Abstract
The efficacy of low-frequency ultrasound pulses in cell disaggregation of Chroococcidiopsis sp. aggregates has been studied as a possible strategy to improve the productivity and operation of the cultures. The modulation of the ultrasound pulses allowed to almost disaggregate most of the cyanobacterial aggregates completely while retaining cellular viability in terms of photosynthetic efficiency. In this study we used a strain isolated from the endolithic habitat of the Atacama Desert, the driest desert in the world due to the extremely scarce rainfall, low level of relative humidity and extremely high incident solar radiation. To survive these conditions and reduce the cell exposure to the incident UV radiation, Chroococcidiopsis sp. grows in the form of aggregates, diminishing the associated photo-oxidative damage. However, this adaptation strategy can reduce the availability of both light and nutrients to the growing cells. This study showed that the low-frequency ultrasound pulses were efficient in disaggregating Chroococcidiopsis sp. aggregates, improving light and nutrient availability to the cells. Our results revealed also that the modulated use of ultrasound pulses resulted in a decreased cell sedimentation velocity which becomes advantageous at large scale. The length of the ultrasound pulses can be optimized to achieve complete disaggregation of the aggregates without affecting cell viability. The preservation of cell viability is considered an advantage for eventual large-scale production as disaggregating of the aggregates can result in more homogeneous cultures with less energy needed to perform mechanical agitation. Additionally, our results indicated an improved growth of cyanobacterium in disaggregated cultures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of extremotolerant microorganisms allows us to understand the limits of the conditions in which life is possible, thus, providing answers to fundamental questions about the origin, distribution, and evolution of life (Varshney et al. 2015). In addition, the adaptation capacity of extremotolerant microorganisms to the harsh conditions of their habitats is partly based on the expression of secondary metabolic pathways leading to the biosynthesis of unique, valuable molecules with applications in human and animal health. These molecules include highly antioxidant phenolic compounds, peptides, indole derivates and terpenes, among other valuable biomolecules groups (Raddadi et al. 2015; Dumorné et al. 2017).
In particular, the genus Chroococcidiopsis is known for its extremotolerant character and capability to adapt to polyextreme environments such as extreme aridity, high salinity, high and low temperatures, and ionizing radiations. The cyanobacterium was isolated from the endolithic habitat of gypsum rocks of the hyper-arid Atacama Desert (north Chile) (Wierzchos et al. 2015). This desert is one of the most challenging polyextreme environment on Earth due to its hyper-aridity, extreme solar irradiance, both ultraviolet radiation (UVR) and photosynthetic active radiation (PAR), high day/night temperature fluctuations and in some cases high salinity (Wierzchos et al. 2018). The high solar radiation in the Atacama Desert makes photosynthesis difficult due to the photo-oxidative damage suffered by the species growing in that habitat (Solovchenko and Merzlyak 2008).
To overcome these conditions, Chroococcidiopsis sp. produces a layer of exopolysaccharides (EPS) that surround the cells allowing them to retain some water and create a more humid endolithic environment to live (Singh et al. 2019), thereby promoting photosynthesis. This EPS layer favors the formation of cellular aggregates (Das et al. 2018), allowing the cyanobacteria to dissipate part of the light received, eventually reducing the oxidative damage (Flemming and Wingender 2010). To achieve this, the cells accumulate the UV-filtering phenolic molecule scytonemin within the outermost EPS sheath (Vítek et al. 2014; Casero et al. 2021). As a cyanobacterium, Chroococcidiopsis strains can be of biotechnological interest also due to the potential accumulation of valuable pigments including phycobiliproteins (Vítek et al. 2014; Montero et al. 2020).
In spite of the potential value of such extremotolerant microorganisms from hyperarid habitats, they have never been exploited commercially and almost no attempts to produce them systematically have been reported in the literature. The latter can be explained based on the limited growth rates and productivities of these microorganisms in liquid medium (Das et al. 2018). The cyanobacterial exopolysaccharides have been reported to play a major role in microbial adhesion and, consequently, in keeping the structure of cellular aggregates (Li et al. 2001). Independently on the variety of biological functions and the biotechnological applications of cyanobacterial exopolysaccharides (Cruz et al. 2020), the chemical interaction between them induces formation of aggregates which tends to make biomass production slower. In the particular case of Chroococcidiopsis sp., the growth in the form of cellular aggregates can favor the economic feasibility of their harvest since high yields are obtained with significantly reduced energy and biomass production costs (Molina et al. 2003). However, despite the above-referred advantage of the aggregates, the cell morphological configuration in aggregates can limit their growth due to the lowered availability of light and nutrients reaching the central cells of the aggregates compared to the peripheral ones (Conradi et al. 2019). As a result, growth can occur at different rates within each cell aggregate, depending on each cell position and availability of light and nutrients. According to it, the hypothesis of this work is that avoiding aggregation could improve the productivity of cyanobacterium, eventually increasing the production and even extraction yields of valuable molecules. The strategies and intensity used to keep cells disaggregated should guarantee the cells viability, this would potentially address improved nutrient availability and thus cell growth and hypothetically facilitate production on a large scale.
