Abstract
Here, we evaluated the effect of culture depth on the growth and nutrient removal rate of Scenedesmus sp. grown in anaerobically digested abattoir effluent (ADAE) and cultivated semi-continuously in raceway ponds at depths of 14 cm, 17 cm, 20 cm, and 23 cm during Austral winter and summer. Culture medium pH was kept constant at pH 6.5 for all treatments throughout the experimental period using a pH–stat system. Algal cultures grown in summer had 2.3- 2.7 times higher biomass productivity than the same grown in winter. In both seasons, maximum volumetric productivity of this alga was achieved at 14 cm depth (14 cm depth⩾ 17 cm depth⩾ 20 cm depth⩾ 23 cm depth). However, areal biomass productivity of culture grown at 23 cm depth was 12% and 29% higher than that of culture grown at 14 cm depth in winter and summer, respectively. In addition, nitrogen, phosphorus and COD areal removal rates were significantly higher in cultures operated at 23 cm among all treatments in both seasons. The effective quantum yield (Fq'/Fm') in summer was 23 cm depth = 20 cm depth > 17 cm depth = 14 cm depth while it followed 14 cm depth⩾ 17 cm depth⩾ 20 cm depth⩾ 23 cm depth in winter, indicating significance of operational conditions on algal photosynthesis. The outcome of this study shows that, irrespective of the season, operating the culture in higher depths significantly increased areal biomass productivity as well as areal nutrient removal rates when treating ADAE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The red meat processing sector is among the largest contributors to the food industry which generates a large volume of wastewater referred to as abattoir effluent that primarily contains blood, oil, minerals, organic matters and water used for the slaughterhouse cleaning & operation (Bustillo-Lecompte and Mehrvar 2015). Currently, anaerobic digestion is the most commonly used method for the removal of organic matter from abattoir effluent (Jensen et al. 2014). Anaerobic digestion typically converts organic waste to end products such as methane (for electricity and heat generation) and carbon dioxide (Hakawati et al. 2017). Although anaerobic digestion represents an attractive biological treatment that significantly reduces the concentration of organics in the initial effluent, the treated slurry that is produced contains substantial amount of inorganic components (e.g. N and P), chemical oxygen demand (COD), and pathogens (Xia and Murphy 2016). Recent studies showed the potential use of microalgae to assimilate various nutrient typically present in anaerobically digested abattoir effluent (ADAE) resulting in production of valuable algal biomass and nutrient-depleted effluent which is safe for potential discharge and reuse (Vadiveloo et al. 2020, 2022; Matos et al. 2021).
Among the common challenges typically associated with AD effluent is its turbidity produced by suspended solids, dissolved colored compounds, finely divided organic and inorganic matter as well as microscopic organisms (Chuka-ogwude et al. 2020). High turbidity generally means reduction in the clarity of the liquid medium resulting in less light penetration into microalgal cultures (Wang et al. 2010). Light is by far the most important limiting factor that affects growth of photo-autotrophs by its direct role in photosynthesis (Raeisossadati et al. 2019a). Pretreatment methods which have been proposed for reducing turbidity (see de Godos et al. 2010; Kim et al. 2014; Wang et al. 2015) are energy intensive and costly to be implemented at large scale of operation (Chuka-ogwude et al. 2020).
One way to enhance the light availability in a turbid algal culture is reduction of culture depth (Chuka-ogwude et al 2022). Indeed, culture depth is among the operational parameters that can affect biomass productivity of algal cultures due to its direct impact on growth factors such as light utilization efficiency and culture temperature (Kim et al. 2018). Culture depth can also influence photosynthesis due to self-shading of the algal cells affecting the availability of light received by cells. Photosynthesis is the driving force for algal nutrient uptake for growth and biomass production (Grobbelaar 2010). Photolimitation and photoinhibition are the two limiting factors for the growth of an outdoor microalgae culture (Raeisossadati et al. 2019a). Photoinhibition stems from inadequate light availability to cultures and photoinhibition occurs when there excess of irradiance available to a culture. Cell concentration also determines the degree of light attenuation whereas mixing rate regulates frequency of cell movement between dark and illuminated zones (Sutherland et al. 2014b).
Raceway ponds are the main cultivation system in use for the treatment of wastewater and resource recovery (Park et al. 2011; Rayen et al. 2019). Maximizing biomass productivity and the removal of nutrients in the shortest possible time is vital to improve the economics of algal phytoremediation (Grobbelaar 2010). To the best of our knowledge, there have been no studies looking at the effects of culture depth on the growth on microalgae when cultivated in abattoir wastewater in both summer and winter. Previous studies showed that a lower depth enhances the surface-to-volume ratio and increases the availability of light to algal cells whereas increasing culture depth can limit light penetration into cultures (Borowitzka 2005). The turbidity of the wastewater along with different light frequency is expected to significantly affect microalgal photosynthesis and biomass productivity in outdoor scenarios. As such, the overarching aim of this study was investigating the effect of culture depth on the growth, photo-physiology and nutrient removal rate of Scenedesmus sp. cultures grown in non-pretreated AD effluent. The experiment was conducted in both Western Australia summer and winter conditions in which ambient temperature can go as high as above 40 °C and as low as 4 °C, respectively. The research objective of this study was to identify if seasonal variation in light availability and its penetration into the culture would affect the wastewater treatment process and microalgal growth in it. The major highlight of this work was the depth optimization of developed microalgal wastewater treatment system to specifically treat AD abattoir effluent under local climatic conditions.
