (Steinhagen et al. 2019), serves in our study as a unique model system to investigate the requirements for withstanding environmental stresses, such as micropollutants (MPs) (Ghaderiardakani et al. 2020; Vockenberg et al. 2020). MPs include antibiotics, hormones, and herbicides, and given their known negative effects on humans and the environment, exposure should be minimized wherever possible. Thus, Ulva might prove to be a powerful tool for efficient water purification and bioremediation. At the same time, herbicides and antibiotics may, however, have negative impacts on the macroalga and its microbiome.
Ulva mutabilis is excellently suited for researching bacterial–algal interactions and investigating specific bacteria-induced growth under environmental stress because it relies on the presence of bacteria for its complete development and morphogenesis (Spoerner et al. 2012). Notably, the natural microbiome of U. mutabilis can be reduced to two bacterial strains, Roseovarius sp. MS2 and Maribacter sp. MS6. These strains supply all essential algal growth- and morphogenesis-promoting factors (AGMPFs) (Ghaderiardakani et al. 2019), and the resulting tripartite community is stable and replicable in the laboratory (Wichard et al. 2015; Wichard 2023). This reductionistic microbiome minimizes bacterial interactions, thereby enabling experimental focus on algal remediation.
In addition, the ability of Ulva to act as a biofilter for various substance raise concerns about its usage as a source of food and feed. Indeed, antibiotics like chloramphenicol (CAP), erythromycin (ERY), oxytetracycline (OTC), and sulfamethoxazole (SMX) are often used in aquaculture (Chen et al. 2020). Additionally, xenoestrogens like bisphenol A (BPA), and 17α-ethinylestradiol (EE2) as well as the most active natural estrogen, 17β-estradiol (E2), can be found in water, thereby affecting the hormone homeostasis of vertebrates and invertebrates (Hoga et al. 2018). BPA is a highly concerning MP as it is almost ubiquitous and pseudo-persistent (Zhang et al. 2019; Zielińska et al. 2019; Hardegen et al. 2021). Herbicides such as triazine, chloracetamide, and glyphosate herbicides were selected to cover a broad range of herbicides that target enzymes identified in the U. mutabilis genome (De Clerck et al. 2018). Notably, atrazine (ATZ) blocks the quinone-binding site in photosystem II resulting in plant death through increased reactive oxygen species generation (Rose et al. 2016). Metolachlor (MTC) inhibits several enzymes involved in cell division and elongation. Fatty acid elongation and geranylgeranyl pyrophosphate cyclization enzymes are two sensitive targets. Glyphosate (PMG, N-(phosphonomethyl) glycine) inhibits enolpyruvylshikimate-3-phosphate synthase, which is required for the synthesis of aromatic amino acids via the shikimate pathway (Rose et al. 2016).
In our study, we thus investigated the removal of various MPs by U. mutabilis and its associated bacteria. Ten substances were chosen to represent antibiotics, endocrine disruptors, and herbicides (Fig. 1a) given their frequent detection in wastewater effluents, surface water, and groundwater (Hirsch et al. 1999; Kummerer 2009; Luo et al. 2014; Baldwin et al. 2016; Das et al. 2017; Sousa et al. 2018; Tran et al. 2018; Montiel-Leon et al. 2019; Nguyen et al. 2022). We examined the effects of these substances on the survival and growth of U. mutabilis during two phases of its lifecycle using dose–response tests (Fig. 1b) and determined the ability of the alga and its associated bacteria to remove the MPs using ultra-high-performance liquid chromatography (UHPLC) coupled with electrospray ionization high-resolution mass spectrometry (ESI-HRMS). Our study highlights the resilience of the U. mutabilis tripartite community against the tested MPs as well as its suitability as an ecotoxicological model system under standardized conditions for investigating the mortality and sub-lethal effects of specific MPs, such as germination and developmental delays.
Micropollutants and experimental setup. (a) Selected micropollutants in the study, (b) parthenogenetic lifecycle of Ulva mutabilis (morphotype "slender") (Wichard 2015), and (c) experimental setup for the removal test. The effect of the micropollutants on the survivability and germination of gametes as well as on the chlorophyll-a fluorescence of growing gametophytes within 14 days was tested. The removal of the micropollutants by the tripartite community Ulva mutabilis sp.-Roseovarius sp.-Maribacter sp. and abiotic dark and light controls was tested over 14 days. Experiments were carried out in three independent biological replicates with 20 gametes or four to seven growing specimens in each case
Material and methods
Ulva and bacteria cultivation
Haploid gametes and gametophytes of Ulva mutabilis Føyn (sl-G[mt +]; morphotype 'slender'; locus typicus: Ria Formosa, Portugal) were used in all experiments. The cultivar is referred to as Ulva. mutabilis throughout this study. Ulva mutabilis was cultivated in artificial seawater Ulva culture medium (UCM) at 18 °C ± 2 °C with a light/dark cycle of 17/7 h and at a light intensity of 40–80 µmol photons m−2 s−1 (Califano and Wichard 2018; Nahor et al. 2021). The bacterial strains, Roseovarius sp. MS2 (GenBank EU359909) and Maribacter sp. MS6 (GenBank EU359911), were propagated in a 50/50 mixture of marine broth and UCM. Gametes were purified to axenic conditions and inoculated with these two strains. To avoid sporulation, Ulva older than two weeks were only supplemented with 50% new UCM in all experiments. Therefore, the medium for experiments with Ulva consisted of 50% each of fresh and spent UCM. This procedure ensures the suppression of gametogenesis in the presence of the sporulation inhibitors (Stratmann et al. 1996; Kessler et al. 2018).
Chemicals
Analytical standards of the model substances atrazine (ATZ), erythromycin (ERY), 17α-ethinylestradiol (EE2), 17β-estradiol (E2), metolachlor (MTC), oxytetracycline (OTC), N-(phosphonomethyl)glycine (PMG; glyphosate), and sulfamethoxazole (SMX) were purchased from Sigma Aldrich/Merck (Germany), while bisphenol A (BPA) and chloramphenicol (CAP) were purchased from Alfa Aesar (USA) and Fluka/Honeywell (USA), respectively. The isotopologue sulfamethoxazole-13C6 (phenyl-13C6) was purchased from Sigma Aldrich/Merck (Germany), atrazine-D5 (ethylamino D5), chloramphenicol-D5 (ring D4, benzyl D), and metolachlor-D6 (propyl D6) were purchased from LGC Group (UK), Bisphenol A-D16 was purchased from CDN Isotopes (Canada), erythromycin-N-methyl-13C, D3, and glyphosate-2-13C were purchased from TRC Canada (Canada), and β-estradiol-D4 (2,4,16,16-D4) and 17α-ethinylestradiol-D4 (2,4,16,16-D4) were purchased from Cambridge Isotope Laboratories (Canada).
Toxicity and inhibition assay for gametes
Freshly discharged gametes were purified from bacteria using their phototactic behavior and diluted in UCM to 200 gametes mL−1 (Califano and Wichard 2018). The axenic gamete suspension was inoculated with Roseovarius sp. MS 2 and Maribacter sp. MS6 cultures (final OD620 = 0.0001). In 96-well plates, a two-fold geometric dilution series of the MPs was prepared in triplicate at 100 µL per well. The gamete-bacterial suspension (100 µL, i.e., 20 gametes) was added to each well to reach the targeted concentration (Table S1). Additionally, controls without MP were prepared for the axenic gametes and inoculated with both bacterial strains. The plates were incubated in the dark for two days before switching to the typical diurnal light cycle.
