Abstract
Methane is an extremely potent yet short-lived greenhouse gas and is thus recognized as a promising target for rapid climate change mitigation. About 35% of anthropogenic methane emissions are associated with livestock production, and most of these emissions are the outcome of enteric fermentation in ruminant animals. The red seaweed Asparagopsis is currently considered the most efficient feed additive to suppress methane emissions from enteric fermentation but is not currently available on commercial scale. The ongoing effort to successfully commercialize Asparagopsis requires the development of pest control frameworks which rely on the quantitative assessment of biological contamination in cultures. Here we present a low-cost readily available approach for quantifying biofouling in Asparagopsis taxiformis cultures based on microscopic examination and automated image analysis. The proposed methodology is demonstrated to estimate contamination associated with Asparagopsis biomass with error rates lower than 2% over a wide range of contamination levels and contaminating organisms, while significantly cutting down image processing time and allowing for frequent contamination quantification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methane is a greenhouse gas that is 80 times more potent than carbon dioxide gas in terms of its warming potential over a 20-year time horizon (IPCC 2014). Atmospheric methane concentration has more than doubled since pre-industrial times (Dlugokencky 2022) due in large part to anthropogenic activities. However, methane has a relatively short lifespan in the atmosphere (~ 12 years) and is mainly produced by specific industrial and agricultural sectors, making it a high priority for global greenhouse gas mitigation (Ming et al. 2022). About 35% of anthropogenic methane emissions are associated with livestock production, most of which are the outcome of enteric fermentation in ruminant animals (Eisen and Brown 2022). The red algal genus Asparagopsis when fed in small quantities has been shown to be highly effective at reducing methane emissions associated with enteric fermentation (up to 90%). Results have been consistent both in vitro and in live ruminant trials (Kinley et al. 2016, 2020; Min et al. 2021; Roque et al. 2021), yielding no apparent trade-offs in product quality, animal production or any negative environmental effects (Glasson et al. 2022). However, to date, the Asparagopsis used in these trials has been primarily wild-harvested and as such it is not commercially available (Nin-Pratt et al. 2022). As research with ruminant animals continues there is a need to better understand this seaweed with particular focus on developing efficient and sustainable approaches for cultivation.
As is true with the domestication of most wild organisms, developing a productive and scalable cultivation system can be challenging. Developing Asparagopsis production systems has been challenging due to the alga’s size and unique morphology. The genus is known for having a heteromorphic triphasic alternation of generations life cycle including a macroscopic haploid gametophyte, an attached diploid carposporophyte and filamentous diploid tetrasporophyte. Current efforts to develop land-based cultivation systems have been largely focused on the filamentous tetrasporophyte stage. When grown in a tumble culture, the filaments, with diameter of up to 0.05 mm and length of up to several cm, usually take on a “puffball” shape with diameter of roughly 0.5–5 cm (Fig. 1). While the development of cultivation techniques for both microalgae and macroalgae has made great progress in the past decades, the intermediate size and filamentous nature of Asparagopsis tetrasporophytes dictates a hybrid “micro–macro” approach calling for the adoption and adaptation of tools from both industries, as well as the development of new Asparagopsis specific technologies. Together with cultivation optimization and scaling up of systems, overcoming or controlling biological contamination is of the utmost importance for the successful development of a commercial industry for Asparagopsis.
Asparagopsis taxiformis filamentous tetrasporophyte—“Falkenbergia” phase. Representative tumble culture of A. taxiformis tetrasporophytes at (a) 200X and (b) 25X light microscopy, and (c) its appearance in a 500 mL flask. Branches are built of tiers of 3 pericentral cells with a total diameter of ~ 15–50 μm. While individual branches are quite small in diameter, branch length can exceed ~ 5 cm and weave together to form “puffballs”. Scale bars = 0.2 mm
In aquaculture, biological contamination is defined as the invasion of cultures by any organism other than the target species. In the context of algal aquaculture, contaminating organisms may have adverse effects on the algae either by directly damaging the target species, by feeding on them, or by competing for resources (such as light and/or nutrients), subsequently impacting target species or products.