Materials and methods
Anaerobically digested abattoir effluent (ADAE) and culture medium
The anaerobically digested abattoir effluent (ADAE) used in this study was collected from the discharge of the anaerobic digestion pond of a local beef abattoir in Western Australia. The physiochemical composition of the ADAE is described in Table 1. The raw ADAE was not subjected to any further pre-treatment (i.e. centrifugation and filtration). The ADAE was diluted with freshwater at a ratio of 1:3. The turbidity of ADAE after dilution was approximately 12 NTU. Other than freshwater dilution, no other nutritional supplements were added since we have previously demonstrated that Scenedesmus can grow efficiently in diluted ADAE without any further addition of nutrients (Shayesteh et al. 2021).
Microalgae Culture
Scenedesmus sp. (MUR 272) was obtained from the culture collection of the Algae Research & Development Centre at Murdoch University. Scenedesmus was previously cultivated on ADAE under outdoor condition for over 6 months and found to have high tolerance to ammonia rich wastewater with up to 50 mg L−1 NH3-N (Shayesteh et al. 2021).
Experimental Setup
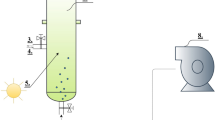
Scenedesmus cultivation was carried out in 0.1 m2 paddle wheel-driven raceway ponds with dimension of 13 × 26 × 80 cm (W × H × L). Thse micro-ponds were operated at four different culture depths of 14 cm, 17 cm, 20 cm, and 23 cm (Fig. 1). The working volumes for the depths of 14, 17, 20, and 23 cm were 14, 17, 20, and 23 L, respectively. These raceway ponds were not operational in depth below 14 cm as the culture would not be appropriately mixed (paddle wheel mixing could not occur). We could also not operate these ponds in depth above 23 cm due to overflowing (spilling of culture). The culture was mixed using a four-blade paddle wheel at the speed of 10, 11.4, 10.8 and 10.4 cm s−1 for the lowest to highest depths, respectively. Variation in mixing velocity was due to the working volume of culture in each treatment. The pH of all treatments was standardized at pH 6.5 (based on the results of our previous study; Shayesteh et al. 2021) and kept constant through the periodical dosage of CO2 using a pH–stat system (Aqua Geek Fukurow controller, Taiwan). The pH–stat is the system which consisted of a pH probe and control system was connected to a CO2 source and was able to control the pH at specific point. Whenever, culture pH increased above 6.5 due to algal photosynthesis, the system pumped in CO2 to reduce the pH back to 6.5.
A) Schematic and B) structure view of paddle wheel-driven raceway ponds used in this study. Four micro-ponds were used for four treatments (14, 17, 20, and 23 cm culture depth) in semi-continuous mode. All micro-ponds were covered from all sides during the cultivation period to simulate the condition of a large-scale raceway pond receiving sunlight only from the top part
All cultures were observed regularly (at least twice weekly) under the microscope to ensure there no biological contaminants such as fungi, protozoa, and other microalgae were present. Evaporative losses of cultures were compensated daily with the addition of tap water. Therefore, evaporative loses did not affect the culture depth. Cultures were operated semi-continuously with total four harvesting regime being performed (four replicates) and the exponential growth period ranging between 3 days in summer and 7 to 9 days in winter. The harvest period was determined based on the biomass concentration of cultures when they reached early stationary phase (Nwoba et al. 2020a). The initial ammoniacal nitrogen concentrations in all treatments were standardized at 50 ± 5 mg L−1 NH3-N. All the cultures were harvested at the same time in a way (different harvesting %) to bring back all the cultures to same initial amount of biomass (0.327 ± 0.011 g L−1). Studies were conducted in Austral winter from 26 July 2020 to 28 August 2020 and summer from 25 January 2021 to 6 February 2021 at the Algae R&D Centre of Murdoch University (32.0734° S, 115.8392° E). Air temperature, solar irradiance, evaporation, rainfall data were obtained from the Murdoch University weather station (http://wwwmet.murdoch.edu.au). Culture temperature was measured using HOBO data loggers (Onset Data Loggers, USA).
Analytical methods
50 mL samples were collected from all treatments at 11 am every second day after compensating for the evaporative losses of each pond. Scenedesmus cell number and biomass concentration (AFDW) was used to calculate specific growth rate and biomass productivity based on the methods described in Moheimani et al. (2013). It is important to note that the areal productivity was calculated by multiplying volumetric productivity (g L−1 day−1) with working culture volume (L) before dividing it with the size (m2) of the algal pond [(volumetric productivity*culture volume)/pond size]. The water chemistry analyses were carried out using a Hanna HI 83,099 multiparameter photometer (Hanna Instruments, USA). To calculate the average of light energy available per cell, solar irradiance received by each pond divided by the total number of cells in the culture volume. The areal nutrient removal rate was also calculated by multiplying volumetric removal rate (g L−1dat−1) with working volume (L) to calculate the whole nutrient removal for each pond per day and then divided by culture surface (m2) [(volumetric removal rate*culture volume)/pond size]. The percentage of nutrients (nitrogen and phosphorus) assimilated by microalgae was calculated based on the elemental ratios of Scenedesmus biomass grown in ADAE. In this regard, the amount of biomass produced as well as N and P that was removed from the microalgae culture was measured. Then, based on the concentration of N and P in Scenedesmus biomass, the percentage that was fixed into the microalgae was calculated from the overall removal rates.
Saturation pulse-based chlorophyll a fluorescence measurement
The photosynthetic performance of the cultures grown at different depths was evaluated through chlorophyll fluorescence measurements using a Water-PAM fluorometer (Walz GmbH, Germany). The maximum quantum yield of light adapted samples (Fq'/Fm') was measured and calculated using the saturation light method (Cosgrove and Borowitzka 2006). Samples were taken from outdoor cultures before harvest at 12 pm for both winter and summer experiments and were immediately subjected for measurements.
