Abstract
The idea of delivering bromoform from Asparagopsis using edible oil has gained momentum recently due to the improved processing time and that it is already a feed that many livestock producers use. The stability of bromoform in oil compared to freeze-dried product is still not well understood. To fill this gap, a systematic study was carried out to determine the effects of storage temperatures (40 °C, 25 °C, 4 °C and -20 °C), fluorescent light and exposure to open air, on the retention of bromoform in freeze-dried Asparagopsis (FD-Asp) and Asparagopsis oil (Asp-Oil) over 24-week period. In the absence of fluorescent light, Asp-Oil was a more effective way to preserve bromoform compared to FD-Asp due to either no change or higher Asp-Oil bromoform content (storage temperature dependent) after 24-week storage. Under the same conditions, FD-Asp bromoform content decreased by 74% at 40 °C, 53% at 25 °C, 6% at 4 °C, and no change of FD-Asp bromoform content at -20 °C. The presence of fluorescent light negatively affected Asp-Oil bromoform content at both 25 °C and 40 °C while the effect was insignificant on FD-Asp. The exposure of Asp-Oil to open air resulted in the decrease of bromoform content to below quantification limit (0.18 mg g−1) on week 8 for 40 °C sample and on week 16 for 25 °C sample. This study provides empirical evidence on the stabilising effect of oil in preserving bromoform extracted from Asparagopsis, confirming it is a more attractive medium to deliver bromoform compared to the freeze-dried powder form.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability of seaweeds to mitigate enteric methane coming from ruminant livestock is heavily dependent on the production and conservation of bioactive compounds that are synthesized and stored within the algal cell walls (Paul et al. 2006; Abbott et al. 2020). Different types of seaweeds synthesize different types of bioactive compounds such as carbohydrates (Holdt and Kraan 2011), lipids (Bikker et al. 2020), peptides (O’Brien et al. 2022), phlorotannins (Lopes et al. 2012), and halogenated methane analogues (Carpenter and Liss. 2000). Asparagopsis has been identified as one of the most potent methane mitigating seaweeds to date and its effectiveness can be attributed to the production of halogenated methane analogues, with bromoform being the predominant compound (Machado et al. 2016). The production of bromoform is largely dependent on the environmental growing conditions, such as temperature and geographic location (Zanolla et al. 2022) as well as sex and life cycle stage (Verges et al. 2008). The conservation of bromoform in seaweeds, however, relies on the application of processing techniques such as collection, dewatering, storage, and transportation conditions (Vucko et al. 2017; Abbott et al. 2020; Vijn et al. 2020). Current processing recommendations for Asparagopsis includes a saltwater rinse, spin dry, freeze at -80 °C, then freeze dry and has been identified as the dewatering technique that maximizes bromoform content (Vucko et al. 2017). The freeze-dried Asparagopsis (FD-Asp) has been the predominant technique to create the final Asparagopsis product used in methane mitigation studies to date. The effectiveness of freeze-dried Asparagopsis (FD-Asp) for methane mitigation has been consistently demonstrated in vitro (Kinley et al. 2016, 2021; Roque et al. 2019a) and in vivo using sheep (Li et al. 2018), dairy cows (Roque et al. 2019b), and beef steers (Kinley et al. 2020; Roque et al. 2021). A comprehensive review of these studies is available from Glasson et al. (2022).