Contamination of algae cultures may be caused by many organisms, including competing algae (e.g. Diatoms: Shea et al. 2006, Winckelmann et al. 2016; cyanobacteria: Meseck 2007; and green algae: Grivalský et al. 2021, Behera et al. 2022), epiphytes (Borburema et al. 2021; Behera et al. 2022), fungi and bacteria (Fott 1967; Berland et al. 1972; Gutman et al. 2009; Carney and Lane 2014; Fulbright et al. 2018), and zooplankton grazers (mainly ciliates, rotifers, copepods, amoeba, and dinoflagellates: Deore et al. 2020; Di Caprio 2020). Biological contamination of both micro and macroalgal cultures is one of the greatest challenges for efficient, reliable and scalable production of algal biomass (Fernandes et al. 2011; Deore et al. 2020; Zhu et al. 2020; Behera et al. 2022). To ensure effective management of algae cultures, frequent assessments are required for the early detection of contaminants thus allowing for early action to prevent culture collapse (Zhu et al. 2020). Further, because contamination is inevitable at some level in large-scale algae production, it is valuable to have a protocol to quantitatively assess the extent of contamination (rather than just monitoring the presence/absence of contaminants) to track trends over time and develop management strategies. Despite the enormous significance of this problem in aquaculture there are no standardized approaches for quantifying or tracking contaminating organisms in culture and as such there is a lack of quantitative information on this issue (Di Caprio 2020).
Current approaches for assessing the level of biological contamination in algal cultures have mostly focused on microalgae and include the following: Plate counting is used to quantify bacterial populations by counting Colony Forming Units (CFU) based on their growth on agar plates. This approach is labor intensive and is biased toward bacterial species that can grow on the specific medium chosen. Furthermore, this method is dependent on culturing bacteria for a few days, during which contamination may take over the culture and make contamination mitigation more difficult. Flow-through microscopy may be used to count and identify microorganisms continuously. Automated systems (e.g. the FlowCam, Yokogawa Fluid Imaging Technologies, USA), are available to characterize microplankton (by their size, shape, or fluorescence) in the size range of 20–200 μm, and have been shown to be effective in quantifying grazers in microalgae cultures (Sieracki et al. 1998; Day et al. 2012; Havlik et al. 2013). While being capable of continuously monitoring various contaminants, flow-through microscopy is relatively costly and is not applicable for larger macroalgal cultures that are too large for the measurement zone. Molecular-based methods are being used to identify contaminating organisms in aquaculture systems across scales. These methods are usually based on the PCR amplification of specific markers and are sensitive and becoming increasingly available. However, these tools rely to some extent on prior knowledge of contaminating organisms and are limited to identifying organisms that have previously been sequenced (Deore et al. 2020). Detection of specific metabolites may be used to monitor the onset and progress of specific biochemical processes within cultures. Assuming a metabolite is unique to the contaminant and does not vary in concentration (grams metabolite per gram of contaminant), taxon-specific metabolites, such as pigments or toxins, can be measured in cultures as a proxy for the level of contamination (Di Caprio 2020). However, careful attention is needed when picking these metabolites as their concentrations can be significantly affected by changes in growth conditions (e.g., Grossman et al. 1993). Optical characteristics of a culture can also be used as indicators of contamination. This approach is especially promising since optical sensors are non-invasive, fast, robust with no moving parts, and rather inexpensive compared to some non-optical methods (Havlik et al. 2022), and has the potential to monitor aspects of contamination in a real-time, continuous manner. However, spectrometers (at their various kinds and specificities) are still not available in most aquaculture facilities, and their use may face some challenges due the heterogeneity of macroalgae cultures (as opposed to fairly homogeneous microalgae suspensions). Microscopic inspection. Usually involves counting contaminants in a given water volume using a Hemocytometer or Sedgwick Rafter counting chamber. Counting can be done manually or can be automated with image analysis software in the case of well-defined contaminants (e.g., spherical cells with a fairly uniform size and color). Contaminant identification can be further assisted by using various selective staining techniques (Carney and Lane 2014). Generally, microscopy is laborious and reliant on the observer's skill and experience, and it has been shown to underestimate the number of algae grazers found in cultures (Day et al. 2012; Deore et al. 2020). The time intensive nature of this approach limits the intervals of sampling for screening efforts, which may hamper proper early detection of contamination. However, a significant decrease in labor and processing time may be achieved with the automation of image analysis. Microscopic examination may be focused either on suspended particles or on particles associated with the algae biomass. Specifically for A. taxiformis tetrasporophyte cultures, the focus of contamination quantification should be on epiphytic communities rather than on suspended contaminants, dictating the quantification of contamination per unit of algae rather than per water volume. Furthermore, because of the diversity in shape and size of contaminating organisms, counting individuals is not an appropriate metric. Rather, quantification of area or the proportion of biomass that is fouled is more valuable. Contamination in A. taxiformis cultures has been noted in previous studies (e.g. (Schuenhoff et al. 2006; Mata 2008)) but has not been effectively quantified. The quantification of biological contamination in Asparagopsis tertrasporophyte cultures may use tools and techniques from the above descriptions but will require some fundamental adaptations due to the filamentous nature of this alga. In this paper, we describe a low-cost contamination quantification methodology specific for Asparagopsis tetrasporophyte cultures, based on an automated image analysis of microscope slide photos. While these techniques were developed specifically for Asparagopsis tetrasporophyte cultures they could be applied to any filamentous algal cultivation setting.