After 14 days, the effect of the MPs on survivability and longitudinal growth after germination was evaluated using light microscopy. The no observed effect concentration (NOEC), lowest observed effect concentration (LOEC), and lethal concentration (LC) were determined by length comparison with the controls using a two-tailed t-test (see chapter data processing). Owing to the use of a two-fold geometric dilution series, the LOEC was typically twice as high as the NOEC. An observed LC refers to the death of over 98% of gametes. The average length of the individuals in the biological replicates was plotted against concentration. Dose–response curves were then calculated using the logistic function (Eq. 1) in OriginPro software (v. 2022, OriginLab, USA) using the Levenberg–Marquardt algorithm (with A1 = bottom asymptote; A2 = top asymptote; p = hill slope; B = inflection point). The EC values were then calculated (with x = causes x% change in response), as follows (Eq. 2) (Table S2):
NOEC and LOEC allow for comparison with the available literature, which is especially useful when data are unsuitable for dose–response fitting. Both LOECs/NOECs and ECx are valuable complementary values when describing the toxicity of MPs because they are derived in different ways (Green et al. 2013). However, as has been discussed in several studies, ECx values contain quantitative information and are generally more robust than NOEC and LOEC (Landis and Chapman 2011; Green et al. 2013; Tanaka et al. 2018).
Toxicity assessment based on Ulva vegetative growth
Five-week-old Ulva individuals (0.5–1.0 cm long) were used for toxicity experiments and incubated in 48-well plates with different concentrations of MPs in UCM for 14 days. As we were primarily interested in changes in chlorophyll fluorescence in response to the application of the MPs, the normalized reflectance/fluorescence was averaged across the waveband of chlorophyll fluorescence (670–760 nm) to calculate the normalized average reflectance in the fluorescence spectrum (nARFS) upon excitation of actinic light.
During the incubation experiments, the MP-induced change in the chlorophyll fluorescence intensity (F665 nm) was used as a proxy for vitality. The effect of the MPs on Ulva was measured in biological triplicates with four to seven individuals each under the standardized conditions. ECx values were calculated based on the decrease in chlorophyll-a fluorescence, while NOEC was determined by comparison with controls under the light microscope. The range of the increasing effect of the MP was first evaluated using a twofold geometric dilution series, followed by a time-independent arithmetic dilution series measured within the area of interest.
The multimode microplate reader Varioskan Flash (Thermo Scientific, USA) was used for measurements. Fluorescence was measured in a grid of 49 points per well at 430 and 665 nm excitation and emission wavelengths, respectively, with a 12-nm bandwidth. Each well was measured three times, and the average of the top 10 values of each measurement was used for plotting and calculating the ECs (Eqs. 1 and 2) (Table S3). The atrazine concentration was log transformed for the calculations.
Determination of micropollutant removal
For the removal experiments, the effect of the MPs ATZ, BPA, CAP, ERY, PMG, MTC, and OTC on Ulva growth were tested at their EC10 concentrations in 12-well plates (Table 1). E2, EE2, and SMX were not toxic at their saturated concentrations and were tested slightly below the saturated concentrations in the UCM (Table 1). Nine wells per MP were filled with 2.2 mL of the respective solution in the UCM. Next, 200 µL were immediately sampled as the starting concentration (d0), spiked with IS, and stored at –20 °C until measurement. The remaining 2 mL were incubated in the dark (‘abiotic dark’, n = 3) with a standard light cycle (‘abiotic light’, n = 3) or with young Ulva in the tripartite community in a standard light cycle (‘biotic’, n = 3) (Fig. 1c). Approximately 3–5 individuals of 6-week-old (1–2 cm long) Ulva were added to each biotic well. After 14 days, 200 µL was sampled (d14), spiked with IS, and stored at –20 °C until measurement without further sample preparation upon appropriate dilution with water. For dry weight measurement, the algae were washed three times with ultra-pure water, frozen in liquid nitrogen, and freeze-dried until there was no further weight loss.
For all compounds that displayed no or only minor removal after 14 days (ATZ, ERY, PMG, MTC, and SMX), removal was tested over 42 days in a similar experiment. This was done to provide more time for the tripartite community to adapt to the introduced MPs. As the five MPs tested did not show any light sensitivity in the 14-day experiment, a dark control was excluded.
UHPLC-ESI-HRMS method
The MPs were separated over 10 min with a 0.3 mL min−1 flow rate and 7 min ramp from 100% A (aqueous) to 100% B (organic) in an ACQUITY UPLC BEH C18 column (130 Å, 1.7 µm, 100 × 2.1 mm, Waters, Ireland) with column guard. The pH of both mobile phases was between 9.4 and 9.6, with A containing 90% water, 10% methanol, and 2 mmol L−1 (NH4)2CO3 and B containing 100% methanol and 2 mmol L−1 (NH4)2CO3. The injection volume was 2 µL.
Electrospray ionization (ESI) was employed with 3 kV voltage, 360 °C capillary temperature, and 400 °C auxiliary gas temperature. High-resolution mass spectrometry (HRMS) was performed in alternating negative–positive mode with a mass range between 100 and 1200 m/z and a resolution of 70,000 at 200 m/z.
Quantification of micropollutants by mass spectrometry
Isotopically labeled internal standard concentrations and appropriate analyte concentrations were used for calibration. For analysis, all samples were spiked with IS at half the molar starting concentration, diluted with water (by a dilution factor between 100 and 10,000 depending on the MPs) to reach the working range of the calibration and filtered with 0.22-µm PVDF filters. Protonated and deprotonated ions of the MPs were used for quantification with a 5 ppm mass range (Table 2). As no isotopologue was available for oxytetracycline, an external calibration was performed.
All calibration parameters are listed in Table S4. Critical signal, limit of detection (LOD), and limit of quantification (LOQ) refer to the calibration used and do not represent the full potential of the analysis method and the selected mass spectrometer. Linearity was tested using the Mandel and lack-of-fit tests (Reichenbächer and Einax 2011) using OriginPro (v. 2022) software.
Data processing
Concentrations were measured before and after incubation to ascertain whether each of the three treatments eliminated MPs from the culture medium. All MPs with a significant decrease in darkness were assumed to have been eliminated through hydrolysis or sorption into the plastic well. Removal by photolysis was assumed for all MPs, with a significantly higher removal rate in light than in the dark. Elimination by sorption, uptake, or biotransformation was assumed for all MPs, with a significantly higher removal rate with the tripartite community than in light controls. For areas below the critical signal, the concentration was set as the detection limit (corrected by the dilution factor) for statistical analysis (Student´s t-test), calculations, and visualization. The relative decrease for each MP was determined and separated into the various contributing effects using the relative removal under four different conditions: (i) the removal by Ulva and its associated bacteria was calculated by subtracting the removal in the treatment of ‘light abiotic controls’ from the ‘biotic treatment’. (ii) Removal by photolysis was calculated by subtracting the removal in the treatment of the ‘dark abiotic controls’ from the ‘light abiotic controls’. (iii) Removal by sorption and hydrolysis was calculated directly from the treatment ‘dark abiotic controls’. (iv) Removal below the LOD was regarded as possibly higher but not lower. A one-tailed two-sample unpaired Student's t-test or Welch's test was used for all statistical tests on removal, depending on the homogeneity of variances. A p-value > 0.05 was deemed insignificant (NS).
Results
The toxic and inhibitory effects of ten different MPs were investigated in U. mutabilis gametes and juvenile gametophytes (1–2 cm), representing two crucial developmental phases in the parthenogenetic lifecycle of Ulva (Fig. 1b).
Survival and inhibition of the germination of gametes
Overall, there was no trend in toxicity regarding the three tested substance groups (herbicides, antibiotics, and hormonal disruptors, Table 3). The curves for eight MPs followed a clear dose–response relationship (Fig. 2 and Table S2), whereas CAP and EE2 were not fitted owing to insufficient and variable data. All MPs inhibited the germinating and growth of the gametes of U. mutabilis; however, ERY significantly promoted longitudinal growth (up to 56% at 0.095 mg L−1) compared to the control (Fig. 2c).