While FD-Asp has historically been the best method to preserve bromoform (Vucko et al. 2017), alternative products that maximize bromoform content are being investigated. Magnusson et al. (2020) has shown that when fresh harvested Asparagopsis is immersed and homogenized into edible oils (Asp-Oil), there is a transfer and stabilization of bromoform. Furthermore, Asp-Oil has been shown to be as effective as FD-Asp at methane mitigation in vitro (Kinley et al. 2022). While continuous improvements toward Asparagopsis derived bromoform conservation are being made, it is also important to understand the retention of bromoform within FD-Asp and Asp-Oil which will ultimately be used to identify their respective shelf-life for on farm use. The retention of bromoform in FD-Asp has been suggested to deteriorate over time, with one study demonstrating as much as 84% bromoform loss over a 120-day period (Stefenoni et al. 2021). The same study also tested the effects of temperature ranging between -20 and 23 °C and found little impact on bromoform content. However, other studies have not seen this result with consistent bromoform concentrations in FD-Asp, and respective methane reductions in vivo, between 90 to 147 days (Kinley et al. 2020; Roque et al. 2021) which indicates that there may be an additional parameter that has not been explored such as exposure to air. As for Asp-Oil, initial testing of 12-week (84 days) samples indicates that the bromoform is not only stable but also but appears to increase in bromoform content over time (Magnusson et al. 2020), however no indication as to whether fluorescent light or exposure to air may also impact bromoform content.
There are a few shelf-life studies on seaweed from different perspectives, such as on stability of bioactive compounds phlorotannin from Brown seaweed Sargassum (Anwar et al. 2018), stability of pigments from brown seaweed Sargassum (Indrawati et al. 2015), and the stability of fatty acid profile, anti-inflammatory activity, antioxidant activity and sensory property of the red seaweed dulse Palmaria palmata (Regal et al. 2020; Stevant et al. 2020). There is very little information on the shelf-life stability of halogenated metabolites even though the antimethanogenic effect of red seaweed Asparagopsis has been well studied. The empirical evidence for the storage and release of halogenated metabolites from the gland cells of Asparagopsis armata was provided by Paul et al. (2006), however very limited information is available on an Asp-Oil product.
This study serves the purpose of filling a knowledge gap and as a compliment to the development of both FD-Asp and Asp-Oil for methane mitigation moving forward. The hypothesis is that an increase in storage temperature and exposure to fluorescent light will decrease bromoform content of both FD-Asp and Asp-Oil over time. It is also hypothesized that FD-Asp will lose bromoform at a faster rate than Asp-Oil. The objectives of this study are to quantify the changes in bromoform content of FD-Asp and Asp-Oil using the following parameters: 1) over a 24-week period; 2) at different storage temperatures (-20 °C to 40 °C); 3) exposure to fluorescent light; and 4) exposure to open air.
Methodology
Preparation of experiment samples
Freeze-dried Asparagopsis (FD-Asp): The Asparagopsis taxiformis was collected in September 2015, near Humpy Island, Keppel Bay, Queensland (23°13′01"S, 150°54′01"E) by Centre for Macroalgal Resources and Biotechnology (MACRO) of James Cook University (JCU) in Townsville, Queensland. The collected biomass was frozen and stored at -20 °C then shipped to Forager Food Co. in Red Hills, Tasmania, where it was freeze-dried and milled to 2–3 mm particle size.
Asparagopsis oil (Asp-Oil): The Asparagopsis taxiformis was collected in July 2021, from Middle Reef Southwest corner of Garden Island in Perth, Western Australia (32°14.519'S, 115°40.757'E). The collected biomass was spun to remove excess seawater and placed into a drum. Canola oil was added into the drum (oil-to-seaweed ratio of 1:1) and mixed well. The content was kept in cool room and mixed daily. On day 10, the oil was separated from the seaweed using a sieve into a new drum.
Both FD-Asp and Asp-Oil samples were shipped to Townsville by express delivery service. The FD-Asp was stored at -20 °C while the Asp-Oil was stored at 4 °C until used.
Shelf-life study
FD-Asp samples (6 sets of 24 samples, 5 g each) were double-bagged (clear zip-lock bags, polyethylene, 200 µm thickness, 7 × 9.5 cm), sealed and each set of bags was stored in one of six scenarios covering the likely range of use conditions: 40 °C in incubator oven and exposed evenly to fluorescent light, 40 °C in incubator oven without fluorescent light, 25 °C at room temperature and exposed to fluorescent light, 25 °C at room temperature without fluorescent light, 4 °C in a fridge with no light and -20 °C in a freezer with no light. The source of fluorescent light was Philips Tornado 12 W Compact fluorescent lamp (CFL) T2, 685 lm, cool daylight colour. Asp-Oil samples (6 sets of 24 samples, 18 mL each) were prepared in sealed sample vials (clear glass vials, solid caps with PTFE liner) and stored in the same conditions as described above. Asp-Oil in sample vials without lids were also prepared and stored at 40 °C and 25 °C and exposed to fluorescent light.