Methods
Biological material
A number of individual cultures of A. taxiformis tetrasporophytes were used to develop a contamination quantification protocol. Specifically, samples were collected from a number of southern California locations, and standard approaches were used to isolate and purify samples as best as possible (based on Kawai et al. (2005)). Despite these efforts, occasional contaminants would find their way into cultures resulting in various contamination levels in the cultures used for quantification purposes.
Cultures were grown in various environmental conditions and contained a wide range of contaminants. Indoor lab cultures were grown in natural seawater filtered to 0.2 μm and autoclaved and were supplemented with commercially available F/2 solutions (Proline F/2 Algae Food, Pentair Aquatic Eco-Systems, Inc., USA) to bring major and minor nutrients to around half-strength F/2 levels (by adding 75 μL L−1 from each part of the ‘Algae Food’ product). Cultures were constantly stirred and aerated by bubbling air, were held at ~ 21 °C, and were illuminated with 50–100 μmol photons m−2 s−1 PAR at 12:12 h light: dark cycles. Additional samples were taken from cultivation experiments to include algae originally collected at various Southern California locations and cultivated under different light conditions (ranging 50–250 μmol photons m−2 s−1 PAR). In order to further increase the range of environmental conditions and contaminants, outdoor cultures, grown at the Scripps outdoor aquarium facility, fed by natural seawater, and exposed to natural variation in environmental conditions, were sampled as well.
Sample preparation for microscopic examination
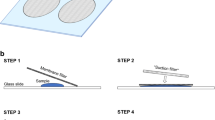
A small amount of the tetrasporophyte stage of A. taxiformis was placed in a petri dish in sterile seawater, and a small amount was then transferred using fine-tipped forceps to a standard glass microscope slide. The sample was then viewed under a stereomicroscope and was spread out to ensure that there were very few overlapping branches and no prominent clumps. The amount of A. taxiformis on the slide was adjusted until it covered slightly less than the area of a coverslip. The cover slip was gently placed on top of the algae with a drop of seawater, and gently depressed to remove excess water and push out any bubbles. Photographs were taken with Olympus SZX16 microscope at 25 × magnification and bright field background using standardized capture settings (in Infinity Capture software: R: 1.85, G: 1.65, B: 2.10, Gain: 1.24, Exposure: 13). 4 to 5 photos were taken per slide, making sure that there was no overlapping area between the photos.