In addition, a 12-h diurnal study was also conducted during the semi-continuous cultivation during winter to evaluate the changes in photosynthetic response of algal cells grown at different depths. Rapid light curves (RLCs) were evaluated to eight pre-set incremental irradiances (34, 52, 79, 122, 178, 274, 409 and 579 μmol photons m−2 s−1) followed by a saturation pulse that lasted for 0.8 s. Saturation light pulses (Fq'/Fm') and curve fitting parameters of the RLC such as electron transport rate maximum (ETRmax) and initial slope of rETR (αETR) was derived to indicate the photo-physiology status of the cultures (Vadiveloo and Moheimani 2018). Measurements were done on day 4 of each semi-continuous run at 6:30 am (before sunrise), 9:30 am (after sunrise), 12:30 am (midday), 3:30 pm, 6:30 pm (after sunset). Samples collected were immediately subjected to the photosynthesis measurement.
Statistical analysis
The statistical difference of measurements during each season was determined by one-way repeated measures ANOVA (p < 0.05). Student’s t-test was used to evaluate the significant difference between the same culture depths during the two different seasons (p < 0.05). For each treatment, the mean and standard error (SE) were calculated using four biological replicates (n = 4). All statistical analysis was performed using SigmaPlot version 14.0 for Windows.
Results
Culture conditions
Open outdoor algal cultivation systems such as raceway ponds are exposed and subjected to a wide range of fluctuating environmental conditions (i.e., temperature and irradiance). Here, we studied the effect of various culture depths on the growth, photo-physiology and nutrient removal rate Scenedesmus grown on ADAE (Fig. 2). In winter, the availability of solar radiation was reduced which corresponded with a higher frequency of rainfall with the highest value at 20.5 mm per day. However, almost no rainfall was recorded during summer with high solar radiation. The average daily solar radiation ranged between 38 to 220 in winter and and 120 to 400 Wm−2 in summer (Fig. 2A). The average light energy available for cells grown at different depths is shown in Table 2. Culture temperature did not differ significantly between different depths during each season(Fig. 2C). Ambient and culture temperature trended similarly and ranged from 4.5 to 25.4 °C and 5.7 to 25.5 °C, respectively, in winter and between 13.8 to 37.78 °C and 11.9 to 33.4 °C in summer (Fig. 2B and C). There was no significant difference in evaporation loss between different depths in both seasons (One Way ANOVA, p > 0.05). However, evaporation loss was significantly higher in summer compared to winter (student t-test, p < 0.05). Evaporation loss even was different between different days of each season. Daily evaporation loss in microponds ranged from 5.5 to 24.5 mm in winter and and 16.6 to 52.55 mm in summer (Fig. 2D).
Microalgae cultures were grown in ADAE with initial 50 mg L−1 NH3-N concentration at depth of 14, 17, 20 and 23 cm in raceway pond. A) Average daily solar irradiance, B) maximum (filled circle) and minimum (empty circle) air temperature, C) maximum (filled circle) and minimum (empty circle) culture temperature, D) evaporation, and 14 cm culture depth (filled circle), 17 cm culture depth (empty circle), 20 cm culture depth (filled triangle), 23 cm culture depth (empty triangle) E) microalgal cell concentration, F) microalgal biomass yield, G) NH3-N concentration and H) phosphorus concentration during the experimental period in winter and summer
Growth and Biomass productivity
Winter growth
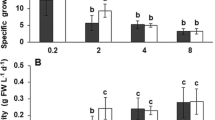
Cell concentration and biomass yield of cultures showed to be highest at 14 and 17 cm culture depths (Fig. 2E and F). The biomass yield and volumetric productivity were 14 cm depth⩾ 17 cm depth⩾ 20 cm depth⩾ 23 cm depth (One Way RM ANOVA, p < 0.05) (Table 2). The specific growth rate of the cultures at 14 cm depth was also significantly higher compared to other treatments (One Way RM ANOVA, p < 0.05) (Table 2). However, Scenedesmus grown at higher depths had significantly higher areal productivity when compared to lower depths (23 cm depth = 20 cm depth⩾ 17 cm depth = 14 cm depth, (Table 2) (One Way RM ANOVA, p < 0.05).
Summer growth
The general pattern of growth in summer was similar to winter (Fig. 2E and F) and cultures grown at lower depths showed significantly higher biomass yield and volumetric productivity among all treatments; 14 cm depth⩾ 17 cm depth⩾ 20 cm depth⩾ 23 cm depth (One Way RM ANOVA, p < 0.05) (Table 2). The specific growth rate was also 14 cm depth > 17 cm depth⩾ 20 cm depth = 23 cm depth (One Way RM ANOVA, p < 0.05) (Table 2). However, the areal biomass productivity obtained from culture grown at 23 cm depth was over 30% higher than that of culture grown at 14 cm depth; 23 cm depth > 20 cm depth > 17 cm depth > 14 cm depth (Table 2) (One Way RM ANOVA, p < 0.05).