FD-Asp samples and Asp-Oil samples were analysed (in triplicate) for the bromoform content on day 0. A total of 3 FD-Asp and 3 Asp-Oil samples from each storage condition described above were collected on week 1, week 2 and week 4, and every 4 weeks thereafter for a total of 24 weeks and the bromoform content was analysed to determine the changes in bromoform content over time under different storage conditions. The samples were sacrificed at the timepoints once analysed.
Bromoform content analysis method
FD-Asp was milled and sieved (500 µm), then weighed (0.1 g) and extracted in methanol (5 mL) containing 5 µg mL−1 naphthalene as an internal standard. Asp-Oil was used as is (0.3 g) and extracted as described above. The sample and solvent mix was sonicated (15 min, 20 °C) and then continuously mixed using an incubator shaker (24 h, 150 rpm, 21 °C). The extract was then filtered using a 0.2 µm filter head prior to GC–MS analysis.
Gas Chromatography (GC) was performed using an Agilent Technologies 7890A GC with Agilent DB-WAX column (122–7032, 30 m/0.250 mm/0.25 µm, 7-inch cage). Helium was used as the carrier gas. All injections (1 µL) were carried out in the splitless mode with an inlet pressure of 8 psi. The injection port was maintained at 250 °C and the GC–MS interface at 300 °C. The GC was first maintained at 40 °C for 1 min, then ramped up to 250 °C (16 °C min−1) and maintained at this temperature for 2 min.
Mass spectrometry was performed on an Agilent Technologies 5975C Mass Selective Detector (MSD). Ions characteristic of the internal standard naphthalene and bromoform were monitored in the selected ion monitoring (SIM) mode (bromoform, 172–174 amu; naphthalene, 127–129 amu). Bromoform standard of known concentration (from Sigma Aldrich, 40,212, certified reference material) was run at regular intervals within sample set. The GC–MS data was processed using Agilent MassHunter Qualitative Analysis B.07.00. The peak area ratio of bromoform over the internal standard was calculated and converted to bromoform concentration (mg mL−1) by reference to a standard curve (6 points, 0.033–0.200 mg mL−1). The bromoform concentration was reported as per g sample analysed (mg g−1).
Statistical analysis
Three samples for each time point for each storage temperature in the presence and absence of fluorescent light were analysed to obtain a mean ± standard error value. The effect of storage time at each temperature, with or without the presence of fluorescence light was also analysed based on bromoform content data by one way ANOVA with Tukey's Honest Significant Difference (HSD) test. Effects were declared significant at p < 0.05.
Results
At 40 °C, the bromoform content of FD-Asp decreased continuously over time, from 7.7 mg g−1 to less than 2 mg g−1 after 24 weeks irrespective of the presence of fluorescent light (74–79% reduction, p < 0.001) (Fig. 1, Table 1). The effect of fluorescent light was not significant throughout the 24 weeks of storage (0.533 ≤ p ≤ 1). At the same storage temperature and storage period, the bromoform content of Asp-Oil decreased gradually over time in the presence of fluorescent light (11% reduction, p = 0.007) (Fig. 1, Table 1) while the bromoform content of the Asp-Oil samples stored in dark fluctuated significantly over time (p < 0.001). The bromoform content decreased from 1.35 to 1.30 mg g−1 initially but increased after that to 1.41 mg g−1 on week 16, and back to the initial bromoform level after that. The bromoform content of Asp-Oil exposed to fluorescent light was consistently lower than the samples stored in dark at 40 °C from week 16.