Image analysis to quantify contamination
In order to quantify the contamination associated with A. taxiformis biomass, two parameters were measured per microscope slide photo: (1) the total area of all algal biomass, including both A. taxiformis and contaminants, and (2) contamination area. These two parameters were used to calculate the contamination percentage per photo (Fig. 2) as Contamination % = Contamination Area / Total Area X 100. All image analyses were conducted with ImageJ (version 1.53b) primarily through an automated procedure, based on color threshold selections for the areas of interest. The automation of this procedure was achieved using a macro script consisting of three adjustable elements: color space, individual color channels, and particle size. The total area of A. taxiformis and associated contaminants in each photo required the use of the HSB color space. To quantify contamination alone, either RGB or LAB was selected as the color space, depending on the type of contaminants present in a sample. The RGB color space was used primarily to detect green contaminants, while the LAB color space was used primarily to detect contaminants with dark green or dark brown hues. The values for the individual color channels (R, G, and B for the RGB color space; L, A, and B for the LAB color space), each ranging between 0 and 255, could be adjusted to selectively target the contaminants while disregarding the A. taxiformis branches. Lastly, we adjusted the minimal particle size according to the nature and size of the contaminants, to avoid the unintended selection of small non-contaminant particles. Individual images could be analyzed by opening an image in the ImageJ program and running the macro script of interest (Plugins > Macros > Edit). Alternatively, an automated process, hereafter referred to as the batch process, could be used to run the macro script on all images within a single dataset (Process > Batch > Macro), so long as the macro script could be used to consistently and accurately quantify the contamination in all the images of a given dataset (Full description can be found in Online Resource 1).
Illustration of the image analysis procedure. (a) Samples were taken from A. taxiformis sporophyte cultures and photographed on a microscope slide. (b) The total biomass area (including A. taxiformis as well as contaminant area) was selected and quantified based on brightness differences from the background. (c) Contamination area was selected and quantified based on RGB differences from A. taxiformis. Contamination % = contamination area / Total area × 100
Quantification of the total area of A. taxiformis and associated contaminants in each photo was achieved through a single macro script (based mostly on brightness differences between biomass and background) across all datasets, given that the sample preparation and microscope settings for each dataset were consistent enough to yield similar color tones among all images. To quantify contamination, however, each dataset (i.e. set of photos prepared and photographed using similar settings and containing similar contamination patterns) typically required some adaptations to the macro script to accurately highlight the contaminants. A full description of the macro scripts as well as the specific thresholds used in this study can be found in Online Resource 1.
To tune the macro script for a specific dataset before running the automated batch process, around 5 representative images, ranging in different contamination levels and occasionally different contamination types, were used to determine the most optimal quantification settings. Ideal settings aim to maximize the selection of the contamination present while minimizing selection of areas consisting of non-contamination components. Based on these representative images, the elements of the macro script were optimally adjusted. After testing the macro script on these representative images, the batch process was used to quantify contamination in all the images from a single dataset (e.g., contained in a single folder). Output images of the selected area for each sample were stored in a separate folder. After running the batch process, each output image was compared to its original image to evaluate whether the contamination was accurately selected. This last step was taken while developing the protocol, to verify the success of the batch process in accurately capturing contamination and gaining confidence in its reliability, and is not necessary for all future analyses.
Image analysis validation
In order to quantitatively validate the above workflow, contamination areas were traced manually using ImageJ selection tools in 100 microscope photos taken at various occasions. Contaminants were mostly green microalgae and filamentous cyanobacteria. The image analysis-based quantification was then applied to those 100 photos and correlated with the manual tracing results (Fig. 3). The prediction error was calculated as the Standard Error of Estimates (SEE) following Eq. (1).
where Y—Y’ is the difference between the predicted and actual contamination value and N is the number of samples.
Manual validation of ImageJ contamination quantification. Contamination percentage results were correlated with manual contamination tracing for 100 microscope slide photos taken by various users across various culture conditions and contamination types. Symbols represent different datasets (specified in Online Resource 3). Blue and red lines represent the 95% confidence and prediction intervals respectively. N = 100 Standard Error of Estimates (SEE) = 1.43%
Assessing sample size required for contamination quantification from microscope photos
A power analysis was run to determine the minimum number of microscope photographs needed to accurately predict the concentration of contamination within a culture of A. taxiformis. To run the analysis, four cultures, with varying types and amounts of contamination, were analyzed. These cultures included (I) one with a minimal amount of filamentous cyanobacteria, (II) one with a competitive green alga, (III) one with an encrusting unicellular green alga, and (IV) a final culture with a high concentration of diatoms. For each culture, 10 slides were prepared, and 5 microscope photos were taken per slide for a total of 50 microscopic photos per culture. The image capture and contamination quantification analysis were completed using the protocol described above.