The application of ultrasound pulses to cyanobacterial suspensions has shown efficacy in controlling aggregates (Joyce et al. 2010; Wu et al. 2011). The development of cyanobacterial blooms in water bodies imparts undesirable characteristics to the water due to the potential presence of toxins (Rajasekhar et al. 2012). Several chemical and physical methods have been used to control the blooms but have limitations in terms of pollution and application on a large scale (Lee et al. 2001). Ultrasound treatment can lead to structural and functional disruption of cyanobacterial cells (Phull et al. 1997) and its use as a treatment option to control cyanobacterial blooms has been under consideration in recent decades. However, only a few works have addressed the potential of ultrasound to improve microalgal growth (Joyce et al. 2014). This might be useful for species which grow forming aggregates, then eventually requiring higher energy inputs for agitation and having less light and nutrients availability. The rationale behind our work is minimizing the energy of the ultrasound pulses applied to cyanobacterial aggregates in liquid cultures to that required, to splitting the cyanobacterial outer polysaccharide sheath over the cell membrane while avoiding cell disruption, thus promoting cell disaggregation. Based on this concept, we studied in culture flasks the efficacy of low-frequency ultrasound in cell disaggregation as a treatment to eventually improve the productivity of the Chroococcidiopsis sp. liquid cultures.
Materials and methods
Biological material and culture conditions
The cyanobacterium Chroococcidiopsis sp. was isolated from the gypsum rock fragments of the Atacama Desert, (23°53′S, 068°08′W and 2,720 m a.s.l.) located in the north–south trending depression of the Cordon de Lila range, in northern Chile. The isolated cyanobacterium was supplied by researchers from the (National Museum of Natural Sciences, CSIC, Madrid) to the Algae Biotechnology research group (BIO-214) of the University of Huelva, Spain. A picture of the microorganism is shown in Fig. 1. The aggregate is representative of the cell cluster morphology of the samples studied. Chroococcidiopsis sp. was cultivated at pH 7 in sterile BG11culture medium, which is widely used for the growth of freshwater green algae and cyanobacteria (Richmond 2004). The constituents of BG11 (per liter) were as follows: 1.5 g NaNO3; 0.04 g KH2PO4·3H2O; 0.075 g MgSO4·7H2O; 0.036 g CaCl2·2H2O; 0.006 g citric acid; 0.006 g ferric ammonium citrate; 0.222 µg ZnSO4.7H2O; 0.079 µg CuSO4·5H2O; 1.81 µg MnCl2·4H2O; 2.86 µg H3BO3; 0.391 µg Na2MoO4·2H2O; 0.049 µg Co(NO3)2·6H2O; 0.001 g Na2EDTA. The cultures were grown in an algal cultivation room at 25 °C illuminated with white fluorescent light of 50–60 µmol photons m–2 s–1, bubbled with a mix of CO2 in the air (2.5%, v/v), which was the only used carbon source.
Ultrasound treatment
The Chroococcidiopsis sp. cultures or samples were subjected to ultrasound pulses of a given intensity and variable duration at the beginning of each experiment, in order to promote disaggregation of cell clusters before performing further experiments or measuring other parameters. Cultures (250 mL in 500 mL Erlenmeyer flasks) or culture samples (15 mL in Falcon tubes) were added to the BG11 culture medium to reach an initial optical density (OD750) between 0.5 and 0.7 and subjected to 180 W ultrasound pulses using a model Ultrasons H–D bath (Selecta, Spain) for a variable duration ranging from a few seconds to 10 min. In the test performed to study the effect of ultrasound pulses on the growth and viability of Chroococcidiopsis sp., the cultures were incubated in the microalgal culture room under the conditions indicated above.
Turbidity measurement and kinetics
The optical density at 750 nm (OD750) was measured using a Cary 60 UV–Vis spectrophotometer (Agilent Technologies, USA) equipped with a temperature control system adjusted to 25 °C. The OD750 data provided the information of the culture turbidity, which was proportional to the amount of biomass present in the culture (Shuler and Kargi 2005).
The decay in the turbidity of Chroococcidiopsis sp. samples was measured through kinetics, showing a decrease in the OD750. The kinetic data are shown in Fig. S1 (Supporting Information). The data also provided information on the resistance to light passage through the cell aggregates (Richmond 2004). The biomass recovery was calculated using the data obtained from the turbidity kinetics, which was expressed as a percentage and determined using the following expression:
where \({OD}_{750}{t}_{0}\) is the turbidity of the sample taken at time zero while \({OD}_{750}t\) is the turbidity of the sample taken at time t of the turbidity kinetics.