Inhibitory and toxic effects of different micropollutants on germinating gametes of Ulva mutabilis using three biological independent replicates with 20 gametes each. After 14 days of germination, the average length was fitted in a dose–response curve. Atrazine (a), bisphenol A (b), erythromycin (c), estradiol (d), glyphosate (e), metolachlor (f), oxytetracycline (g), and sulfamethoxazole (h) showed a dose–response relationship. Representative microscopic images of different inhibitory concentrations of erythromycin (i). Scale bar: 500 µm. The length after 14 days of uninhibited growth varied between the groups due to variability between different batches of Ulva but always matched the respective group control. The red triangles indicate the average length of the negative controls (n = 3)
The herbicide ATZ was most toxic for Ulva gametes, with an EC50 value for longitudinal growth inhibition of 0.05 mg L−1, followed by BPA with an EC50 value of 1.28 mg L−1 (Table 3). The EC50 values of ERY, EE2, CAP, and E2 were approximately between 3–4 mg L−1, whereas MTC, OTC, SMX, and PMG were less active, with EC50 values of 6.11, 17.5, 50.7, and 108 mg L−1, respectively. Similarly, ATZ and BPA were the most toxic, with LCs of 0.47 and 4.97 mg L−1, respectively. The toxicity of the antibiotic CAP was comparable with that of the endocrine disruptor BPA, with an LC of 6.36 mg L−1, whereas the other MPs were less toxic, with LCs ranging from 25–201 mg L−1. PMG was non-toxic at the highest concentration tested (100.8 mg L−1).
EC50 and slope can be used to determine the full range of activity for MPs and are relevant in bioremediation and aquaculture maintenance. The slopes of the dose–response curves of ATZ and CAP represent their potency at toxically relevant inhibition levels; the slopes are steep and exhibit the lowest relative distance between the no effect and maximum effect points. In contrast, ERY had the highest relative range of increasing effect over three orders of magnitude.
Toxicity and survival of growing Ulva (gametophyte)
The toxic effects of all MPs were recorded for Ulva in the tripartite community. After 14 days of incubation with varying concentrations of MPs, the chlorophyll-a activity of biological triplicates was evaluated. Eight of the ten MPs exhibited dose-dependent response relationships and were fitted (Fig. 3 and Table S3), whereas SMX and E2 had no impact, even at saturated concentrations. NOECs were determined by visual comparison, and EC values were determined using dose–response curves (Table 4). Some concentrations had no statistical effect on fluorescence values, while damage to the algae was visible under the microscope. In these cases, the NOEC represents a more conservative estimate of toxicity compared to the EC10.
Toxicity of different micropollutants for growing Ulva mutabilis. Chlorophyll-a fluorescence was fitted in a dose–response curve using three independent biological replicates with four to seven specimens each. In alphabetical order: (a) atrazine, (b) bisphenol A, (c) chloramphenicol, (d) erythromycin, (e) ethinylestradiol, (f) glyphosate, (g) metolachlor, (h) and oxytetracycline decreased chlorophyll-a fluorescence, while estradiol and sulfamethoxazole showed no effect, even in saturated concentrations. The red triangles indicate the average fluorescence of the negative controls (n = 3)
ATZ had a dynamic range of over two orders of magnitude and was measured in a geometrical dilution series (Fig. 3a). BPA exhibited a high distribution in the biological triplicates (Fig. 3b). Three wells in the active CAP range demonstrated high fluorescence (Fig. 3c). Presence of ERY decreased the fluorescence of Ulva by only 56% (Fig. 3d), whereas the other compounds bleached the algae. EE2 (Fig. 3e), PMG (Fig. 3f), MTC (Fig. 3g), and OTC (Fig. 3h) indicated standard dose–response relationships.
ATZ had the highest activity with a NOEC (4.72 mg L−1) followed by BPA (6.2 mg L−1) (Table 4). The NOECs of all other drugs ranged between 50.1 and 402 mg L−1, with SMX having the lowest activity. Similarly, ATZ and BPA were the most hazardous, with EC50 values of 7.19 and 18.2 mg L−1, respectively. E2 and SMX were not toxic within the investigated concentration range, whereas the other MPs had EC50 values ranging from 93.0 to 339.9 mg L−1.
Quantification of micropollutants in the algal culture medium
A robust quantification methodology was required to determine the removal of MPs from the algal culture medium. For all analytes, symmetrical-shaped chromatographical peaks with minimal tailing were obtained by detecting the respective quasimolecular ions of the MPs (Fig. 4). To combine the analyses on the same reversed-phase chromatographic platform, PMG was derivatized with Fmoc reagent. Using isotopically labeled internal standards, a base calibration was performed for all compounds except OTC, where an external calibration was utilized (Fig. S4). All calibrations passed the Mandel and lack-of-fit tests for linearity. The relative standard deviations of the calibrations of ATZ, BPA, CAP, E2, EE2, PMG, MTC, and SMX were below 10%, whereas the remaining two were over 10% (Table 5). Owing to the lack of an internal standard, the calibration of the OTC exhibited large variability throughout the calibration.
Removal of micropollutants by the Ulva tripartite model system
High concentrations of MPs were preferentially evaluated to determine the suitability of U. mutabilis for bioremediation. Thus, the EC10 limits of growing Ulva were used to identify the highest amounts possible while minimizing cell damage and toxicity. Micropollutant levels were measured before and after 14 days of incubation with a tripartite community of Ulva mutabilis-Roseovarius-Maribacter and compared with that of abiotic control treatments incubated in the dark and light (Fig. 5). Depending on the biotic and abiotic conditions, four cases were observed: (i) no changes at all, (ii) biotic-dependent changes, (iii) light-dependent changes, and (iv) treatment-independent changes.
Concentrations of micropollutants in the growth medium of Ulva mutabilis before and after 14 or 42 days of cultivation. The EC10 value of each individual micropollutant for Ulva was first adjusted in the growth medium. The tripartite community Ulva-Roseovarius-Maribacter (biotic) treatment was compared to controls with (light) and without light (dark). The values of the detection limit (corrected by the dilution factor) are shown, when the analyte signal was below the critical signal. Relative removal rates (%) of the micropollutants during incubation were plotted for a significant decrease (t-test, p ≤ 0.05). Diamonds represent the concentrations of biological replicates (n = 3) and error bars show mean ± standard deviation. NS = not significant
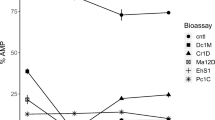
The concentrations of ATZ and SMX did not change significantly under any conditions (Fig. 5a, b). Owing to the high variability in ERY measurements, no significant difference was found (Fig. 5c). The situation was different for CAP, where the concentration decreased significantly by approximately 70% (p = 0.001) in both the biological and the light controls but not in the dark (Fig. 5d). Similarly, the OTC concentration decreased below LOD in the presence of light (in both the biological and light controls), but also decreased by 23.7% in the dark (Fig. 5e).
Notably, the BPA concentration decreased significantly (p = 0.003) under biological conditions below the LOD but did not change under abiotic conditions (Fig. 5f). Over 98% of BPA was removed in the presence of Ulva and the associated bacteria. Similarly, the hormones E2 and EE2 were significantly reduced (98.2% and 94.3%, respectively; p < 0.001) to below the LOD in the presence of the Ulva-Roseovarius-Maribacter model system, were not significantly reduced in the dark, and decreased only slightly in the light by 24.3% (p = 0.019) and 29.9% (p = 0.014) (Fig. 5g, h). MTC and PMG herbicides exhibited low but significant removal rates under biological and dark conditions, respectively (Fig. 5i, j).
The concentrations of the more stable compounds ATZ, SMX, ERY, MTC, and PMG were monitored for 42 days under biological and light treatments only. The concentration of PMG was reduced by 30.4% under biotic conditions (p = 0.005) but, in contrast, increased slightly in the light control (Fig. 5k). The concentrations of ATZ, ERY, MTC, and SMX were stable for this extended period under both biotic and abiotic light conditions (data not shown).