At 25 °C, the bromoform content of FD-Asp decreased continuously over time at a lower rate compared to that of 40 °C, from 7.7 to 3.6 mg g−1 after 24 weeks irrespective of fluorescent light (53% reduction, p < 0.001). The effect of fluorescent light on FD-Asp was not significant throughout the duration of this study (0.992 ≤ p ≤ 1). The bromoform content in Asp-Oil again decreased only gradually over time after 24-week storage at the presence of fluorescent light (17% reduction, p < 0.001) while the bromoform content of Asp-Oil stored in the dark fluctuated significantly over time (p < 0.001) similar to the 40 °C Asp-Oil samples. The bromoform content decreased initially from 1.35 to 1.21 mg g−1 at week 8 but increased after that to 1.41 mg g−1 at week 24.
There was no difference in bromoform content of Asp-Oil exposed to fluorescent light in comparison to the Asp-Oil stored in the dark up to week 12, and from week 16 onwards, the bromoform content of samples exposed to fluorescent light were consistently lower.
At a refrigeration temperature of 4 °C, a 5.5% reduction in FD-Asp bromoform content was observed after 24 weeks (7.7 vs 7.3 mg g−1; p = 0.012). At -20 °C, there was no change of bromoform content (p = 0.057) after 24-week storage. As for Asp-Oil, an increase in bromoform content was observed over 24-week storage period (p < 0.001). The bromoform content of Asp-Oil stored at 4 °C and -20 °C increased 7.1% and 8.8%, respectively.
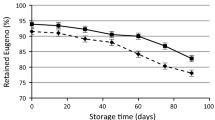
When Asp-Oil was exposed to air at 40 °C, the bromoform content decreased significantly after 4 weeks, from 1.35 to 0.39 mg g−1 (71% reduction) (Fig. 2, Table 1). Asp-Oil exposed to air at 25 °C decreased to the same bromoform level (0.39 mg g−1) after 8 weeks.
The bromoform content of both samples then decreased further to below the quantification limit of 0.18 mg g−1, on week 8 for 40 °C samples and on week 16 for the 25 °C samples. Bromoform was not detected in the 40 °C samples from week 16 onwards, that is, no bromoform peak was observed in the chromatograms while a trace amount of bromoform was still detected in the 25 °C sample at the end of the 24-week shelf-life study (limit of detection = 0.06 mg g−1). The bromoform content of FD-Asp in sealed bags at both 25 °C and 40 °C was not stable, with 16.8% and 27.8% reduction, respectively, after 4-week storage, thus the effect of exposure to air was not studied.
Discussion
This study demonstrates excellent shelf-life stability of FD-Asp stored in a -20 °C freezer with no change of bromoform content after 24-week storage. It is less ideal to store FD-Asp at 4 °C refrigerator as a small decrease was observed (5.5% reduction) after 24 weeks. The decrease in bromoform maybe due to the breakdown of the algal cell wall over time resulting in a release of bromoform into the air space within the sealed bag. After 12-weeks of storage at 4 °C, the decrease in bromoform for FD-asp was not significant (3.5% reduction). This is consistent with the study by Magnusson et al. (2020). The study reported 1.3 ± 1.0% reduction and 5 ± 4.1% reduction in bromoform content when freeze-dried samples were stored for 12 weeks at -20 °C and 4 °C, respectively. The large standard error values reported suggests that the samples used in the study were reasonably dispersed from the mean indicating the change is most likely insignificant. At 25 °C, Magnusson et al. (2020) reported 37.8 ± 6.1% reduction after 12-week storage, very similar to the findings from this study of 40.2% reduction within the same storage period. A storage temperature of 40 °C had a definitively negative effect on the retention of bromoform over time as demonstrated in this study, which showed increased release of bromoform from FD-Asp at the higher temperature. Stefenoni et al. (2021) on the other hand, concluded that storage temperature did not have an effect on bromoform concentration in FD-Asp, this may simply be due to the shorter storage period (4 months) and lower storage temperature used in their study (23 °C maximum).