In some cases the relative optical density at time zero was expressed as a percentage according to the following expression:
where OD750 is the optical density at 750 nm; t0 is the time zero for a sample not subjected to ultrasound treatment, while ti corresponds to the ultrasound-treated samples at time zero.
Optical microscopic observations
The observations and images were acquired using an optical microscope model CX40 (Olympus, UK) with an objective of 10× or, in some cases, 40×. The resulting images allowed us to prepare figures for the distribution of aggregates and cells according to their size and abundance. Next, the cell aggregates were classified into the following two groups: (i) large and medium aggregates with the number of cells greater than 5, and (ii) small and non-aggregated cells, wherein the small aggregates had cell numbers equal to or less than 5. The size of the cell aggregate and individual cells was determined based on microscopic observations and CellProfiler cell image analysis software (CellProfiler.org).
Maximum quantum yield (Fv/Fm) of Photosystem II
Based on the chlorophyll fluorescence measurements, the maximum quantum yield (Fv/Fm) of Photosystem II (PSII) was determined according to the method described by Schreiber (2004). Quantum yield was measured by placing Chroococcidiopsis sp. culture samples into the measuring chamber of portable pulse amplitude modulated fluorimeter (AquaPEN AP-C 100, Czech Republic). Fv represents the minimum level of fluorescence observed after exposing the cells to a non-actinic beam and acclimatizing them in the dark for 10 min, while Fm was the maximum fluorescence observed in cells after exposing them to a short but saturating actinic light pulse.
Photosynthetic and respiratory activities
The measurement of photosynthetic and respiratory activities was performed using a Clark electrode (Hansatech, UK). The working temperature of the system was maintained at 25 °C, which received light intensity of 110 µmol photons m–2 s–1. A 2.5 mL of culture sample was placed into the electrode cuvette and 30 µL of 0.2 M bicarbonate was added. The net oxygen consumption by endogenous respiration was recorded in darkness, while the net oxygen production was recorded during the illumination period. The photosynthetic activity was calculated as the sum of slopes obtained in both light and dark conditions. The evolution of oxygen was quantified as µmoles of O2 produced (photosynthetic activity) or consumed (endogenous respiratory activity) per unit of time (h) and biomass (mg of chlorophyll). Photosynthetic and respiratory activity of culture samples of Chroococcidiopsis sp. was calculated (Fig. S4, Supporting Information).
Pigment extraction
The extraction and subsequent determination of the total content of chlorophylls and carotenoids in the culture of Chroococcidiopsis sp. was performed by the method described by Cuaresma et al. (2011). Total content of photosynthetic pigments of culture samples of Chroococcidiopsis sp. was calculated (Fig. S3, Supporting Information).
Specific growth rate
The maximal specific growth rate was calculated during the early-mid exponential phase, where cell density showed an increase with time according to the following logarithmic expression:
where Ct and C0 represent the cell density for times t and zero, respectively, and μ represents the specific growth rate, expressed in day−1. In this study, the optical density at 750 nm was used as an indirect measure of the biomass density, while the above equation was used to calculate the specific growth rates.
Sedimentation velocity
For the calculation of sedimentation velocity, the expression based on Stokes' Law (Bürger and Concha 1998) was applied. This expression assumes that the sedimentation velocity (V), in the case of particles with spherical shape and laminar regime, is proportional to the square of the radius of a particle, and to the difference of the density of the particle and liquid medium. The formula is as follows:
where (i) r and ρs represent the average radius and density of the particles, respectively, (ii) g indicates the gravitational acceleration, and (iii) ρl and ɳ denote the density and dynamic viscosity of the liquid, respectively. The average radius of Chroococcidiopsis sp. was calculated from the observations of the cell clusters and individual cells under light microscopy (Fig. S2, Supporting Information).
According to De Schryver et al. (2008), bacteria smaller than 100 µm always represent a laminar regime. Since bacteria are more or less spherical particles and have a chemical composition similar to that of cyanobacteria, it is assumed that the culture of Chroococcidiopsis sp. represents a laminar regime because of their cells having an average size of less than 100 µm. The density of the liquid culture medium is similar to that of water at 25 °C (ρl = 997.13 kg m–3) as the medium contains mostly water and traces of inorganic salts. Similarly, the viscosity of the liquid medium can be close to that of the water (ɳ = 1 mPa s–1) with an error of 10% (Georgi 1996). Finally, as per the method of Milledge and Heaven (2013), the approximate value of the density of the cyanobacterial biomass was found to be 1100 kg m–3.
Statistics
Unless otherwise indicated, the presented data indicate the means of three independent experiments while standard deviations are represented in the corresponding figures and tables.