Nutrient removal rates
Volumetric removal rate
Ammoniacal nitrogen (NH3-N) represents the major nutrient that is present in the AD effluent (Table 1). The removal rate of NH3-N for cultures operated at 14 cm depth was 15% higher than that of cultures grown at 20 cm depth in winter. However, NH3-N removal rate of cultures at the depth of 14 and 17 cm were not significantly different in winter (One Way RM ANOVA, p > 0.05) (Table 3). Further, NH3-N removal rate of cultures in summer showed that the cultures at the depth of 14, 17, and 20 cm had the same removal rate with no significance difference (One Way RM ANOVA, p > 0.05). Also, there was no significant difference for phosphorus and COD removal rates in all treatments during both summer and winter (One Way RM ANOVA, p > 0.05) (Table 3).
Areal removal rate
As summarized in Table 3, NH3-N areal removal rate was 23 cm depth > 20 cm depth > 17 cm depth > 14 cm depth (One Way RM ANOVA, p > 0.05). The areal removal rate of NH3-N and P for cultures grown at 23 cm depth was around 31% and 35% higher than that of cultures grown at 14 cm depth in both winter and summer. Similarly, areal COD removal rate was 39% and 36% greater for Scenedesmus grown at 23 cm depth compared to 14 cm depth in winter and summer (Table 3).
The initial N-NO2 concentration of all cultures was 2 mg L−1 and the final concentration of N-NO2 was 0.2 mg L−1 at the end of the experiment for all treatments meaning no significant difference among the various treatments. The initial concentration of N-NO3 was low for all treatments (1.5 mg L−1) (Data not shown).
Mass balance analysis
The elemental composition of Scenedesmus sp. biomass grown in the ADAE was previously characterized and identified to contain 48% carbon, 8.5% nitrogen, and 1.5% phosphorus. Based on the nitrogen (NH3-N) removal rates and algal biomass yield (g L−1), the nitrogen mass balance was also calculated (Table 4). Nitrogen assimilation into the biomass was 11% higher in the cultures with the depth of 14 cm compared to that in the cultures with the depth of 20 cm in winter (Table 4). Phosphorus assimilation was 16% higher in cultures at the depth of 14 cm compared to that in cultures with the depth of 20 cm in winter (Table 4). Further, nitrogen and phosphorus assimilation into the biomass for cultures at the depth of 14 cm were 5 and 9.5% higher that of those in cultures with the depth of 20 cm in summer (Table 4).
Photosynthetic performance
Figure 3 illustrates the effective quantum yield (Fq'/Fm') of cultures before harvest (at 12 pm) during the cultivation period in both winter and summer. In general, Fq'/Fm' values of cultures in winter (between 0.692 and 0.723) were significantly higher than that recorded in summer (between 0.595 and 0.628) (student t-test, p < 0.05). In addition, the Fq'/Fm' values of cultures with lower depths were significantly greater than those of cultures grown at higher depths in winter (One Way RM ANOVA, p < 0.05). However, it was not the case in summer. Overall, Fq'/Fm' in summer was M23D = M20D > M17D = M14D while it followed while it followed 14 cm depth⩾ 17 cm depth⩾ 20 cm depth⩾ 23 cm depth in winter (One Way RM ANOVA, p > 0.05) (Fig. 3).
The diurnal photosynthetic performance of cultures grown at different depths over 12 h and performed four times was only conducted during the winter season and is summarized in Fig. 4. Fq'/Fm' and αETR values ranged between 0.68 – 0.74 and 0.22–0.27, respectively, and trended similarly in all ponds particularly from 9:30 am to 3:30 pm. These values began to decrease mainly after 9:30 am until midday (12:30). Subsequently, values began recovering and reach to their initial values at 6:30 pm. No significant difference was observed in Fq'/Fm' and αETR values for the cultures grown in 14 and 17 cm depths while values in 20 and 23 cm depths trended lower (One Way RM ANOVA P > 0.05). Overall, Fq'/Fm' and αETR values trended as 14 cm depth⩾17 cm depth⩾ 20 cm depth⩾ 23 cm depth.
12-h diurnal photosynthetic measurement of effective quantum yield (Fq'/Fm'), initial slope of rETR (αETR) and maximum relative electron transport rate (ETRmax) of cultures in all ponds during winter. 14 cm culture depth (empty triangle), 17 cm culture depth (filled triangle), 20 cm culture depth (empty circle), and 23 cm culture depth (filled circle). Data shown are the mean ± SE, n = 4
On the other hand, ETRmax values of all ponds began to increase immediately after sunrise (6:30 am) and reached their highest at 12:30 pm. ETRmax values stabilized after 12.30 pm for all treatment except for the 23 cm one, and began decreasing after 6.30 pm. No significant difference was observed among the ETRmax values of cultures at 14 and 17 cm depths as well as between 20 and 23 cm depths (14 cm depth = 17 cm depth > 20 cm depth = 23 cm depth) (One Way RM ANOVA, p > 0.05).
Discussion
The main advantage of microalgal phytoremediation is cost effectiveness of the process in taking up inorganic nutrients such as ammonia, nitrate and phosphate. However, for the process to be most efficient, it needs to be optimized. As highlighted previously, one of the potential operational variables is culture depth.
Algal cultures grown in ADAE at different depths in summer had higher productivity, yield, and nutrient removal rates when compared to the same in winter. This was expected due to higher availability of solar irradiance and higher ambient temperature in summer than winter (Sutherland et al 2014a). The solar irradiance recorded in summer (400 W m−2) was twice that than winter. As previously noted, light is the main limiting factor for any algal culture when temperature and nutrients are at optimum levels (Khan et al. 2018). Light intensity plays a core role in microalgal growth due to its direct impact on photosynthesis for carbon fixation (Zeng et al. 2021). Gomez-Villa et al. (2005) also showed that Scenedesmus cultures grown in wastewater in raceway ponds had 65% higher biomass production in summer compared to that in winter.