The recovery rates for all chemicals were between 90–120%, except for PMG (78.5%), BPA (138.0%), and E2 (148.1%) (Table 5). The relative decrease for each MP within 14 days was determined and separated into the various contributing modes of action for the removal (Fig. 6a). PMG and OTC were significantly removed by hydrolysis or sorption; photolysis removed significant amounts of CAP, E2, EE2, and OTC. Importantly, BPA, E2, EE2, and MTC were all significantly removed via biosorption, uptake, or biotransformation accounting for the strongest effect on these MPs. Indeed, the tripartite community entirely removed BPA, E2, and EE2 (< LOD). The dry weight removal capacity of Ulva (0.8–12.8 mg g−1) suggests that it could be used as a biofilter in wastewater treatment (Fig. 6b).
Removal of micropollutants after 14 days. (a) Total removal was divided into effects in the dark (hydrolysis and sorption), with light (photolysis), and removal by biota. Error bars represent the mean ± standard deviation (n = 3). For removal below the limit detection, the error bars show only the positive range to 100% because removal could be higher but not lower. (b) Biological removal below LOD of endocrine disruptors normalized to Ulva dry weight
Discussion
In general, the harmful effects of MPs on Ulva were minimal compared with those concentration observed in surface water, groundwater, and wastewater effluents (Das et al. 2017; Sousa et al. 2018; Tran et al. 2018). Environmental concentrations are typically many orders of magnitude below the levels that influenced Ulva in this study. Accordingly, as a holobiont, Ulva can be grown even in harsh conditions and, in some cases, can contribute to the bioremediation of wastewater (Qiu et al. 2017). In only one case, an environmental concentration of ATZ (40.2 µg L−1, Baldwin et al. 2016) was reported that was higher than the NOEC and EC10 values but lower than EC50 determined for gametes of U. mutabilis in our study.
Lifecycle dependent toxicity: differences between germinating gametes and growing algae
We discovered that the tested MPs had a lifecycle stage-dependent sensitivity, which has also been observed in flowering plants (Boutin et al. 2014). Gametes were more sensitive to MPs than mature Ulva specimens: Based on the EC50 value, the gametes were 2 to 138 times more sensitive compared to growing thalli of U. mutabilis for the tested various MPs.
Although target enzymes for all three herbicides are present in the U. mutabilis genome (De Clerck et al. 2018), only ATZ demonstrated enhanced activity relative to the other MPs, whereas MTC and PMG did not. Notably, PMG had the lowest activity among all tested MPs in gametes. BPA was the second most active substance against germination.
Erythromycin displayed algistatic effects
ERY displayed the widest range of increasing effects on gametes, and longitudinal growth was inhibited over a large concentration range. The LC/LOEC ratio of ERY was 32, while the ratios of the other MPs were ≤ 8. Therefore, the slope of the ERY dose–response curve was shallowest. For the growing Ulva, ERY decreased fluorescence by only 56%, whereas the other compounds killed and even bleached the algae. This effect could be explained by the inhibition of protein synthesis in chloroplasts and mitochondria by ERY. Machado and Soares (2019a) observed an algistatic effect of ERY for the green microalga Raphidocelis subcapitata at concentrations ranging from 38–200 µg L−1. They observed a decrease in chlorophyll-a content and mitochondrial function, which decreased growth without harming the cell membrane. This was attributed to the similarity of the chloroplast and mitochondria ribosomes to prokaryotic ribosomes, thereby inhibiting protein synthesis in these cell organelles. Ulva appears to be more resistant than Raphidocelis, with inhibitory effects in gametes at 3–49 mg L−1 (Fig. 2c, i), whereas the EC50 value for decreasing chlorophyll-a fluorescence in growing Ulva was 340 mg L−1.
Chloramphenicol treatment indicated decoupling of photosystem
Higher fluorescence was observed in the CAP treatment of growing Ulva with multiple repetitions at certain concentrations. CAP can serve as an electron acceptor for photosystems I and II and transfers electrons to oxygen, decoupling the photosystem and generating superoxide (Rehman et al. 2016). Therefore, CAP reduces photosynthetic efficiency by competing for electrons and damaging the cell. This effect reached a maximum in rice leaves (Oryza sativa) at 323 mg L−1 (Okada et al. 1991) and probably caused higher electron transfer rates in the remaining U. mutabilis at a CAP concentration between 254–354 mg L−1 (Fig. 3c), resulting in higher chlorophyll-a fluorescence by decoupling the photosystems in this study.
Comparison of the resilience of U. mutabilis with other Ulva species, microalgae and macrophytes
The existing literature suggested that Ulva and its associated bacteria can withstand high concentrations of MPs under harsh conditions. In the following sections, the individual MPs are discussed in the context of Ulva. Overall, U. mutabilis was highly resistant to antibiotics, whereas its gametes were more susceptible to CAP and ERY (Table 6). Endocrine disruptors displayed opposing effects on U. mutabilis; the algae were highly resilient against EE2, whereas the gametes were susceptible to BPA and E2 (Table 7). Ulva gametes displayed similar susceptibility to herbicides as green microalgae, whereas growing U. mutabilis was considerably more resilient than green microalgae, macrophytes, and most other Ulva species (Table 8).
Chloramphenicol (CAP): U. mutabilis was considerably more resilient to CAP in our study (about 20 times higher EC50) than has been reported during gametogenesis (Hoxmark 1975), which was most likely linked to cell damage and additional stress owing to thallus fragmentation. Similar to our study, the growth of U. lactuca was found not to be negatively affected by CAP at concentrations up to 50 mg L−1 (Leston et al. 2013). The toxic effects observed after CAP addition are caused by the photodegradation product p-nitrobenzaldehyde, which can be detoxified by algae—presumably by oxidation—to p-nitrobenzoic acid (Hoxmark and Nordby 1977). As Ulva gametes were tested at lower cell densities, they could not detoxify higher concentrations of p-nitrobenzaldehyde and were more susceptible than the growing Ulva. Furthermore, microalgae display similar susceptibility as gametes (Xiong et al. 2019b; Nguyen et al. 2022) (Table 6).
Erythromycin (ERY): Growing Ulva mutabilis was extremely resilient to ERY, especially when compared with U. pertusa, even though Ulva gametes were only marginally more resistant than most green microalgae. Specifically, ERY shows a wide range of effects on different species of green microalgae (Liu et al. 2011; Machado and Soares 2019a; Ma et al. 2021; Zhang et al. 2021; Li et al. 2022) and was highly effective against U. pertusa (Chen et al. 2017).
Oxytetracycline (OTC): Compared to microalgae, gametes and growing Ulva, in particular, were more resilient (10–50 times higher EC50) to OTC (Oh et al. 2005; Ferreira et al. 2007; Kolar et al. 2014; Siedlewicz et al. 2020). Interestingly, OTC can even enhance the growth of Ulva sp. significantly at a concentration of 0.12 mg L−1 (Rosa et al. 2019). To date, no adverse effects on green macroalgae have been reported (Table 6).
Sulfamethoxazole (SMX): Gametes and growing U. mutabilis were distinctly more resilient to SMX than U. pertusa and green microalgae (up to 100 times higher EC50) like Raphidocelis subcapitata used as bioindicator species for toxic substances (Table 6) (Liu et al. 2011; Chen et al. 2017; Xiong et al. 2019a; Zhang et al. 2021).
Bisphenol A (BPA): U. mutabilis displayed similar resilience as other Ulva species and microalgae (Yang and Hong 2012; Zhang et al. 2019; Azizullah et al. 2022; Ludmila et al. 2022), whereas Ulva gametes were the most susceptible (EC50 = 1.28 mg L−1) in this comparative group (Table 7).