Results
Our study aimed to evaluate the efficacy of low-frequency ultrasound (LFU) in cell disaggregation as a treatment to promote the growth of the extremotolerant cyanobacterium Chroococcidiopsis sp. in liquid cultures. This goal should be achieved by optimizing the ultrasound pulse duration in order to simultaneously limit the impact of the LFU treatment to the cell cluster disaggregation while avoiding any negative effect on the cell viability, in order to keep the cultures viable. According to the previous studies detailed in the Introduction section, the use of LFU pulses could help in disaggregating cellular aggregates of microalgae suspended in a liquid medium (Phull et al. 1997). Accordingly, we hypothesized that using ultrasound on the cyanobacterial cultures could allow greater accessibility of light and nutrients into the cultivated cells. Consequently, if there is no negative effect on the cell viability by the ultrasound, an improvement in growth could be expected which might be further advantageous for an eventual production process at a larger scale.
To verify whether the proposed hypothesis is valid, we first performed a test to analyze whether the application of ultrasound pulses had any effect on the aggregation state of the Chroococcidiopsis sp. culture in a liquid medium. Based on the obtained results, a second test was performed to study the effect of ultrasound on the growth and viability of Chroococcidiopsis sp. subjected to different durations of ultrasound pulse.
Effect of ultrasound treatment on the aggregation of Chroococcidiopsis sp. cultures
To test the expected ultrasound-mediated disaggregation effect in cyanobacterial aggregates, culture samples were subjected to LFU pulses of different duration. The evolution of the aggregation state of treated samples was assessed by following variations in optical density of the samples and comparing the obtained data with those of the non-treated samples.
Figure 2 shows the variation in the relative optical density, plotted versus the ultrasound pulse duration in culture samples of Chroococcidiopsis sp. Details of the relative optical density meaning are provided in the Materials and Methods section. The curve (Fig. 2A) shows a linear trend for ultrasound pulse duration lower than 3 min, after which a constant maximum value of relative optical density is reached. A similar trend is also observed in the light micrographs of Chroococcidiopsis sp. samples (Fig. 2B), where an increase in cellular disaggregation was observed as increasing the ultrasound pulse duration, resulting in the maximum disaggregation after an ultrasound pulse duration of 3 min.
Variations in the relative optical density as a function of ultrasound pulse duration, expressed as a percentage, where 0.7 is the relative optical density value at time zero (A); Images of Chroococcidiopsis sp. obtained using the optical microscope (bright field) (B).Culture sample not subjected to ultrasound pulses (a); culture sample after ultrasound pulses of 3 min (b) and 5 min (c). Error bars indicate standard deviation of three independent measurements. Procedure details are provided in the Materials and Methods section
We have quantified the impact of the pulse duration on the aggregation state of the cyanobacterial cell clusters by counting under the microscope the number of large, medium and small aggregates, as per the criteria described in the Materials and Methods section. Specifically, the experiment aimed at determining the ultrasound pulse duration required for obtaining a fully disaggregated culture sample that eventually reached the highest optical density value. The variations seen in the abundance of cellular entities (Fig. 3) were expressed as a percentage and as a function of ultrasound pulse duration. The number of small aggregates and non-aggregated cells was shown to be increased with an increase in the ultrasound pulse duration. A higher percentage of 88% was obtained for an ultrasound pulse duration of equal to or greater than 3 min. From the observations under the optic microscope (Fig. 2B), the size of the large aggregates in ultrasound-treated samples, measured as average radius, decreased by 50% from 11 to 6 µm approximately while the size of the small aggregates and non-aggregated cells remained constant around 1 µm.
Effect of ultrasound treatment on the growth and viability of Chroococcidiopsis sp. culture
In this section, the eventual impact of the ultrasound treatment on cyanobacterial growth and cell viability, measured as Fv/Fm, was evaluated. Our results were useful in discussing the suggested hypothesis of enabling greater availability of nutrients and light to disaggregated cultures. Hence, we subjected the cultures to different durations of ultrasound pulses (Fig. 4) according to the procedure described in the Materials and Methods section.
Effect of ultrasound pulse duration on the specific growth rate (A) and on the maximal photosynthetic efficiency of photosystem II (Quantum yield, Fv/Fm) along the cultivation time (B). Culture not subjected to ultrasound treatment (-♦-), and cultures subjected to ultrasound pulses of 2 min (-■-), 4 min (-▲-), 6 min (-●-), and 10 min (-○-). Error bars indicate standard deviation of three independent measurements. Procedure details are provided in the Materials and Methods section
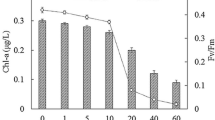
The variations in the maximal specific growth rate of Chroococcidiopsis sp. subjected to different durations of ultrasound pulse are shown in Fig. 4A. The curve for the control culture and cultures subjected to ultrasound for 2 min began at a rate of 0.12 day–1, approximately. A slight increase was observed in the growth rate of the cultures subjected to a pulse of 4 and 6 min duration, respectively, showing the highest value of approximately 0.13 day–1, which was 14% higher than the control rate.