Volumetric productivity, cell concentration, and yield of cultures with 14 cm depth were at maximum in both winter and summer. Temperature and light availability are the most important factors typically affected by culture depth which could influence the performance of the system. In this study, no significant difference was found in the temperature of cultures grown at different depths. However, cells grown at lower depths on average had a greater exposure to light compared to those grown at higher depths (Table 2). Cultures with 14 cm depth have a lower culture volume than the cultures with a higher depth. Having a lower culture volume means that algal cells are exposed more frequently to sunlight due to the greater surface-to-volume ratio. Thus, light availability is higher for cells grown in these cultures. Furthermore, it has been reported that sunlight cannot penetrate an algal culture in raceway ponds when the depth is greater than 5 cm (Raeisossadati et al. 2019a). As highlighted above, ADAE was used as a culture medium for Scenedesmus cultures in this study. Considering those inherent properties of abattoir wastewater, an increase in cellular density of Scenedesmus sp. coupled with the turbidity of the effluent will most certainly reduce the light availability to the cells, thus restricting culture growth. Assuming that only the top 5 cm of algal cultures in raceway ponds can receive sunlight at a given time (Raeisossadati et al. 2019a), it is estimated that 65% of Scenedesmus cultures volume operated at 14 cm depth was in darkness while approximately 78% of cultures is in darkness at 23 cm depth. In line with our result, Kim et al. (2018) also found 65% decrease in volumetric productivity when HARP water depth increased by 10 cm, affecting light penetration into the microalgal culture. Similarly, Nwoba et al. (2020b) found lower volumetric productivity when Nannochloropsis sp. was grown in a closed photobioreactor with an optical pathlength of 10 cm vs. 1.3–5 cm used in similar studies. The larger light paths lead to light deficient zones in the photobioreactor, making it less productive (Nwoba et al. 2020b).
Fq'/Fm' is a light-adapted test that indicates the amount of energy used in photochemistry by photosystem II under steady-state photosynthetic lighting conditions and usually used as an indicator of stress level of algae cells (Cosgrove and Borowitzka 2006). The higher Fq'/Fm' values recorded for culture grown at lower depths in winter show that the open PSII reaction centers of microalgal cells grown at lower depths functioned more effectively in capturing energy from the sun (Masojídek et al. 2021). It also shows the improved physiological state of the cells which is due to higher available light in these ponds. On the other hand, excessive photon flux density in summer (around midday) was seen to negatively impact Fq'/Fm' values of ponds with lower depths. Microalgae are adapt to changes in light intensity through different mechanisms in order to protect their PSII (Morosinotto et al. 2022). The most common response to excess light irradiance is non-photochemical quenching (NPQ) which dissipates excessive light as heat, primarily by proteins of the family LHCSR/LHCX in microalgae (Ruban 2016). In addition, excess light triggers the production of zeaxanthin from violaxanthin by the enzyme violaxanthin de-epoxidase (VDE). Apart from enhancing NPQ, zeaxanthin also actively scavenges reactive oxygen species (ROS) produced by chlorophyll excited states interacting with oxygen molecules (Dall'Osto et al. 2012). The zeaxanthin pigment is converted back into violaxanthin by zeaxanthin epoxidase (ZE) as the incident irradiance decreases to increase light harvesting ability of microalgal cells (Morosinotto et al. 2022).
Sutherland et al. (2014b) reported significantly lower photosynthetic rate for microalgal culture grown at 20 cm compared to cultures grown at 30 and 40 cm depth at noon in summer. αETR is the slope of the linear portion of photosynthetic irradiance curve and represent efficiency of photosynthetic electron transfer (Malapascua et al. 2014). On the other hand, ETRmax shows the maximum flow of electrons through the photosystem II complex at a given time (Vadiveloo and Moheimani 2018). Diurnal pattern of αETR and Fq'/Fm' values for all treatments in winter was similar with the lowest values during the noon which is probably due to high temperature and too much solar irradiance. Conversely, maximum values of ETRmax near mid-day followed by declining in the afternoon is the typical response in various species (Behrenfeld et al. 2004; Yoshikawa and Furuya 2006). The highest ETRmax values recorded for cultures at 14 and 17 cm depths suggest improved photosynthesis of the Scenedesmus cells in lower depths which could be due to higher exposure of microalgal cells to light under these growth conditions.
The volumetric NH3-N removal rate of Scenedesmus cultures operated at 14 cm depth in winter and summer was about 10–12% higher than that of cultures with 23 cm depth. The results obtained for ammoniacal nitrogen removal rates (5- 9 mg L−1 day−1) and efficiencies (50- 85%) were comparable to that of the available literature. Previous studies also indicated that NH3-N removal rate ranged between 5.2 and 33 mg L−1 day−1 (69- 99%) can be achieved depending on the microalgal species, initial N concentration, cultivation system and condition (see Table 5 for the summary of the information). It must be noted that the removal of ammonia in microalgal cultures is either by direct ammonia uptake by microalgae or other confounding factors. For example, inherent nitrifying and denitrifying bacteria available in wastewater can also influence removal and conversion of ammonia (Bohutskyi et al. 2015). In a recent study, Ayre et al. (2021) demonstrated that microalgal-bacterial consortia can remove NH4+ and NO2− from AD piggery effluent, possibly through nitrification and denitrification and shift nitrogen pathways towards NO3− accumulation. Moreover, volatilization and stripping of ammonia represent another significant factor in open systems for the removal of ammonia (Zimmo et al. 2003; Shayesteh et al. 2021). For instance, due to lack of proper optimization, a significant amount of nitrogen was lost to the atmosphere when microalgae grown in AD piggery effluent with the initial N concentration of 800 mg L−1 (Ayre et al. 2017).