The contamination quantification of the 50 samples from each culture were used to determine the accuracy of the sample mean as it compares to the culture mean for sample sizes ranging from 3 to 49 measurements. The average percent contamination of the 50 samples was used to represent the population mean of the culture. For each sample size, samples were randomly selected using the sample() function in R and the average % contamination of the samples was calculated. Each sample size was re-sampled 10,000 times and the standard deviation of the sample means were calculated to show the variability in the sample mean from the culture mean (Table 1).
Results
Overall, 280 microscope slide photos were analyzed to quantify contamination levels associated with A. taxiformis tetrasporophyte tissue. These samples, collected from cultures of various origins and cultivated under a wide variety of culture conditions, contained mostly three groups of contaminants: (1) Cyanobacteria, mostly filamentous, forming dark tangled matts within A. taxiformis branches, (2) Green microalgae, mostly unicellular, growing attached (encrusting) on A. taxiformis branches to various levels, and (3) Diatoms, mainly attached to A. taxiformis branches as individuals or colonies. While sampling for this analysis, red algae contaminants (rarely present but in some cultures) were avoided due to difficulties in color-based separation. Batch processing of microscope photos for contamination was efficient and required only minor changes in macro script parameters (Online Resource 2) to be adjusted between different contaminants.
100 samples were used to validate the automated contamination quantification results in comparison to manual tracing in microscope slide photos. Due to the small size and complex shape of many diatom species, it was not feasible to manually trace them accurately, so validation was only conducted for green algae and cyanobacteria. There was a strong correlation between the automated contamination quantification and manual tracing (R2 = 0.93) with an error of ± 1.43% (Standard Error of Estimation) over a wide range of contamination levels ranging from 0–22%, including photos taken across various cultures and at various occasions (Fig. 3).
The number of microscope photos required to accurately determine contamination levels per culture was assessed for four cultures using a power analysis. The accuracy of the sample size, as it relates to variability from the culture mean, was found to be dependent on the concentration and patchiness of the contamination, with patchiness referring to the extent to which the contamination was homogenous throughout the culture. Level of patchiness was determined by taking the standard deviation of the 50 samples. The contamination pattern in these four cultures differed in both level of concentration and patchiness from less than 1% contamination and inherently low patchiness (indicated by low STDV levels; 0.38% STDV) for culture (I), to almost 40% contamination concentrated in diatom clumps causing high patchiness (8.6% STDV) for culture (IV). Thus, the accuracy of each sample size varied between the cultures. (Fig. 4). If an accuracy of 1% (calculated as standard deviation from the mean) is sought, a sample size of 3 is required for a culture with low concentration, low patchiness (II), while a sample size of 10 is required for a culture with high contamination low patchiness (III) and a sample size of 30 is required for a culture with high contamination, high patchiness (IV) (Fig. 4).
A) Sample size power assessment of standard deviations associated with the number of photos analyzed for contamination quantification in various A. taxiformis cultures. The different lines represent the types of contamination found in the cultures and represent different contamination and patchiness levels (I to IV from low to high patchiness). (b) Microscope photos (25X magnification) on the bottom demonstrate the appearance of slides for each dataset assigned with contamination-patchiness categories (I-IV), the average contamination percentage, and standard deviation of the 50 samples
Discussion
In aquaculture, most conventional contamination assessments have been developed for microalgae cultures and have largely focused on contaminants suspended in culture media. With a growing interest in macroalgal cultivation, especially for the red alga Asparagopsis taxiformis as a methane mitigating supplement for livestock, there is an urgent need to develop approaches that specifically target contaminating organisms associated with this species. The biological contamination of Asparagopsis poses a significant challenge in achieving reliable large-scale production and should not be overlooked. Realizing that at full scale production contamination needs to be controlled rather than eliminated, quantitative assessments of contamination are a prerequisite to developing mitigation tools and pest control frameworks. The microscopic examination procedure shown here has been demonstrated to be applicable for reliably quantifying contaminants associated with A. taxiformis tetrasporophyte biomass, yielding error rates lower than 2%. While sample preparation for microscopic examination is still time consuming (estimated time for slide preparation and photographing is around three minutes per slide) and may not be carried out as part of continuous in-line monitoring of cultures, the automated image analysis described here allows for batch processing of hundreds of microscope photos in minutes, and may be used to quantitatively assess contamination in small scale experiments as well as in commercial scale systems.