Stable and productive growth is associated with the photosynthetic capability of cells and cell aggregates. To verify whether the application of ultrasound pulses did not cause the losses in cell viability of cyanobacterial cultures, the variations in the time-course evolution of the photochemical activity taking place in photosystem II (PSII) was analyzed in the samples of Chroococcidiopsis sp. cultures subjected to different durations of ultrasound pulses (Fig. 4B). The light photochemical usage by ultrasound-treated cultures seems to have the same efficiency as the control culture since the values obtained were between 0.2 and 0.4, which are typical of photosynthetically active cultures of cyanobacteria (Zijffers et al. 2010; Schuurmans et al. 2015). Only the early values of Fv/Fm of ultrasound treated cultures, varying between 0.14 and 0.18, were lower than the expected values of healthy cyanobacterial cells.
Accordingly, if the pulse duration is optimized, as shown above, the productivity of Chroococcidiopsis sp. in the liquid medium can remain unaltered or even increase slightly by the LFU treatment. In addition, a side consequence of the LFU treatment is the improved extraction of the biomass with organic solvents, as proven by testing the pigment extraction which resulted in increased extracted contents of chlorophylls and carotenoids (Fig. S3, Supporting Information). This might be of interest to improve extraction yields at large scale.
Effect of ultrasound treatment on the sedimentation velocity of Chroococcidiopsis sp. culture
It has been demonstrated that the disaggregating effect of ultrasound pulses on cell aggregates of Chroococcidiopsis sp. allows the maximum optical density to be achieved at approximately 3 min of ultrasound pulse. As shown in Fig. 2, one of the main consequences of cell disaggregation is the variation in the sedimentation velocity (Fig. 5) of ultrasound-treated cultures. According to Stokes' Law, particle size has the greatest influence on the sedimentation velocity. In this study, the average aggregates’ radius was calculated and shown in Fig. S2, which was used to obtain the sedimentation velocities of Chroococcidiopsis sp. culture samples. According to the figure, the sedimentation velocity decreased with an increase in the ultrasound pulse duration. This resulted in approximate variations between 0.015 and 0.002 m h–1 for control samples and samples subjected to ultrasound pulses equal to or greater than 3 min, respectively.
Based on these results, it can be suggested that under suitable culture conditions, the disaggregation produced by low-frequency ultrasound pulses in cultures of Chroococcidiopsis sp. can favor their growth. This could be at least partly attributed to the greater availability of light and nutrients per cell. Also, the operating conditions of the cultures could reduce economic and energy costs in the agitation processes necessary for the homogenization of cultures, which is discussed in the next section.
Discussion
Effect of ultrasound treatment on the aggregation of Chroococcidiopsis sp. cultures
The results indicated a direct relationship between the duration of the applied ultrasound pulses and the increase in the optical density value of ultrasound-treated samples. We attribute this relationship to the disaggregation caused by the ultrasound treatment in the cell aggregates, which was evidenced by the light micrographs. In a more disaggregated sample of liquid culture, the cell suspension becomes homogeneously distributed with an increase in the number of single cells and smaller aggregates (Fig. 2). An increased density of cell entities in the ultrasound-treated samples interferes with the light passage in the spectrophotometer cuvette, resulting in a higher spectrophotometer reading. The higher optical density of ultrasound treated samples could be explained by the fact that the number of non-aggregated cells and small aggregates descend slower than larger aggregates, as evidenced by the kinetics obtained through the time-course evolution of optical density (Figure S1, in Supporting Information). As mentioned in the Materials and Methods section, Stokes’ law assumes the particle size to be the parameter that exhibits a greater influence on the sedimentation velocity. Hence, our results are in good agreement with the hypothesis suggesting that the non-aggregated cells and small aggregates to descend slower than larger aggregates (Fig. 5). This has a consequence in the culture sedimentation velocity, with a side consequence in the energy costs of the production process (discussed below). In this work, we have intended to infer a conclusion which can be generally applied to cyanobacterial cultivation: the chemical interaction between cyanobacterial exopolysaccharides in viable cells can be energetically exceeded by an optimized low energy ultrasound pulse. The key aspect of this work is that a minimum ultrasound pulse time has been defined that ensures cell disaggregation and retention of the full photosynthetic capacity, as shown in Results and discussed below.
Effect of ultrasound treatment on the growth and viability of Chroococcidiopsis sp.