The mass balance results showed that the cultures at 14 cm depth had a significantly higher N and P assimilation rate than those at 23 cm depth in winter and summer. This can be explained by greater biomass productivity for cultures at lower depths compared to those at higher depths in both seasons. N assimilation rates for different culture depths (51- 83%) obtained here were higher than previously reported by De Godos et al. (2009) who found 35% of N removed in biomass in a raceway pond culture with a 50-day growth period and Raeisossadati and Moheimani (2020) who reported 6.4% for nitrogen assimilation in Scenedesmus biomass (Table 5). Between 56 and 80% of ADAE phosphorus was assimilated when Scenedesmus cultivated at different depths during both seasons. These results are greater than 15% phosphorus uptake of Chlorella sp. grown in undiluted ADAE supplemented with 1% CO2 under laboratory conditions (Vadiveloo et al. 2020 and Table 5). This shows the robustness of the isolated microalgae in treating AD wastewater as well as producing algal biomass. It is to be noted that algal cultures in ponds were harvested when cultures had reached stationary phase. The harvest and dilution regime were carried out to prevent culture from collapsing. Our aim has been to optimize the ADAE treatment while growing microalgae to achieve optimum biomass productivity. It is quite possible that the ADAE treatment process would require second stage treatment post algal harvest since the treated ADAE seems to still have a fair amount of N and P.
Although no significant difference was recorded for volumetric COD and phosphate removal rates between treatments during each season, these values were significantly higher in summer when compared to winter (more than twice). This was in accordance with higher biomass productivity in summer. The successful microalgae growth in ADAE with high COD (initial COD was 178 and 110 mg L−1 in winter and summer, respectively) indicated the ability of Scenedesmus to grow mixotrophically and utilize the energy of the organic carbon for their growth and biomass formation (Wang et al. 2012; Raeisossadati et al. 2019b). The COD removal rate obtained in the current study (around 30% in summer) is comparable to the findings of other research. Microalgal treatment of piggery effluents was reported to remove up to 39% of initial COD concentration (see Wang et al. 2012; Raeisossadati et al. 2019b and Table 5).
Based on the results of this study, cultures operated at 23 cm depth showed significantly higher areal productivity and nutrient removal rates due to over 40% more culture volume when compared to lower depths. Previous studies indicated that areal biomass productivity increases with increasing the culture depth (see Kumar et al. 2015; Chuka-ogwude et al. 2021 and Table 5). For instance, increasing the pond depth from 20 to 40 cm led to 134–200% increase in areal biomass productivity throughout the year when microalgae was used for treating domestic effluent (Sutherland et al. 2014b and Table 5). However, Béchet et al. (2016) found an 8% increase in annual areal productivity by decreasing the pond depth from 50 to 10 cm when growing Chlorella vulgaris in Mediterranean climate conditions. This is due to the fact that lower depths allow daytime temperatures to reach optimal levels for photosynthesis and causes the temperature to drop faster at night, which reduces respiration levels during night (Béchet et al. 2016). In another study, Moheimani and Borowitzka (2007) found the maximum areal productivity at 13 cm (lowest depth) in autumn and 21 cm (highest depth) in summer when growing Pleurochrysis carterae in a 1 m2 raceway pond (Table 5). Based the above findings, increasing the pond depth does not necessarily result in increasing areal productivity. This could be related to different factors like algal species, cell concentration, cultivation system, climate condition and type of medium (wastewater or synthetic medium). It would be beneficial to conduct further optimization studies examining other operational factors such as initial cell density, percentage and frequency of harvest to evaluate the effect of these variables on the overall process of biomass production and wastewater treatment.
Conclusion
Areal biomass productivity of Scenedesmus culture at 23 cm depth was 12% and 29% higher than the culture when operated at 14 cm depth in winter and summer, respectively. In addition, there was more than 30% higher areal nutrient removal rate for the culture operated at higher depth in both winter and summer. Based on the above, it can be concluded that the operation of wastewater-grown microalgae at higher depth (i.e., 23 cm) is seen to be more appropriate to fulfil the objectives of wastewater treatment and algal production in summer. However, in winter, 14 cm depth would be more appropriate as the due to the natural increase in pond volume due to increase in rainfall (overfilling the ponds) as well as significantly lower biomass yield in higher depths.
Data availability
Available upon request.
Change history
25 February 2023
Missing Open Access funding information has been added in the Funding Note.