The study by Magnusson et al. (2020) reported an increase of bromoform content in Asp-Oil after 12 weeks of storage at 4 °C which is in agreement with the findings of this study. However, the increment observed in this study was only 8.4%, compared to the 26.7% reported by Magnusson et al. (2020) at the same storage temperature. The increase of bromoform over time is counter intuitive. However, it may be due to the particulate algal biomass (< 100 µm) remaining in the oil after filtering Asp-Oil to remove biomass, that continues to release bromoform into the oil during storage. Differences in processing of Asp-Oil between the studies may further explain differences in the level of bromoform increases reported. Magnusson et al. (2020) combined the seaweed oil treated with and without homogenisation and used it in the shelf-life stability study while the seaweed and oil mix used in this study was not homogenised implying potentially less particulate biomass in the oil, thus less release of bromoform into the oil over time. The increase in bromoform content of samples stored at -20 °C in this study presumably due to the same reason. The process of homogenising the seaweed-oil mixture had an apparent positive effect on the filtered oil’s bromoform content during long-term storage. The initial intention of homogenisation in the Magnusson study was to investigate the efficiency of bromoform infusion into the oil during the steeping process (Magnusson et al. 2020). Higher bromoform content of oil was reported on day 1 of steeping for the homogenised sample compared to the oil with intact seaweed. The bromoform content, however, did not increase further after day 1 while the bromoform level of the oil with intact seaweed increased gradually over 10 days and the final bromoform content was no different to that of homogenised seaweed in oil.
The negative effect of fluorescent light on bromoform stability of FD-Asp was not observed in this study at 40 °C. This may be due to the mild/moderate power of light bulb used (12 W). More powerful light bulbs or longer exposure period to fluorescent light is expected to have more apparent negative effects on the retention of bromoform content of samples stored at this temperature and at 25 °C. The result from this study is in disagreement with the findings by Stefenoni et al. (2021). They reported that fluorescent light had effects on the bromoform content of FD-Asp samples stored at 23 °C, 4 °C, -20 °C with 17% lower bromoform concentration on average compared to samples stored in the dark. The power of the light bulb used in the study is not known and percentage reduction for each temperature was not reported. Therefore, further comparisons between the two studies and further discussion on the effects of fluorescent light at different temperatures is not possible.
The effect of fluorescent light was more apparent for Asp-Oil compared to FD-Asp. In the presence of fluorescent light at both 25 °C and 40 °C, Asp-Oil bromoform content decreased over time. However, in the dark environment, the bromoform content decreased initially up to week 8 (from 1.35 to 1.21–1.30 mg g−1, p < 0.05), then increased and maintained at a level similar to the initial bromoform level for the rest of the storage period. This observation is rather encouraging as a confirmation of Asp-Oil stability at temperatures as high as 40 °C for at least 24 weeks in an airtight and dark environment. At 4 °C and -20 °C storage, which are related to storage conditions of a fridge and freezer, respectively, continuous exposure to fluorescent light is not usually an issue as lights are only activated upon opening of doors, therefore these conditions were not investigated for both FD-Asp and Asp-Oil in this study.
All fluorescent lamps emit a certain level of UV (FDA 2017). Even though majority of the light emitted by the fluorescent light bulb used in this experiment is localized within the visible region of the light spectrum (approximately 400–700 nm wavelength), a small amount of UV, such as UVA (315–400 nm) and UVB (280–315 nm) and infrared (> 700 nm) radiation are also emitted. Photodissociation of bromoform is imminent at the presence of UV (Huang et al. 2004). The breaking of C–Br bond, into other compounds such as Br, CHBr2, CHBr, CBr, HBr and Br2 was reported by Zou et al. (2004). This is largely the reason why it is not an option to dry Asparagopsis directly under the sun. Bromoform is characterised as a very short-lived substance (Orkin et al. 2013). It has an atmospheric lifespan of approximately 24 days due to photodissociation as well as reactions with OH in the troposphere (WMO 2010). The effect of UV is a critical parameter, nonetheless, it is not mentioned as a consideration in the shelf-life study section of the Guidance for the preparation of dossier for zootechnical additives (EFSA 2012). The guidance recommends only temperature and humidity settings appropriate for a shelf-life study.