As expected, our data supported the positive impact of modulated ultrasound pulses on the growth of Chroococcidiopsis sp. cultures. The ultrasound-mediated cultures increased disaggregation and greater light and nutrient accessibility were provided to individual cells in liquid cultures previously occupying inner positions in the large Chroococcidiopsis sp. cell clusters before the ultrasound treatment. The data obtained on the efficiency of the photochemical processes, in turn, demonstrated that the ultrasound treatment with the optimized duration of the pulse did not affect cell viability since the Fv/Fm values obtained were within the range of values typical for cyanobacteria in liquid cultures (Schuurmans et al. 2015). The decrease in Fv/Fm after the first 24 h (Fig. 4B) is a common situation in almost each culture treatment for the cultures being adapting to the new conditions. In this study, we hypothesized that the initial decrease in Fv/Fm for ultrasound-treated cultures was due to the cells being more disaggregated. Therefore, they experienced a more intense light exposure compared to the cells in non-disaggregated cell clusters, which needed to acclimatize to the newly experienced light conditions. Such acclimatization process can be temporarily linked to a less productive photochemical yield of the photosynthetic light fixation process (Richmond 2004). The sudden increase in the light intensity experienced by the individual cells derived from the ultrasound-mediated disaggregation of Chroococcidiopsis sp. could be the reason behind the Fv/Fm decrease, and the later increase of Fv/Fm to regular values is on favor of this argument. Interestingly, the reduced disaggregation of the cyanobacterial cultures subjected to ultrasound pulses results in a higher ratio between photosynthesis and respiration (Fig. S4, Supporting Information). This demonstrates a greater predominance of the light-dependent oxygen production reactions in relation to those involved in oxygen consumption (the so-called dark phase), thus leading to suggest that light is more available to the cyanobacterial cells in a disaggregated culture, in coherence with the results of growth. Below the compensation point there is a net loss of organic matter (Kirst et al. 2014), and conversely, above the compensation point – in viable cultures with regular values of Fv/Fm – the growth is favored, as unveiled by the obtained results. Whether the maintenance of this pattern of improved photosynthetic capacity in disaggregated cells requires frequent application of LFU treatment, remains to be investigated. Investigating the light-harvesting antenna size in disaggregated cyanobacterial cultures of high cell density would be valuable to understand if rearrangements of the photosynthetic apparatus are taking place in disaggregated cells being subjected to higher irradiance. Such rearrangements might be expected in order to improve growth and productivity of cells adapted to higher irradiance. It has been suggested that substantial improvements in the photosynthetic efficiency and productivity of cyanobacterial mass cultures can be obtained upon minimizing the phycobilisome light-harvesting antenna size (Perrine et al. 2012), which has practical application in cyanobacterial mass production.
Cultivation strategies for effective biomass production
The sedimentation velocity is a key parameter in gravitational sedimentation that allows the comparison between different methods for biomass harvesting. Hence, it is useful in designing and optimizing cyanobacterial production processes (Quijano et al. 2017). Acceleration or deceleration of the culture sedimentation has a direct impact on the economy of the production. This fact makes it possible to discuss two possible cultivation strategies for the eventual effective production of Chroococcidiopsis sp. at a pilot scale and helps in deciding production conditions that can lead to savings in process costs. On the one hand, according to our results, the use of ultrasound decreased the state of cell aggregation and sedimentation velocity of the cultures saving on the agitation costs and allowing higher productivities, while on the other hand, the cultures not treated with ultrasound favored aggregation and sedimentation, resulting in a cheaper harvest of biomass compared to that of centrifugation. As shown in Table 1, this may represent up to 30% of the total costs in the production of microalgae (Molina et al. 2003).
Effect of ultrasound treatment on the sedimentation velocity of Chroococcidiopsis sp. cultures
As mentioned above, obtaining high biomass recovery levels during the harvest may help reduce energy costs. Here, we highlight that biomass recovery for Chroococcidiopsis sp. cultures seems to be significantly faster compared to other microalgal and cyanobacterial species, which were selected in the literature based on their commercial use (Table 1). As well-known and inferred from Stokes’ Law, the particle size influences the sedimentation velocity greatly. The large size of Chroococcidiopsis sp. aggregates, whose diameter is in the average range of 20 to 30 μm (S2, Supporting Information), is by far much larger than most of the unicellular microalgal cells (Table T1, Supporting Information), which obviously becomes advantageous for harvesting. The great stability of Chroococcidiopsis sp. aggregates is based on the natural linkage between cells through their outer EPS sheath that surrounds them. This also makes them resistant to mechanical agitation required to keep the aggregates suspended in liquid cultures (Montero et al. 2020), as evidenced by the time-course stability of the aggregate average radius. This stability feature can guarantee sedimentation at a larger scale. However, further studies are required to prove it. The eventual advantage of the stability of the aggregates needed for harvesting becomes a disadvantage for growth, which can be overcome by subjecting the cultures to modulated ultrasound pulses, as shown in this study. The decision of either producing or not producing disaggregated cultures depends on the aim of each bioprocess.