References
Ayre JM, Moheimani NR, Borowitzka MA (2017) Growth of microalgae on undiluted anaerobic digestate of piggery effluent with high ammonium concentrations. Algal Res 24:218–226
Ayre JM, Mickan BS, Jenkins SN, Moheimani NR (2021) Batch cultivation of microalgae in anaerobic digestate exhibits functional changes in bacterial communities impacting nitrogen removal and wastewater treatment. Algal Res 57:102338
Béchet Q, Shilton A, Guieysse B (2016) Maximizing productivity and reducing environmental impacts of full-scale algal production through optimization of open pond depth and hydraulic retention time. Environ Sci Technol 50:4102–4110
Behrenfeld MJ, Prasil O, Babin M, Bruyant F (2004) In search of a physiological basis for covariations in light-limited and light-saturated photosynthesis. J Phycol 40:4–25
Bohutskyi P, Liu K, Nasr LK, Byers N, Rosenberg JN, Oyler GA, Betenbaugh MJ, Bouwer EJ (2015) Bioprospecting of microalgae for integrated biomass production and phytoremediation of unsterilized wastewater and anaerobic digestion centrate. Appl Microbiol Biotechnol 99:6139–6154
Borowitzka MA (2005) Culturing microalgae in outdoor ponds. In: Anderson RA (ed) Algal Culturing techniques. Elsevier Academic Press, London, pp 205–218
Bustillo-Lecompte CF, Mehrvar M (2015) Slaughterhouse wastewater characteristics, treatment, and management in the meat processing industry: A review on trends and advances. J Environ Manage 161:287–302
Chuka-ogwude D, Ogbonna J, Moheimani NR (2020) A review on microalgal culture to treat anaerobic digestate food waste effluent. Algal Res 47:101841
Chuka-ogwude D, Mickan BS, Ogbonna JC, Moheimani NR (2022) Developing food waste biorefinery: using optimized inclined thin layer pond to overcome constraints of microalgal biomass production on food waste digestate. J Appl Phycol 34:2917–2928
Chuka-ogwude D, Nafisi M, Vadiveloo A, Taher H, Bahri PA, Moheimani NR (2021) Effect of medium recycling, culture depth, and mixing duration on D. salina growth. Algal Res 60:102495
Cosgrove J, Borowitzka M (2006) Applying pulse amplitude modulation (PAM) fluorometry to microalgae suspensions: stirring potentially impacts fluorescence. Photosynth Res 88:343–350
Dall’Osto L, Holt NE, Kaligotla S, Fuciman M, Cazzaniga S, Carbonera D, Frank HA, Alric J, Bassi R (2012) Zeaxanthin protects plant photosynthesis by modulating chlorophyll triplet yield in specific light-harvesting antenna subunits. J Biol Chem 287:41820–41834
de Godos I, Blanco S, García-Encina PA, Becares E, Muñoz R (2009) Long-term operation of high rate algal ponds for the bioremediation of piggery wastewaters at high loading rates. Bioresour Technol 100:4332–4339
de Godos I, Vargas VA, Blanco S, González MCG, Soto R, García-Encina PA, Becares E, Muñoz R (2010) A comparative evaluation of microalgae for the degradation of piggery wastewater under photosynthetic oxygenation. Bioresour Technol 101:5150–5158
Gomez-Villa H, Voltolina D, Nieves M, Pina P (2005) Biomass production and nutrient budget in outdoor cultures of Scenedesmus obliquus (Chlorophyceae) in artificial wastewater, under the winter and summer conditions of Mazatlan, Sinaloa, Mexico. Vie et Milieu:121–126
Grobbelaar JU (2010) Microalgal biomass production: challenges and realities. Photosynth Res 106:135–144
Hakawati R, Smyth BM, McCullough G, De Rosa F, Rooney D (2017) What is the most energy efficient route for biogas utilization: heat, electricity or transport? Appl Energy 206:1076–1087
Jensen PD, Sullivan T, Carney C, Batstone DJ (2014) Analysis of the potential to recover energy and nutrient resources from cattle slaughterhouses in Australia by employing anaerobic digestion. Appl Energy 136:23–31
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact 17:1–21
Kim H-C, Choi WJ, Ryu JH, Maeng SK, Kim HS, Lee B-C, Song KG (2014) Optimizing cultivation strategies for robust algal growth and consequent removal of inorganic nutrients in pretreated livestock effluent. Appl Biochem Biotechnol 174:1668–1682
Kim B-H, Choi J-E, Cho K, Kang Z, Ramanan R, Moon D-G, Kim H-S (2018) Influence of water depth on microalgal production, biomass harvest, and energy consumption in high rate algal pond using municipal wastewater. J Microbiol Biotechnol 28:630–637
Kumar K, Mishra SK, Shrivastav A, Park MS, Yang J-W (2015) Recent trends in the mass cultivation of algae in raceway ponds. Renew Sust Energy Rev 51:875–885
Malapascua JR, Jerez CG, Sergejevová M, Figueroa FL, Masojídek J (2014) Photosynthesis monitoring to optimize growth of microalgal mass cultures: application of chlorophyll fluorescence techniques. Aquat Biol 22:123–140
Masojídek J, Ranglová K, Lakatos GE, Silva Benavides AM, Torzillo G (2021) Variables governing photosynthesis and growth in microalgae mass cultures. Processes 9:820
Matos AP, Vadiveloo A, Bahri PA, Moheimani NR (2021) Anaerobic digestate abattoir effluent (ADAE), a suitable source of nutrients for Arthrospira platensis cultivation. Algal Res 54:102216
Moheimani NR, Borowitzka MA (2007) Limits to productivity of the alga Pleurochrysis carterae (Haptophyta) grown in outdoor raceway ponds. Biotechnol Bioeng 96:27–36
Moheimani NR, Borowitzka MA, Isdepsky A, Fon Sing S (2013) Standard methods for measuring growth of algae and their composition. In: Borowitzka MA, Moheimani NR (eds) Algae for Biofuels and Energy. Springer, Dordrecht, pp 265–284
Morosinotto T, Perin G, Gambaro F (2022) Knowledge of regulation of photosynthesis in outdoor microalgae cultures is essential for the optimization of biomass productivity. Front Plant Sci :751