As the bromoform content of Asp-Oil was shown to be stable at different temperature studied, the effect of exposure to open air was also studied. A significantly lower bromoform content was observed in Asp-Oil exposed to open air compared to Asp-Oil in airtight sample vials at both 25 °C and 40 °C. More than 50% of the bromoform was lost after 3 weeks at 40 °C or 5 weeks at 25 °C and the rate of decrease started to slow down after week 8 at both temperatures. This result demonstrates the importance of storing Asp-Oil in air-tight containers, suggesting that without an airtight lid/seal, bromoform in oil will escape from the oil medium into the headspace and then into surrounding air. Results from this study confirm that storage temperature plays an important role in bromoform stability, in that higher storage temperatures result in faster loss of bromoform from the product into container head space and eventual release into the immediate environment.
This study is the first to investigate a relatively long-term storage of FD-Asp and Asp-Oil in various storage conditions (different temperatures, presence of fluorescent light, and exposure to open air). It clearly demonstrates a differential shelf-life stability of bromoform content in FD-Asp compared to Asp-Oil. Asp-Oil was a comparatively more attractive way to preserve bromoform compared to FD-Asp. FD-Asp was only stable at -20 °C while Asp-Oil had higher/no change in bromoform content after 24-week storage at 40 °C, 25 °C, 4 °C and -20 °C in dark conditions. Besides the advantages of shelf-life stability of Asp-Oil, it is also economically competitive. The production cost of FD-Asp is the main concern. It involves blast-freezing of fresh seaweed, followed by freeze-drying, both of which are very energy intensive processes. The hygroscopic characteristic of freeze-dried powder is also a drawback resulting in the increase in moisture content and water activity over time if not stored properly, which affects endogenous chemical reactions and microbial activity (Stevant et al. 2020). On the other hand, the preparation of Asp-Oil is relatively straight forward, it involves steeping of seaweed in oil for a specific period of time followed by removal of seaweed biomass and aqueous fraction from the oil which contains the active compound bromoform. The ability of oil in preserving bromoform has provided a practical alternative to lessen the processing burden of FD material. Additionally, the use of a lipid source in feed mixtures is already implemented as an approach for methane mitigation in ruminants making it a practical candidate to deliver bromoform (Beauchemin et al. 2007). The effectiveness of bromoform in oil for methane mitigation in vitro has been demonstrated recently by Kinley et al. (2022). Animal trials using Asp-Oil are in progress. Considering the effective bromoform concentration for methane mitigation is extremely low, only a small amount of Asp-Oil or FD-Asp will need to be used in ruminant feed mix. Along with the development of different bromoform delivery mechanisms, bromoform analysis methods need to be updated for quantification of very low levels of bromoform (microgram level) in feed. This will be crucial for the checking bromoform content in the mixed feed over time and testing homogeneity of the bioactive in feed mixed with FD-Asp or Asp-Oil.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request and upon approval from FutureFeed Pty Ltd.