The influence of natural aggregation phenomena of microalgae and cyanobacteria on culture productivity seems to be scarcely analyzed in the scientific literature; the existing references mostly deal with analyzing the sedimentation velocity of each species based on the use of various factors or treatments that favor sedimentation (Smith and Davis 2012; Salim et al. 2013; Quijano et al. 2017). Therefore, it is difficult to compare the natural sedimentation velocities of specific species of microalgae and cyanobacteria due to the large number of physicochemical conditions that affect the aggregation. However, the dispersed single cells of microalgae have been reported to commonly have a sedimentation velocity range of 0.001 to 0.026 m h–1 (Choi et al. 2006) and the sedimentation velocities in our study (Fig. 5) were in this range. To improve cyanobacterial productivity, an optimized duration of ultrasound pulse was required to disaggregate culture. This was presented with a low sedimentation velocity, theoretically promoting an increase in the growth rate due to the greater accessibility of light and nutrients. The greater light availability to the cells was evidenced by the increased turbidity of the ultrasound-treated cultures (Fig. 2), indicating that more cells could capture incident photons in the treated cultures than in the aggregated control cyanobacterial cultures.
The natural aggregation of Chroococcidiopsis sp. can favor the economy of the harvesting process since larger particle size eases sedimentation or flocculation techniques, saving energy costs compared to centrifugation (Fasaei et al. 2018). However, additional energy costs may be required to keep cultures of large size aggregates in liquid medium homogeneously mixed (Montero et al. 2020). Meanwhile, since the light and nutrients in principle may be less accessible to the inner cells of the aggregates, maximal biomass productivity cannot be achieved as well.
Conclusions
Low-frequency ultrasound is effective in producing disaggregation of Chroococcidiopsis sp. cells clusters, making it an effective way according to our results. Disaggregation increases the homogeneity of suspension cultures reducing the energy requirements for culture agitation. In addition, cyanobacterial growth has been proven greater in disaggregated cultures, based on a demonstrated greater availability of light and, expectedly, nutrients. Overall, the adequate modulation of LFU pulses to cyanobacterial aggregated cultures should be considered as a valuable strategy to improved growth and save agitation energy costs.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Bürger R, Concha F (1998) Mathematical model and numerical simulation of the settling of flocculated suspensions. Int J Multiph Flow 24:1005–1023
Casero MC, Ascaso C, Quesada A, Mazur-Marzec H, Wierzchos J (2021) Response of endolithic Chroococcidiopsis strains from the polyextreme Atacama desert to light radiation. Front Microbiol 11:3607
Choi SK, Lee JY, Kwon DY, Cho KJ (2006) Settling characteristics of problem algae in the water treatment process. Water Sci Technol 53:113–119
Conradi FD, Zhou RQ, Oeser S, Schuergers N, Wilde A, Mullineaux CW (2019) Factors controlling floc formation and structure in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol 201:e00344-19
Cruz D, Vasconcelos V, Pierre G, Michaud P, Delattre C (2020) Exopolysaccharides from cyanobacteria: Strategies for bioprocess development. Appl Sci 10:3763
Cuaresma M, Janssen M, Vílchez C, Wijffels R (2011) Horizontal or vertical photobioreactors? How to improve microalgae photosynthetic efficiency. Bioresour Technol 102:5129–5137
Das P, Abdulquadir M, Chaudhary A, Thaher M, Khan S, Alghazal G, Al-jabri H (2018) Outdoor continuous cultivation of self-settling marine cyanobacterium Chroococcidiopsis sp. Ind Biotechnol 14:45–53
De Schryver P, Crab R, Defoirdt T, Boon N, Verstraete W (2008) The basics of bioflocs technology: the added value for aquaculture. Aquaculture 277:125–137
Dumorné K, Córdova DC, Eló MA, Renganathan P (2017) Extremozymes: A potential source for industrial applications. J Microbiol Biotechnol 27:649–659
Fasaei F, Bitter JH, Slegers PM, van Boxtel AJB (2018) Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res 31:347–362
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633
Georgi P (1996) Viscosity of algal cultures and estimation of turbulence in devices for the mass culture of microalgae. Arch Hydrobiol 114:99–104
Joyce E, Wu X, Mason T (2010) Effect of ultrasonic frequency and power on algae suspensions. J Environ Sci Health A 45:863–866