Overall, we identify three main limitations for our proposed technique, namely (1) the need for discrete sampling (rather than automated sampling) and the time required for microscope slide preparation, (2) the limited success in quantifying non-target red algae (when their color is too similar to Asparagopsis in culture), and (3) the possible error due to heterogeneity in contamination through cultures (relative to homogenous microalgae cultures) which requires increased sampling efforts.
Despite the limitations, our approach allows for a consistent and comprehensive quantitative contamination characterization of algal cultures across scales, from wild-collected specimens, to lab cultures, to full-scale production. Such data are needed for the development of a data-driven approach for contamination control. This low-cost contamination quantification tool requires merely a microscope with camera and a computer running the ImageJ software (accessible for free at https://imagej.nih.gov/ij/) and can therefore be instantly implemented at most algae research and farming facilities. We urge researchers and cultivators to further investigate and develop more efficient and accurate tools, either within the ImageJ framework described here, or using any other approach to improve contamination quantification tools in Asparagopsis cultivation. Such tools will be critically important for informing the successful development of commercial scale Asparagopsis farming in order to be available as cattle feed for methane mitigation worldwide.
Ultimately, a complete assessment of contamination in Asparagopsis culture should include the identification and quantification of contaminants in a continuous or frequent manner. An accurate identification of contaminants might be especially important in the case of attempts to control microbial populations towards specific beneficial microbial assemblages. The highest level of Identification of contaminants may be achieved with PCR-based techniques. These techniques are currently out of reach for many cultivators but are constantly improving and expected to become more readily available, both for qualitative and quantitative assessment of contaminants. A particularly promising technology is the relatively fast and low-cost long-read nanopore sequencing, demonstrated by van der Loos et al. (2021) to efficiently characterize algal microbiomes. Alternatively, outsourcing these analyses will remove the need for farm-scale molecular equipment and expertise as the price and turnover time for molecular tests decreases in the future. Nevertheless, this approach will probably not provide continuous monitoring of contamination state.
Continuous monitoring of contamination can probably be achieved, at various levels of accuracy, using optical measurements and Artificial Intelligence (AI) data processing. The digital output acquired directly from an optical sensor may be used for inline monitoring of contamination and automatic response by regulation of culture medium or environmental conditions. Recent developments in this field include the promising use of Low-Resolution Raman Spectroscopy (supported by AI algorithms) for the quantification and identification of contaminants (Adejimi et al. 2022), the assessment of contamination of large-scale ponds by reflectance measurements (Reichardt et al. 2020), and the use of spectral absorption together with artificial neural network (ANN) for data processing (Franco et al. 2019). Continuous data acquisition may be also achieved using the “sniffer” approach based on CIMS sensing of airborne metabolites (Sauer et al. 2021), or in situ microscopic imaging systems complemented with AI-based algorithms (Orenstein et al. 2020).
While we expect to see significant technological advancements in contamination quantification, making pest management possible for the emerging Asparagopsis industry, these innovative technologies are not expected to become fully operational in the next couple of years, leaving an urgent need for the interim and readily replicable approach described here. The image analysis-based approach detailed here is demonstrated to cut down processing time while retaining ~ 1% error for most contaminated cultures and may fill the gap until more robust technologies are available.
Data availability
Data for methods validation are available in the Online Supplementary Information.
Code availability
ImageJ macro scripts are available in the Online Supplementary Information.