17β-estradiol (E2): Similar to previous studies on Ulva spp., U. mutabilis was not affected by E2, even at saturated concentrations (Yang and Hong 2012; Lu et al. 2021). No precipitation or crystallization was observed at the LCs of E2 and EE2 despite the tested concentrations being close to reported solubility in distilled water at 25 °C (Shareef et al. 2006). Noteworthy, Ulva gametes were more susceptible (about 5 times lower EC50) than growing Ulva and the green microalgae Chlorella spp. (Huang et al. 2019) (Table 7).
17α-ethinylestradiol (EE2): Gametes and growing Ulva were considerably more resilient to EE2 than green microalgae (Balina et al. 2015; Belhaj et al. 2017; Zhang et al. 2022). The only report on the effect of EE2 on green macroalgae identified metabolome changes in U. lactuca at concentrations as low as 1 mg L−1 (Cabral et al. 2018), which is similar to the observed effects in U. mutabilis at higher concentrations.
Atrazine (ATZ): The inhibitory effect of ATX on Ulva gametes was comparable to the EC50 values reported for green microalgae (Qian et al. 2008; Sun et al. 2012; Kabra et al. 2014; Camuel et al. 2017), U. pertusa, and macrophytes (Fairchild et al. 1998; Lee et al. 2019); however, the growing U. mutabilis was considerably more resilient, but the EC50 value reached the lowest measured value (0.052 mg L−1) compared to the other MPs in our study (Table 8).
Glyphosate (N-(phosphonomethyl) glycine, PMG): Gametes and growing U. mutabilis displayed similar resistance to PMG as Chlorella spp. and U. intestinalis (Kittle and McDermid 2016; Kaeoboon et al. 2021). PMG inhibited photosynthesis in U. lactuca at very low concentrations; however, tolerance mechanisms were observed at higher concentrations (de Carvalho et al. 2022), which could explain the high resilience of gametes and growing U. mutabilis. In a study of four different microalgae, low EC50 values for a PMG formulation were observed after 96 h (Hernandez-Garcia and Martinez-Jeronimo 2020). As one of the microalgae in this previous study was C. vulgaris, the formulation of PMG likely resulted in a substantial change in effectivity (Table 8).
Metolachlor (MTC): Growing U. mutabilis were highly resilient to MTC compared to green microalgae and macrophytes, whereas gametes were more resilient than all but one species (Fairchild et al. 1998; Maronic et al. 2018; Machado and Soares 2019b). This is the first study to investigate MTC toxicity in macroalgae.
Removal of micropollutants
Overall, our analytical approach was well adapted for MP quantification, with an acceptable peak shape, a low relative standard deviation of the calibration, and adequate recovery rates. Furthermore, when calibrated suitably, the developed method can also detect lower, environmentally relevant concentrations. Consideration should be given to enhance the ionization of the phenolic endocrine disruptors when improving these methods. This can be accomplished, for example, through post-column infusion or the use of atmospheric pressure chemical ionization. The observed increase in MP concentrations after 14 days in some cases is probably attributed to water evaporation, which increases the concentration at stable MP concentrations. In summary, ATZ and SMX were stable under all conditions and not removed by the tripartite Ulva-Roseovarius-Maribacter community; PMG and OTC were slightly removed by hydrolysis or sorption; CAP, E2, EE2, and OTC were partially removed by photolysis; and BPA, E2, and EE2 were removed to below LOD, presumably by degradation. The tripartite community partially and reversibly removed ERY and MTC while partially removing PMG after a lag phase.
CAP and OTC undergo photodegradation (Shih 1971; Jin et al. 2017). In contrast, E2 and EE2 are stable under UV-A and visible light conditions but are slowly degraded in the presence of UV-B and UV-C radiation (Liu and Liu 2004; Rosenfeldt and Linden 2004; Mazellier et al. 2008; Leech et al. 2009; Matamoros et al. 2009). Owing to the medium composition and use of 50% spent medium, organic compounds might act as photocatalysts and enhance photodegradation (Leech et al. 2009). Although approximately 12% of ERY and MTC were removed in the presence of Ulva within 14 days, no effective removal was observed after 42 days, indicating a reversible sorption process. The tripartite community likely adapted to PMG, as its removal under biotic conditions occurred only after the longer incubation period of 42 days and not in the first two weeks.
As the tripartite community removed BPA, E2, and EE2 to below the LOD, biotransformation or total metabolization is the most likely mechanism. Removal of these three endocrine disruptors by U. mutabilis have been previously examined alongside other Ulva species. For example, U. prolifera removed approximately 95% of 1 mg L−1 BPA within six days (Zhang et al. 2019), and U. pertusa 90% of 0.01 mg L−1 EE2 and 60% of 0.01 mg L−1 E2 within the first day of incubation (Lu et al. 2021). Notably, other species of Ulva were not found contributing to the removal of BPA, EE2, or E2 (Astrahan et al. 2021). In our study, U. mutabilis displayed better or comparable removal capabilities to other Ulva species for BPA and higher removal efficiencies for E2 and EE2.
Implications for the use of Ulva in aquaculture
To use U. mutabilis in IMTA, the compounds that accumulate and might be passed on via consumption of the algae must be identified when applying Ulva as a biofilter and food and feed source at the same time. Further examination of the underlying removal mechanisms of the phenolic endocrine disruptors BPA, E2, and EE2, as well as the herbicide PMG will facilitate a clearer risk assessment. However, as no biotic processes are involved in the removal of the other studied MPs, Ulva will not likely become contaminated by these under the studied exposure conditions, thus highlighting its potential for use in aquaculture.
Implications for the modes of action of algal growth factors
While U. mutabilis was resistant to the tested herbicides and antibiotics, as evidenced by high EC50 values, all tested hormone disruptors were removed during our experiments. However, the extent to which these hormones are absorbed or biologically transformed requires further study. This is particularly important for hormone-like compounds, such as thallusin (Alsufyani et al. 2020; Dhiman et al. 2022), which are released by associated bacteria and are essential for the development of Ulva. Compounds with a potentially similar spectrum of action may likely be eliminated from Ulva to facilitate the selective action of thallusin.
Conclusions
The green macroalga U. mutabilis is resilient against many MPs at high concentrations including antibiotics, endocrine disruptors, and herbicides. Depending on the MP, Gametes were up to two order of magnitude more vulnerable than a growing tubular thalli of U. mutabilis but still more resilient than green microalgae. The bioaccumulation of the endocrine disruptors remains a critical factor for assessing Ulva in IMTA, which requires further investigation. Our developed analytical method is suitable for the direct measurement and quantification of various compounds in seawater; however, the selected detection limits could be lowered further using the analytical techniques employed here. Ulva mutabilis and its associated bacteria did not remove the tested antibiotics and herbicides from seawater. Nonetheless, the fast growth rates and broad geographical distribution of Ulva make it an ideal target for the bioremediation of endocrine disruptors, BPA, E2, and EE2, and this genus can be considered an effective biofilter for wastewater management.