References
Abbott DW, Aasen IM, Beauchemin KA, Grondahl F, Gruninger R, Hayes M, Huws S, Kenny DA, Krizsan SJ, Kirwan SF, Lind V, Meyer U, Ramin M, Theodoridou K, von Soosten D, Walsh PJ, Waters S, Xing X (2020) Seaweed and seaweed bioactives for mitigation of enteric methane: challenges and opportunities. Animals 10:2432
Anwar E, Erianto H, Putri KS (2018) Preparation of powder from brown seaweed (Sargassum plagyophyllum) by freeze-drying with maltodextrin as a stabilizer. Int J Appl Pharm 10:348–353
Beauchemin KA, McGinn SM, Petit HV (2007) Methane abatement strategies for cattle: Lipid supplementation of diets. Can J Anim Sci 87:431–440
Bikker P, Stokvis L, van Krimpen MM, van Wikselaar PG, Cone JW (2020) Evaluation of seaweeds from marine waters in Northwestern Europe for application in animal nutrition. Anim Feed Sci Technol 263:114460
Carpenter LJ, Liss PS (2000) On temperate sources of bromoform and other reactive organic bromine gases. J Geophys Res 105:20539–20547
EFSA (2012) Guidance for the preparation of dossiers for zootechnical additives. EFSA panel on additives and products or substances used in animal feed (FEEDAP). EFSA J 10:2536
FDA (2017) Compact fluorescent lamps (CFLs) – Fact sheet/FAQ. US Food and Drug Administration, Silver Spring. https://www.fda.gov/radiation-emitting-products/home-business-and-entertainment-products/compact-fluorescent-lamps-cfls-fact-sheetfaq. Accessed 1 July 2022
Glasson CRK, Kinley RD, de Nys R, King N, Adams SL, Packer MA, Svenson J, Eason CT, Magnusson M (2022) Benefits and risks of including the bromoform containing seaweed Asparagopsis in feed for the reduction of methane production from ruminants. Algal Res 64:102673
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: Functional food applications and legislation. J Appl Phycol 23:543–597
Huang HY, Chuang WT, Sharma RC, Hsu CY, Lin KC (2004) Molecular elimination of Br2 in 248 nm photolysis of bromoform probed by using cavity ring-down absorption spectroscopy. J Chem Phys 121:5253–5260
Indrawati R, Sukowijoyo H, Indriatmoko WRDE, Limantara L (2015) Encapsulation of brown seaweed pigment by freeze drying: Characterization and its stability during storage. Procedia Chem 14:353–360
Kinley RD, de Nys R, Vucko MJ, Machado L, Tomkins NW (2016) The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces methane production during in vitro fermentation with rumen fluid. Anim Prod Sci 56:282–289
Kinley RD, Martinez-Fernandez G, Matthews MK, de Nys R, Magnusson M, Tomkins NW (2020) Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J Clean Prod 259:120836
Kinley RD, Tan S, Turnbull J, Askew S, Roque BM (2021) Changing the proportions of grass and grain in feed substrate impacts the efficacy of Asparagopsis taxiformis to inhibit methane production in vitro. Am J Plant Sci 12:1835–1858
Kinley RD, Tan S, Turnbull J, Askew S, Harris J, Roque BM (2022) Exploration of methane mitigation efficacy using Asparagopsis-derived bioactives stabilized in edible oil compared to freeze-dried Asparagopsis in vitro. Am J Plant Sci 13:1023–1041
Li X, Norman HC, Kinley RD, Laurence M, Wilmot M, Bender H, de Nys R, Tomkins N (2018) Asparagopsis taxiformis decreases enteric methane production from sheep. Anim Prod Sci 58:681–688
Lopes G, Sousa C, Silva LR, Pinto E, Andrade PB, Bernardo J, Mouga T, Valentao P (2012) Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 7:e31145
Machado L, Magnusson M, Paul NA, Kinley R, de Nys R, Tomkins N (2016) Identification of bioactives from the red seaweed Asparagopsis taxiformis that promote antimethanogenic activity in vitro. J Appl Phycol 28:3117–3126
Magnusson M, Vucko MJ, Neoh TL, de Nys R (2020) Using oil immersion to deliver a naturally-derived, stable bromoform product from the red seaweed Asparagopsis taxiformis. Algal Res 51:102065
O’Brien R, Hayes M, Sheldrake G, Tiwari B, Walsh P (2022) Macroalgal Proteins: A Review. Foods 11:571