References
Adejimi OE, Ignat T, Sadhasivam G, Zakin V, Schmilovitch ZE, Shapiro OH (2022) Low-Resolution Raman Spectroscopy for the detection of contaminant species in algal bioreactors. Sci Total Environ 809:151138
Behera DP, Ingle KN, Mathew DE, Dhimmar A, Sahastrabudhe H, Sahu SK, Krishnan MG, Shinde PB, Ganesan M, Mantri VA (2022) Epiphytism, diseases and grazing in seaweed aquaculture: A comprehensive review. Rev Aquacult 14:1345–1370
Berland BR, Bonin DJ, Maestrini SY (1972) Are some bacteria toxic for marine algae? Mar Biol 12:189–193
Borburema HD dos S, Barbosa ÊN de A, Miranda GEC de (2021) Decontamination protocol of the macroalga Bostrychia binderi Harvey (Rhodophyta) for unialgal cultures and laboratory studies. Hoehnea 48:e582020
Carney LT, Lane TW (2014) Parasites in algae mass culture. Front Microbiol 5:278
Day JG, Thomas NJ, Achilles-Day UEM, Leakey RJG (2012) Early detection of protozoan grazers in algal biofuel cultures. Bioresour Technol 114:715–719
Deore P, Beardall J, Noronha S (2020) A perspective on the current status of approaches for early detection of microalgal grazing. J Appl Phycol 32:3723–3733
Di Caprio F (2020) Methods to quantify biological contaminants in microalgae cultures. Algal Res 49:101943
Dlugokencky E (2022) Global Monitoring Laboratory - Carbon Cycle Greenhouse Gases. In: NOAA/GML. https://gml.noaa.gov/ccgg/trends_ch4/. Accessed 23 May 2022
Eisen MB, Brown PO (2022) Rapid global phaseout of animal agriculture has the potential to stabilize greenhouse gas levels for 30 years and offset 68 percent of CO2 emissions this century. PLoS Clim 1:e0000010
Fernandes DRP, Yokoya NS, Yoneshigue-Valentin Y (2011) Protocol for seaweed decontamination to isolate unialgal cultures. Rev Bras Farmacogn 21:313–316
Fott B (1967) Phlyctidium scenedesmi spec. nova, a new chytrid destroying mass cultures of algae. Z Allg Mikrobiol 7:97–102
Franco BM, Navas LM, Gómez C, Sepúlveda C, Acién FG (2019) Monoalgal and mixed algal cultures discrimination by using an artificial neural network. Algal Res 38:101419
Fulbright SP, Robbins-Pianka A, Berg-Lyons D, Knight R, Reardon KF, Chisholm ST (2018) Bacterial community changes in an industrial algae production system. Algal Res 31:147–156
Glasson CR, Kinley RD, de Nys R, King N, Adams SL, Packer MA, Svenson J, Eason CT, Magnusson M (2022) Benefits and risks of including the bromoform containing seaweed Asparagopsis in feed for the reduction of methane production from ruminants. Algal Res 64:102673
Grivalský T, Střížek A, Přibyl P, Lukavský J, Čegan R, Hobza R, Hrouzek P (2021) Comparison of various approaches to detect algal culture contamination: a case study of Chlorella sp. contamination in a Phaeodactylum tricornutum culture. Appl Microbiol Biotechnol 105:5189–5200
Grossman AR, Schaefer MR, Chiang GG, Collier JL (1993) The phycobilisome, a light-harvesting complex responsive to environmental conditions. Microbiol Rev 57:725
Gutman J, Zarka A, Boussiba S (2009) The host-range of Paraphysoderma sedebokerensis, a chytrid that infects Haematococcus pluvialis. Eur J Phycol 44:509–514
Havlik I, Beutel S, Scheper T, Reardon KF (2022) On-line monitoring of biological parameters in microalgal bioprocesses using optical methods. Energies 15:875
Havlik I, Reardon KF, Ünal M, Lindner P, Prediger A, Babitzky A, Beutel S, Scheper T (2013) Monitoring of microalgal cultivations with on-line, flow-through microscopy. Algal Res 2:253–257
IPCC (2014): Summary for Policymakers. In: Edenhofer O, Pichs-Madruga R, Sokona Y, Farahani E, Kadner S, Seyboth K, Adler A, Baum I, Brunner S, Eickemeier P, Kriemann B, Savolainen J, Schlömer S, von Stechow C, Zwickel T, Minx JC (eds) Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge
Kawai H, Motomura T, Okuda K (2005) Isolation and purification techniques for macroalgae. In: Anderson RA (ed) Algal Culturing techniques. Elsevier, London pp 133–143
Kinley RD, de Nys R, Vucko MJ, Machado L, Tomkins NW (2016) The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces methane production during in vitro fermentation with rumen fluid. Anim Prod Sci 56:282–289