Availability of data and material
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Alsufyani T, Califano G, Deicke M, Grueneberg J, Weiss A, Engelen AH, Kwantes M, Mohr JF, Ulrich JF, Wichard T (2020) Macroalgal–bacterial interactions: identification and role of thallusin in morphogenesis of the seaweed Ulva (Chlorophyta). J Exp Bot 71:3340–3349
Arumugam N, Chelliapan S, Kamyab H, Thirugnana S, Othman N, Nasri NS (2018) Treatment of wastewater using seaweed: A review. Int J Envron Res Public Health 15:2851
Astrahan P, Korzen L, Khanin M, Sharoni Y, Israel A (2021) Seaweeds fast EDC bioremediation: Supporting evidence of EE2 and BPA degradation by the red seaweed Gracilaria sp., and a proposed model for the remedy of marine-borne phenol pollutants. Environ Pollut 278:116853
Azizullah A, Gao KS, Khan S, Gao G (2022) The interplay between bisphenol A and algae-A review. J King Saud Univ Sci 34:102050
Baghel RS, Trivedi N, Gupta V, Neori A, Reddy CRK, Lali A, Jha B (2015) Biorefining of marine macroalgal biomass for production of biofuel and commodity chemicals. Green Chem 17:2436–2443
Baldwin AK, Corsi SR, De Cicco LA, Lenaker PL, Lutz MA, Sullivan DJ, Richards KD (2016) Organic contaminants in Great Lakes tributaries: Prevalence and potential aquatic toxicity. Sci Total Environ 554–555:42–52
Balina K, Balode M, Muzikante L, Blumberga D (2015) Impact of synthetic hormone 17α-ethinylestradiol on growth of microalgae Desmodesmus communis. Agron Res 13:445–454
Belhaj D, Athmouni K, Frikha D, Kallel M, El Feki A, Maalej S, Zhou JL, Ayadi H (2017) Biochemical and physiological responses of halophilic nanophytoplankton (Dunaliella salina) from exposure to xeno-estrogen 17 α-ethinylestradiol. Environ Sci Pollut Res Int 24:7392–7402
Bolton JJ, Robertson-Andersson DV, Shuuluka D, Kandjengo L (2009) Growing Ulva (Chlorophyta) in integrated systems as a commercial crop for abalone feed in South Africa: a SWOT analysis. J Appl Phycol 21:575–583
Cabral D, Cardoso S, Rocco S, Sforça M, Hengeltraub SF, Bauer C, Rocha M, Maraschin M (2018) 17α-ethinylestradiol analysis of endo- and exometabolome of Ulva lactuca (Chlorophyta) by 1H-NMR spectroscopy and bioinformatics tools. In: Fdez-Riverola F, Mohamad MS, Rocha M, Paz JFD, González P (eds) Practical Applications of Computational Biology and Bioinformatics, 12th International Conference. Springer, Cham pp 225–226
Califano G, Wichard T (2018) Preparation of axenic cultures in Ulva (Chlorophyta). In: Charrier B, Wichard T, Reddy CRK (eds) Protocols for Macroalgae Research. CRC Press, Boca Raton, pp 159–171
Camuel A, Guieysse B, Alcantara C, Bechet Q (2017) Fast algal eco-toxicity assessment: Influence of light intensity and exposure time on Chlorella vulgaris inhibition by atrazine and DCMU. Ecotoxicol Environ Saf 140:141–147
Chen YY, Di YL, Lu S, Wu D, Sun P (2017) Tolerance and indicator characteristics of Ulva pertusa under sulfamethoxazole and erythromycin stress. China Environ Sci 37:3114–3122
Chen JM, Sun RX, Pan CG, Sun Y, Mai BX, Li QX (2020) Antibiotics and food safety in aquaculture. J Agr Food Chem 68:11908–11919
Das S, Ray NM, Wan J, Khan A, Chakraborty T, Ray MB (2017) Micropollutants in wastewater: Fate and removal processes. In: Farooq R, Ahmad Z (eds) Physico-Chemical Wastewater Treatment and Resource Recovery. IntechOpen, London, pp 75–107
de Carvalho RC, Feijao E, Matos AR, Cabrita MT, Utkin AB, Novais SC, Lemos MFL, Cacador I, Marques JC, Reis-Santos P, Fonseca VF, Duarte B (2022) Effects of glyphosate-based herbicide on primary production and physiological fitness of the macroalgae Ulva lactuca. Toxics 10:430
De Clerck O, Kao SM, Bogaert KA, Blomme J, Foflonker F, Kwantes M, Vancaester E, Vanderstraeten L, Aydogdu E, Boesger J, Califano G, Charrier B, Clewes R, Del Cortona A, D’Hondt S, Fernandez-Pozo N, Gachon CM, Hanikenne M, Lattermann L, Leliaert F, Liu XJ, Maggs CA, Popper ZA, Raven JA, Van Bel M, Wilhelmsson PKI, Bhattacharya D, Coates JC, Rensing SA, Van Der Straeten D, Vardi A, Sterck L, Vandepoele K, Van de Peer Y, Wichard T, Bothwell JH (2018) Insights into the evolution of multicellularity from the sea lettuce genome. Curr Biol 28:2921–2933
Dhiman S, Ulrich JF, Wienecke P, Wichard T, Arndt H-D (2022) Stereoselective total synthesis of (‒)-thallusin for bioactivity profiling. Angew Chem Int Ed 61: e202206746
Fairchild JF, Ruessler DS, Carlson AR (1998) Comparative sensitivity of five species of macrophytes and six species of algae to atrazine, metribuzin, alachlor, and metolachlor. Environ Toxicol Chem 17:1830–1834
Ferreira CSG, Nunes BA, de Melo Henriques-Almeida JM, Guilhermino U (2007) Acute toxicity of oxytetracycline and florfenicol to the microalgae Tetraselmis chuii and to the crustacean Artemia parthenogenetica. Ecotox Environ Safe 67:452–458
Ghaderiardakani F, Califano G, Mohr JF, Abreu MH, Coates JC, Wichard T (2019) Analysis of algal growth- and morphogenesis-promoting factors in an integrated multi-trophic aquaculture system for farming Ulva spp. Aquac Environ Interact 11:375–391
Ghaderiardakani F, Quartino ML, Wichard T (2020) Microbiome-dependent adaptation of seaweeds under environmental stresses: A perspective. Front Mar Sci 7: 575228
Green JW, Springer TA, Staveley JP (2013) The drive to ban the NOEC/LOEC in favor of ECx is misguided and misinformed. Integr Environ Asses 9:12–16
Guttman L, Boxman SE, Barkan R, Neori A, Shpigel M (2018) Combinations of Ulva and periphyton as biofilters for both ammonia and nitrate in mariculture fishpond effluents. Algal Res 34:235–243
Hardegen J, Braeutigam P, Abendroth C, Wichard T (2021) Bisphenol A: quantification in complex matrices and removal by anaerobic sludges. Pollutants 1:194–206
Hernandez-Garcia CI, Martinez-Jeronimo F (2020) Multistressor negative effects on an experimental phytoplankton community. The case of glyphosate and one toxigenic cyanobacterium on Chlorophycean microalgae. Sci Total Environ 717:137186
Hirsch R, Ternes T, Haberer K, Kratz KL (1999) Occurrence of antibiotics in the aquatic environment. Sci Total Environ 225:109–118
Hoga CA, Almeida FL, Reyes FGR (2018) A review on the use of hormones in fish farming: Analytical methods to determine their residues. Cyta-J Food 16:679–691
Hoxmark RC (1975) The effect of chloramphenicol and cycloheximide on meiotic zoosporogenesis and mitotic gametogenesis in Ulva mutabilis. Can J Genet Cytol 17:553–561
Hoxmark RC, Nordby O (1977) Warning against using chloramphenicol in light. Plant Sci Lett 8:113–118
Huang B, Tang J, He H, Gu L, Pan X (2019) Ecotoxicological effects and removal of 17β-estradiol in Chlorella algae. Ecotoxicol Environ Saf 174:377–383
Jin X, Xu HZ, Qiu SS, Jia MY, Wang F, Zhang AQ, Jiang X (2017) Direct photolysis of oxytetracycline: Influence of initial concentration, pH and temperature. J Photochem Photobiol A 332:224–231