Nwoba EG, Ayre JM, Moheimani NR, Ubi BE, Ogbonna JC (2016) Growth comparison of microalgae in tubular photobioreactor and open pond for treating anaerobic digestion piggery effluent. Algal Res 17:268–276
Nwoba EG, Parlevliet DA, Laird DW, Alameh K, Moheimani NR (2020a) Outdoor phycocyanin production in a standalone thermally-insulated photobioreactor. Bioresour Technol 315:123865
Nwoba EG, Parlevliet DA, Laird DW, Alameh K, Moheimani NR (2020b) Pilot-scale self-cooling microalgal closed photobioreactor for biomass production and electricity generation. Algal Res 45:101731
Park J, Jin H-F, Lim B-R, Park K-Y, Lee K (2010) Ammonia removal from anaerobic digestion effluent of livestock waste using green alga Scenedesmus sp. Bioresour Technol 101:8649–8657
Park J, Craggs R, Shilton A (2011) Wastewater treatment high rate algal ponds for biofuel production. Bioresour Technol 102:35–42
Raeisossadati M, Moheimani NR (2020) Can luminescent solar concentrators increase microalgal growth on anaerobically digested food effluent? J Appl Phycol 32:3703–3710
Raeisossadati M, Moheimani NR, Parlevliet D (2019a) Luminescent solar concentrator panels for increasing the efficiency of mass microalgal production. Renew Sust Energy Rev 101:47–59
Raeisossadati M, Vadiveloo A, Bahri PA, Parlevliet D, Moheimani NR (2019b) Treating anaerobically digested piggery effluent (ADPE) using microalgae in thin layer reactor and raceway pond. J Appl Phycol 31:2311–2319
Rayen F, Behnam T, Dominique P (2019) Optimization of a raceway pond system for wastewater treatment: a review. Crit Rev Biotech 39:422–435
Ruban AV (2016) Nonphotochemical chlorophyll fluorescence quenching: mechanism and effectiveness in protecting plants from photodamage. Plant Physioy 170:1903–1916
Shayesteh H, Vadiveloo A, Bahri PA, Moheimani NR (2021) Can CO2 addition improve the tertiary treatment of anaerobically digested abattoir effluent (ADAE) by Scenedesmus sp.(Chlorophyta)? Algal Res 58:102379
Sutherland DL, Howard-Williams C, Turnbull MH, Broady PA, Craggs RJ (2014a) Seasonal variation in light utilisation, biomass production and nutrient removal by wastewater microalgae in a full-scale high-rate algal pond. J Appl Phycol 26:1317–1329
Sutherland DL, Turnbull MH, Craggs RJ (2014b) Increased pond depth improves algal productivity and nutrient removal in wastewater treatment high rate algal ponds. Water Res 53:271–281
Vadiveloo A, Moheimani N (2018) Effect of continuous and daytime mixing on Nannochloropsis growth in raceway ponds. Algal Res 33:190–196
Vadiveloo A, Shayesteh H, Bahri PA, Moheimani NR (2022) Comparison between continuous and daytime mixing for the treatment of raw anaerobically digested abattoir effluent (ADAE) and microalgae production in open raceway ponds. Bioresour Technol Rep 17:100981
Vadiveloo A, Matos AP, Chaudry S, Bahri PA, Moheimani NR (2020) Effect of CO2 addition on treating anaerobically digested abattoir effluent (ADAE) using Chlorella sp.(Trebouxiophyceae). J CO2 Utilization 38:273–281
Wang L, Li Y, Chen P, Min M, Chen Y, Zhu J, Ruan RR (2010) Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour Technol 101:2623–2628
Wang H, Xiong H, Hui Z, Zeng X (2012) Mixotrophic cultivation of Chlorella pyrenoidosa with diluted primary piggery wastewater to produce lipids. Bioresour Technol 104:215–220
Wang Y, Guo W, Yen H-W, Ho S-H, Lo Y-C, Cheng C-L, Ren N, Chang J-S (2015) Cultivation of Chlorella vulgaris JSC-6 with swine wastewater for simultaneous nutrient/COD removal and carbohydrate production. Bioresour Technol 198:619–625
Xia A, Murphy JD (2016) Microalgal cultivation in treating liquid digestate from biogas systems. Trends Biotech 34:264–275
Yoshikawa T, Furuya K (2006) Effects of diurnal variations in phytoplankton photosynthesis obtained from natural fluorescence. Mar Biol 150:299–311
Zeng J, Wang Z, Chen G (2021) Biological characteristics of energy conversion in carbon fixation by microalgae. Renew Sust Energy Rev 152:111661
Zimmo O, Van Der Steen N, Gijzen HJ (2003) Comparison of ammonia volatilisation rates in algae and duckweed-based waste stabilisation ponds treating domestic wastewater. Water Res 37:4587–4594
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This Wastes to Profits project was supported by Meat and Livestock Australia through funding from the Australian Government Department of Agriculture, Water and the Environment as part of its Rural R&D for Profit program and project partners.
Author information
Authors and Affiliations
Contributions
Shayesteh: Conceptualization and design of experiment; Data analysis and curation; Primary Investigation; Roles/Writing – original draft; Writing – review & editing.
Raeisossadati: Investigation, Writing – review & editing.
Vadiveloo: Conceptualization and design of experiment; Investigation, Supervision; Writing – review & editing.
Bahri: Funding acquisition; Resources; Supervision; Methodology; Validation; Analysis & Discussion; Writing – review & editing.
Moheimani: Conceptualization and design of experiment; Funding acquisition; Investigation; Methodology; Resources; Supervision; Writing – review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no potential financial or other interests are perceived to influence the outcomes of this research.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shayesteh, H., Raeisossadati, M., Vadiveloo, A. et al. Culture depth effect on Scenedesmus sp. growth, photo-physiology and nutrient removal rate in anaerobically digested abattoir effluent. J Appl Phycol 35, 567–580 (2023). https://doi.org/10.1007/s10811-023-02915-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-02915-2