Kinley RD, Martinez-Fernandez G, Matthews MK, de Nys R, Magnusson M, Tomkins NW (2020) Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J Clean Prod 259:120836
Mata LFR Da (2008) Integrated aquaculture of Bonnemaisoniaceae: physiological and nutritional controls of biomass production and of halogenated metabolite content. Doctoral Thesis, Universidade do Algarve
Meseck SL (2007) Controlling the growth of a cyanobacterial contaminant, Synechoccus sp., in a culture of Tetraselmis chui (PLY429) by varying pH: Implications for outdoor aquaculture production. Aquaculture 273:566–572
Min BR, Parker D, Brauer D, Waldrip H, Lockard C, Hales K, Akbay A, Augyte S (2021) The role of seaweed as a potential dietary supplementation for enteric methane mitigation in ruminants: Challenges and opportunities. Anim Nutr 7:1371–1387
Ming T, Li W, Yuan Q, Davies P, De Richter R, Peng C, Deng Q, Yuan Y, Caillol S, Zhou N (2022) Perspectives on removal of atmospheric methane. Adv Appl Energy 5:100085
Nin-Pratt A, Beveridge M, Sulser TB, Marwaha N, Stanley M, Grisenthwaite R, Phillips MJ (2022) Cattle, seaweed, and global greenhouse gas emissions. IFPRI Discussion Paper
Orenstein EC, Ratelle D, Briseño-Avena C, Carter ML, Franks PJ, Jaffe JS, Roberts PL (2020) The Scripps Plankton Camera system: A framework and platform for in situ microscopy. Limnol Oceanogr Methods 18:681–695
Reichardt TA, Maes D, Jensen TJ, Dempster TA, McGowen JA, Poorey K, Curtis DJ, Lane TW, Timlin JA (2020) Spectroradiometric detection of competitor diatoms and the grazer Poteriochromonas in algal cultures. Algal Res 51:102020
Roque BM, Venegas M, Kinley RD, de Nys R, Duarte TL, Yang X, Kebreab E (2021) Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PLoS One 16:e0247820
Sauer JS, Simkovsky R, Moore AN, Camarda L, Sherman SL, Prather KA, Pomeroy RS (2021) Continuous measurements of volatile gases as detection of algae crop health. Proc Natl Acad Sci U S A 118:e2106882118
Schuenhoff A, Mata L, Santos R (2006) The tetrasporophyte of Asparagopsis armata as a novel seaweed biofilter. Aquaculture 252:3–11
Shea R, Chopin T, Shea R, Chopin · T (2006) Effects of germanium dioxide, an inhibitor of diatom growth, on the microscopic laboratory cultivation stage of the kelp, Laminaria saccharina. J Appl Phycol 191:27–32
Sieracki CK, Sieracki ME, Yentsch CS (1998) An imaging-in-flow system for automated analysis of marine microplankton. Mar Ecol Prog Ser 168:285–296
van der Loos LM, D’hondt S, Willems A, De Clerck O (2021) Characterizing algal microbiomes using long-read nanopore sequencing. Algal Res 59:102456
Winckelmann D, Bleeke F, Bergmann P, Elle C, Klöck G (2016) Detection of weed algae in open pond cultures of Cyanobacterium aponinum using PAM. Int Aquat Res 8:81–90
Zhu Z, Jiang J, Fa Y (2020) Overcoming the biological contamination in microalgae and cyanobacteria mass cultivations for photosynthetic biofuel production. Molecules 25:5220
Acknowledgements
We would like to thank Samantha Clements for assistance with all lab related work and Blue Ocean Barns Inc. for providing funding.
Funding
The study was funded by Blue Ocean Barns Inc.
Author information
Authors and Affiliations
Contributions
GD conceived and wrote the manuscript, and developed the ImageJ workflow; HMR Performed the power analysis and wrote manuscript; BT adjusted ImageJ quantification and reviewed the manuscript; KB collected microscope photos and performed the analyses; JG conducted manual and ImageJ contamination tracing; JES secured funding, conceived the project, and contributed to writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The study was funded by Blue Ocean Barns Inc.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dishon, G., Resetarits, H.M., Tsai, B. et al. Image-based analysis and quantification of biofouling in cultures of the red alga Asparagopsis taxiformis. J Appl Phycol 35, 209–218 (2023). https://doi.org/10.1007/s10811-022-02884-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02884-y