Kabra AN, Ji MK, Choi J, Kim JR, Govindwar SP, Jeon BH (2014) Toxicity of atrazine and its bioaccumulation and biodegradation in a green microalga Chlamydomonas mexicana. Environ Sci Pollut Res 21:12270–12278
Kaeoboon S, Suksungworn R, Sanevas N (2021) Toxicity response of Chlorella microalgae to glyphosate herbicide exposure based on biomass, pigment contents and photosynthetic efficiency. Plant Sci Today 8:293–300
Kessler RW, Alsufyan T, Wichard T (2018) Purification of sporulation and swarming inhibitors from Ulva. In: Charrier B, Wichard T, Reddy CRK (eds) Protocols for Macroalgae Research. CRC Press, Boca Raton, pp 139–157
Kittle RP, McDermid KJ (2016) Glyphosate herbicide toxicity to native Hawaiian macroalgal and seagrass species. J Appl Phycol 28:2597–2604
Kolar B, Arnus L, Jeretin B, Gutmaher A, Drobne D, Durjava MK (2014) The toxic effect of oxytetracycline and trimethoprim in the aquatic environment. Chemosphere 115:75–80
Kummerer K (2009) Antibiotics in the aquatic environment - A review - Part I. Chemosphere 75:417–434
Lai HT, Hou JH, Su CI, Chen CL (2009) Effects of chloramphenicol, florfenicol, and thiamphenicol on growth of algae Chlorella pyrenoidosa, Isochrysis galbana, and Tetraselmis chui. Ecotox Environ Safe 72:329–334
Landis WG, Chapman PM (2011) Well past time to stop using NOELs and LOELs. Integr Environ Assess Manag 7 (4):vi-viii
Lawton RJ, Mata L, de Nys R, Paul NA (2013) Algal bioremediation of waste waters from land-based aquaculture using Ulva: selecting target species and strains. PLoS One 8 (10):e77344
Lee H, Brown MT, Choi S, Pandey LK, De Saeger J, Shin K, Kim JK, Depuydt S, Han T, Park J (2019) Reappraisal of the toxicity test method using the green alga Ulva pertusa Kjellman (Chlorophyta). J Hazard Mater 369:763–769
Leech DM, Snyder MT, Wetzel RG (2009) Natural organic matter and sunlight accelerate the degradation of 17 β-estradiol in water. Sci Total Environ 407:2087–2092
Leston S, Nunes M, Viegas I, Ramos F, Pardal MA (2013) The effects of chloramphenicol on Ulva lactuca. Chemosphere 91:552–557
Li J, Liu K, Li W, Zhang M, Li P, Han J (2022) Removal mechanisms of erythromycin by microalgae Chlorella pyrenoidosa and toxicity assessment during the treatment process. Sci Total Environ 848:157777
Liu B, Liu XL (2004) Direct photolysis of estrogens in aqueous solutions. Sci Total Environ 320:269–274
Liu BY, Nie XP, Liu WQ, Snoeijs P, Guan C, Tsui MTK (2011) Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole on photosynthetic apparatus in Selenastrum capricornutum. Ecotox Environ Safe 74:1027–1035
Liu JJ, Pemberton B, Lewis J, Scales PJ, Martin GJO (2020) Wastewater treatment using filamentous algae - A review. Bioresour Technol 298:122556
Lu J, Zhang C, Wu J (2021) Removal of steroid hormones from mariculture system using seaweed Caulerpa lentillifera. Front Env Sci Eng 16(2):15
Ludmila M, Veronika L, Murashova, Alena MT, Svetlana Z, Victor E (2023) Morphological changes and biochemical reaction of Ulva rigida in response to the toxic effect of bisphenol A under experimental conditions. Aquat Bot 184:103579
Luo YL, Guo WS, Ngo HH, Nghiem LD, Hai FI, Zhang J, Liang S, Wang XCC (2014) A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci Total Environ 473:619–641
Ma ZH, Yang FS, Ren JY, Fan R, Duan QN, Guo JP, Guo JH (2021) Growth inhibition and oxidative stress in two green algal species exposed to erythromycin. J Am Water Resour Assoc 57:628–637
Machado MD, Soares EV (2019a) Impact of erythromycin on a non-target organism: Cellular effects on the freshwater microalga Pseudokirchneriella subcapitata. Aquat Toxicol 208:179–186
Machado MD, Soares EV (2019b) Sensitivity of freshwater and marine green algae to three compounds of emerging concern. J Appl Phycol 31:399–408
Maronic DS, Camagajevac IS, Horvatic J, Pfeiffer TZ, Stevic F, Zarkovic N, Waeg G, Jaganjac M (2018) S-metolachlor promotes oxidative stress in green microalga Parachlorella kessleri - A potential environmental and health risk for higher organisms. Sci Total Environ 637:41–49
Matamoros V, Duhec A, Albaiges J, Bayona JM (2009) Photodegradation of carbamazepine, ibuprofen, ketoprofen and 17 α-ethinylestradiol in fresh and seawater. Water Air Soil Poll 196:161–168
Mazellier P, Meite L, De Laat J (2008) Photodegradation of the steroid hormones 17β-estradiol (E2) and 17α-ethinylestradiol (EE2) in dilute aqueous solution. Chemosphere 73:1216–1223
Mohsenpour SF, Hennige S, Willoughby N, Adeloye A, Gutierrez T (2021) Integrating micro-algae into wastewater treatment: A review. Sci Total Environ 752:142168
Montiel-Leon JM, Munoz G, Duy SV, Do DT, Vaudreuil MA, Goeury K, Guillemette F, Amyot M, Sauve S (2019) Widespread occurrence and spatial distribution of glyphosate, atrazine, and neonicotinoids pesticides in the St. Lawrence and tributary rivers. Environ Pollut 250:29–39
Nahor O, Morales-Reyes CF, Califano G, Wichard T, Golberg A, Israel Á (2021) Flow cytometric measurements as a proxy for sporulation intensity in the cultured macroalga Ulva (Chlorophyta). Bot Mar 64:83–92
Neori A (1991) Use of seaweed biofilters to increase mariculture intensification and upgrade its effluents. Fisheries and Fishbreeding in Israel: Review of Fisheries in Israel 24:171–179
Neori A, Msuya FE, Shauli L, Schuenhoff A, Kopel F, Shpigel M (2003) A novel three-stage seaweed (Ulva lactuca) biofilter design for integrated mariculture. J Appl Phycol 15:543–553
Nguyen LM, Nguyen NTT, Nguyen TTT, Nguyen TT, Nguyen DTC, Tran TV (2022) Occurrence, toxicity and adsorptive removal of the chloramphenicol antibiotic in water: a review. Environ Chem Lett 20:1929–1963
Norvill ZN, Shilton A, Guieysse B (2016) Emerging contaminant degradation and removal in algal wastewater treatment ponds: Identifying the research gaps. J Hazard Mater 313:291–309
Oh M-H, Kang Y-H, Lee C-H, Chung I-K (2005) Effects of six antibiotics on the activity of the photosynthetic apparatus and ammonium uptake of thallus of Porphyra yezoensis. Algae 20:121–125
Okada K, Satoh K, Katoh S (1991) Chloramphenicol is an inhibitor of photosynthesis. FEBS Lett 295:155–158
Qian HF, Sheng GD, Liu WP, Lu YC, Liu ZG, Fu ZW (2008) Inhibitory effects of atrazine on Chlorella vulgaris as assessed by real-time polymerase chain reaction. Environ Toxicol Chem 27:182–187
Qiu S, Ge SJ, Champagne P, Robertson RM (2017) Potential of Ulva lactuca for municipal wastewater bioremediation and fly food. Desalin Water Treat 91:23–30
Rehman AU, Kodru S, Vass I (2016) Chloramphenicol mediates superoxide production in photosystem II and enhances its photodamage in isolated membrane particles. Front Plant Sci 7:479
Reichenbächer M, Einax JW (2011) The lack-of-fit test by ANOVA. In: Challenges in Analytical Quality Assurance. Springer, Heidelberg pp 136–138
Rosa J, Leston S, Freitas A, Vila Pouca AS, Barbosa J, Lemos MFL, Pardal MA, Ramos F (2019) Oxytetracycline accumulation in the macroalgae Ulva: Potential risks for IMTA systems. Chemosphere 226:60–66