Orkin VL, Khamaganov VG, Kozlov SN, Kurylo MJ (2013) Measurements of rate constants for the OH reactions with bromoform (CHBr3), CHBr2Cl, CHBrCl2, and epichlorohydrin (C3H5ClO). J Phys Chem A 117:3809–3818
Paul NA, de Nys R, Steinberg PD (2006) Chemical defence against bacteria in the red alga Asparagopsis armata: Linking structure with function. Mar Ecol Prog Ser 306:87–101
Regal AL, Alves V, Gomes R, Matos J, Bandarra NM, Afonso C, Cardoso C (2020) Drying process, storage conditions, and time alter the biochemical composition and bioactivity of the anti-greenhouse seaweed Asparagopsis taxiformis. Eur Food Res Technol 246:781–793
Roque BM, Brooke CG, Ladau J, Polley T, Marsh LJ, Najafi N, Pandey P, Singh L, Kinley R, Salwen JK, Eloe-Fadrosh E, Kebreab E, Hess M (2019a) Effect of the macroalgae Asparagopsis taxiformis on methane production and rumen microbiome assemblage. Anim Microbiome 1:3
Roque BM, Salwen JK, Kinley R, Kebreab E (2019b) Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J Clean Prod 234:132–138
Roque BM, Venegas M, Kinley RD, de Nys R, Duarte TL, Yang X, Kebreab E (2021) Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PLoS ONE 16:e0247820
Stefenoni HA, Raisanen SE, Cueva SF, Wasson DE, Lage CFA, Melgar A, Fetter ME, Smith P, Hennessy M, Vecchiarelli B, Bender J, Pitta D, Cantrell CL, Yarish C, Hristov AN (2021) Effects of the macroalga Asparagopsis taxiformis and oregano leaves on methane emission, rumen fermentation, and lactational performance of dairy cows. J Dairy Sci 104:4157–4173
Stevant P, Olafsdottir A, Deleris P, Dumay J, Fleurence IB, Jonsdottir R, Ragueneau E, Rebours C, Rustad T (2020) Semi-dry storage as a maturation process for improving the sensory characteristics of the edible red seaweed dulse (Palmaria palmata). Algal Res 51:102048
Verges A, Paul NA, Steinberg PD (2008) Sex and life-history stage alter herbivore responses to a chemically defended red alga. Ecology 89:1334–1343
Vijn S, Compart DP, Dutta N, Foukis A, Hess M, Hristov AN, Kalscheur KF, Kebreab E, Nuzhdin SV, Price NN, Sun Y, Tricarico JM, Turzillo A, Weisbjerg MR, Yarish C, Kurt TD (2020) Key considerations for the use of seaweed to reduce enteric methane emissions from cattle. Front Vet Sci 7:597430
Vucko MJ, Magnusson M, Kinley RD, Villart C, de Nys R (2017) The effects of processing on the in vitro antimethanogenic capacity and concentration of secondary metabolites of Asparagopsis taxiformis. J Appl Phycol 29:1577–1586
WMO (2010) Global Ozone Research and Monitoring Project - Report No. 52 Scientific Assessment of Ozone Depletion. World Meteorological Organization, Geneva. http://www.esrl.noaa.gov/csd/assessments/ozone/2010/. Accessed 30 June 2022
Zanolla M, Romanazzi D, Svenson J, Sherwood A, Stengel DB (2022) Bromoform, mycosporine-like amino acids and phycobiliprotein content and stability in Asparagopsis armata during long-term indoor cultivation. J Appl Phycol 34:1635–1647
Zou P, Shu JN, Sears TJ, Hall GE, North SW (2004) Photodissociation of bromoform at 248 nm: Single and multiphoton processes. J Phys Chem A 108:1482–1488
Author information
Authors and Affiliations
Contributions
Siong Tan—Study conception and experimental design, material preparation, sample collection and analysis, data analysis, original draft preparation, manuscript review and editing.
Jessica Harris—Sample collection and analysis, manuscript review and editing.
Breanna M. Roque—Data analysis, manuscript elaboration, manuscript review and editing.
Shane Askew—Supervision of laboratory work, sample analysis and data elaboration.
Robert D. Kinley—Study conception and experimental design, manuscript review and editing, supervision of study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest regarding the publication of this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tan, S., Harris, J., Roque, B.M. et al. Shelf-life stability of Asparagopsis bromoform in oil and freeze-dried powder. J Appl Phycol 35, 291–299 (2023). https://doi.org/10.1007/s10811-022-02876-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02876-y