Rose MT, Cavagnaro TR, Scanlan CA, Rose TJ, Vancov T, Kimber S, Kennedy IR, Kookana RS, Van Zwieten L (2016) Impact of herbicides on soil biology and function. Adv Agron 136:133–220
Rosenfeldt EJ, Linden KG (2004) Degradation of endocrine disrupting chemicals bisphenol A, ethinyl estradiol, and estradiol during UV photolysis and advanced oxidation processes. Environ Sci Technol 38:5476–5483
Shareef A, Angove MJ, Wells JD, Johnson BB (2006) Aqueous solubilities of estrone, 17β-estradiol, 17α-ethynylestradiol, and bisphenol A. J Chem Eng Data 51:879–881
Shih IK (1971) Photodegradation products of chloramphenicol in aqueous solution. J Pharm Sci 60:1889–1890
Shpigel M, Neori A, Popper DM, Gordin H (1993) A proposed model for “environmentally clean” land-based culture of fish, bivalves and seaweeds. Aquaculture 117:115–128
Siedlewicz G, Zak A, Sharma L, Kosakowska A, Pazdro K (2020) Effects of oxytetracycline on growth and chlorophyll a fluorescence in green algae (Chlorella vulgaris), diatom (Phaeodactylum tricornutum) and cyanobacteria (Microcystis aeruginosa and Nodularia spumigena). Oceanologia 62:214–225
Simon C, McHale M, Sulpice R (2022) Applications of Ulva biomass and strategies to improve its yield and composition: A perspective for Ulva aquaculture. Biology 11:1593
Sousa JCG, Ribeiro AR, Barbosa MO, Pereira MFR, Silva AMT (2018) A review on environmental monitoring of water organic pollutants identified by EU guidelines. J Hazard Mater 344:146–162
Spoerner M, Wichard T, Bachhuber T, Stratmann J, Oertel W (2012) Growth and thallus morphogenesis of Ulva mutabilis (Chlorophyta) depends on a combination of two bacterial species excreting regulatory factors. J Phycol 48:1433–1447
Steinhagen S, Barco A, Wichard T, Weinberger F (2019) Conspecificity of the model organism Ulva mutabilis and Ulva compressa (Ulvophyceae, Chlorophyta). J Phycol 55:25–36
Stratmann J, Paputsoglu G, Oertel W (1996) Differentiation of Ulva mutabilis (Chlorophyta) gametangia and gamete release are controlled by extracellular inhibitors. J Phycol 32:1009–1021
Sun Q, Li Y, Chou PH, Peng PY, Yu CP (2012) Transformation of bisphenol A and alkylphenols by ammonia-oxidizing bacteria through nitration. Environ Sci Technol 46:4442–4448
Sun C, Xu Y, Hu N, Ma J, Sun S, Cao W, Klobucar G, Hu C, Zhao Y (2020) To evaluate the toxicity of atrazine on the freshwater microalgae Chlorella sp. using sensitive indices indicated by photosynthetic parameters. Chemosphere 244:125514
Tanaka Y, Nakamura K, Yokomizo H (2018) Relative robustness of NOEC and ECx against large uncertainties in data. PLoS One 13 (11):e0206901
Tran NH, Reinhard M, Gin KY (2018) Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res 133:182–207
Viegas C, Gouveia L, Gonçalves M (2021) Aquaculture wastewater treatment through microalgal. Biomass potential applications on animal feed, agriculture, and energy. J Environ Manage 286:112187
Vockenberg T, Wichard T, Ueberschaar N, Franke M, Stelter M, Braeutigam P (2020) The sorption behaviour of amine micropollutants on polyethylene microplastics – impact of aging and interactions with green seaweed. Environ Sci: Process Impacts 22:1678–1687
Wichard T (2015) Exploring bacteria-induced growth and morphogenesis in the green macroalga order Ulvales (Chlorophyta). Front Plant Sci 6:86
Wichard T (2023) From model organism to application: Bacteria-induced growth and development of the green seaweed Ulva and the potential of microbe leveraging in algal aquaculture. Semin Cell Dev Biol 134:69–78
Wichard T, Charrier B, Mineur F, Bothwell JH, Clerck OD, Coates JC (2015) The green seaweed Ulva: a model system to study morphogenesis. Front Plant Sci 6:72
Xiong JQ, Govindwar S, Kurade MB, Paeng KJ, Roh HS, Khan MA, Jeon BH (2019a) Toxicity of sulfamethazine and sulfamethoxazole and their removal by a green microalga, Scenedesmus obliquus. Chemosphere 218:551–558
Xiong Q, Hu LX, Liu YS, Wang TT, Ying GG (2019b) New insight into the toxic effects of chloramphenicol and roxithromycin to algae using FTIR spectroscopy. Aquat Toxicol 207:197–207
Yang Y-j, Hong Y-p (2012) Combination effects of bisphenol A and isobutylparaben on the green macroalga Ulva pertusa. J Toxicol Environ 4:37–41
Zhang C, Lu J, Wu J, Luo Y (2019) Phycoremediation of coastal waters contaminated with bisphenol A by green tidal algae Ulva prolifera. Sci Total Environ 661:55–62
Zhang YB, He D, Chang F, Dang CY, Fu J (2021) Combined effects of sulfamethoxazole and erythromycin on a freshwater microalga, Raphidocelis subcapitata: Toxicity and oxidative stress. Antibiotics 10(5):576
Zhang YR, Chen ZX, Tao Y, Wu WY, Zeng YY, Liao KJ, Li XY, Chen LZ (2022) Transcriptomic and physiological responses of Chlorella pyrenoidosa during exposure to 17α-ethinylestradiol. Int J Mol Sci 23(7):3583
Zielińska M, Wojnowska-Baryła I, Cydzik-Kwiatkowska A (2019) Biological wastewater treatment technologies for BPA removal. In: Bisphenol A Removal from Water and Wastewater. Springer, Cham, pp 79–101
Zollmann M, Rubinsky B, Liberzon A, Golberg A (2021) Multi-scale modeling of intensive macroalgae cultivation and marine nitrogen sequestration. Commun Biol 4:848
Acknowledgements
We thank Georg Pohnert for his helpful support and discussion during the preparation of this manuscript. This article is also based upon work from COST Actions CA20106 "Tomorrow's wheat of the sea': Ulva, a model for innovative mariculture", supported by COST (European Cooperation in Science and Technology, www.cost.eu). Findings of the removal mechanisms of bisphenol A were presented at the COST Action conference "From fundamental biology to aquaculture: state-of-the-art, bottlenecks and gaps" held in Cádiz (Spain).
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was funded by the Friedrich Schiller University Jena (Germany) ('Landesgraduiertenstipendium' of the Free State of Thuringia) and was supported by the Deutsche Forschungsgemeinschaft through Grant No. SFB 1127/2 ChemBioSys–239748522.
Author information
Authors and Affiliations
Contributions
Conceptualization: Justus Hardegen, Thomas Wichard; Formal analysis: Justus Hardegen, Thomas Wichard; Funding acquisition: Thomas Wichard; Investigation: Justus Hardegen, Gabriel Amend; Methodology: Justus Hardegen; Resources: Thomas Wichard; Supervision: Thomas Wichard; Validation: Justus Hardegen, Thomas Wichard; Visualization: Justus Hardegen; Writing – original draft: Justus Hardegen; Writing – Review and editing: Gabriel Amend, Thomas Wichard. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hardegen, J., Amend, G. & Wichard, T. Lifecycle-dependent toxicity and removal of micropollutants in algal cultures of the green seaweed Ulva (Chlorophyta). J Appl Phycol 35, 2031–2048 (2023). https://doi.org/10.1007/s10811-023-02936-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-02936-x