Joyce E, King P, Mason T (2014) The effect of ultrasound on the growth and viability of microalgae cells. J Appl Phycol 26:1741–1748
Kirst H, Formighieri C, Melis A (2014) Maximizing photosynthetic efficiency and culture productivity in cyanobacteria upon minimizing the phycobilisome light-harvesting antenna size. Biochim Biophys Acta Bioenerg 1837:1653–1664
Lee TJ, Nakano K, Matsumara M (2001) Ultrasonic irradiation for blue-green algae bloom control. Environ Technol 22:383–390
Li P, Harding SE, Liu Z (2001) Cyanobacterial exopolysaccharides: their nature and potential biotechnological applications. Biotechnol Genet Eng Rev 18:375–404
Milledge J, Heaven S (2013) A review of the harvesting of micro-algae for biofuel production. Rev Environ Sci Bio-Technol 12:165–178
Molina E, Belarbi EH, Acien FG, Robles A, Yusuf C (2003) Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv 20:491–515
Montero Z, Cordero JL, Garbayo I, Ascaso C, Wierzchos J, Vega JM, Vílchez C (2020) Identification, biochemical composition and phycobiliproteins production of Chroococcidiopsis sp. from arid environment. Process Biochem 97:112–120
Perrine Z, Negi S, Sayre RT (2012) Optimization of photosynthetic light energy utilization by microalgae. Algal Res 1:134–142
Phull SS, Newman AP, Lorimer JP, Pollet B, Mason TJ (1997) The development and evaluation of ultrasound in the biocidal treatment of water. Ultrason Sonochem 4:157–164
Quijano Q, Arcila JS, Buitrón G (2017) Microalgal-bacterial aggregates: Applications and perspectives for wastewater treatment. Biotechnol Adv 35:772–781
Raddadi N, Cherif A, Daffonchio D, Neifar M, Fava F (2015) Biotechnological applications of extremophiles, extremozymes and extremolytes. Appl Microbiol Biotechnol 99:7907–7913
Rajasekhar P, Fan L, Nguyen T, Roddick F (2012) A review of the use of sonication to control cyanobacterial blooms. Water Res 46:4319–4329
Richmond A (2004) Biological principles of mass cultivation. In: Richmond A (ed) Handbook of Microalgal Cultures. Biotechnology and Applied Phycology. Blackwell Science, Oxford, pp 125–177
Rosine T, Gifuni I, Lavenant L, Pruvost J, Marchal L (2018) Bead milling disruption kinetics of microalgae: Process modeling, optimization and application to biomolecules recovery from Chlorella sorokiniana. Bioresour Technol 267:458–465
Salim S, Bosma R, Vermuë M, Wijffels RH (2010) Harvesting of microalgae by bio-flocculation. J Appl Phycol 23:849–855
Salim S, Gilissen L, Rinzema A, Vermuë MH, Wijffels RH (2013) Modeling microalgal flocculation and sedimentation. Bioresour Technol 144:602–607
Schreiber U (2004) Pulse-Amplitude-Modulation (PAM) fluorometry and saturation pulse method: an overview. In: Papageorgiou G, Govindjee (eds) Chorophyll a fluorescence: A signature of photosynthesis. advances in photosynthesis and respiration. Springer, Dordrecht, pp 279–319
Schuurmans R, Alphen P, Schuurmans J, Matthijs H, Hellingwerf K (2015) comparison of the photosynthetic yield of cyanobacteria and green algae: Different methods give different answers. PLoS One 10:e0139061
Shuler ML, Kargi F (2005) Bioprocess Engineering: Basic Concepts, 2nd edn. Pearson Education Pearson Education, Singapore
Singh S, Kant C, Yadav RK, Reddy YP, Abraham G (2019) Cyanobacterial exopolysaccharides: composition, biosynthesis, and biotechnological applications. In: Mishra AK, Tiwari DN, Rai AN (eds) Cyanobacteria. Academic Press, Massachusetts, pp 347–358
Smith B, Davis R (2012) Sedimentation of algae flocculated using naturally-available, magnesium-based flocculants. Algal Res 1:32–39
Solovchenko AE, Merzlyak MN (2008) Screening of visible and UV radiation as a photoprotective mechanism in plants. Russ J Plant Physiol 55:719
Ummalyma SB, Mathew AK, Pandey A, Sukumaran RK (2016) Harvesting of microalgal biomass: Efficient method for flocculation through pH modulation. Bioresour Technol 213:216–221
Varshney P, Mikulic P, Vonshak A, Beardall J, Wangikar PP (2015) Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour Technol 184:363–372
Vítek P, Jehlicka J, Ascaso C, Masek V, Gomez-Silva B, Olivares H, Wierzchos J (2014) Distribution of scytonemin in endolithic microbial communities from halite crusts in the hyperarid zone of the Atacama Desert, Chile. FEMS Microbiol Ecol 90:351–366
Wierzchos J, DiRuggiero J, Vítek P, Artieda O, Souza-Egipsy V, Skaloud P, Tisza M, Davila AF, Vilchez C, Garbayo I, Ascaso C (2015) Adaptation strategies of endolithic chlorophototrophs to survive the hyperarid and extreme solar radiation environment of the Atacama Desert. Front Microbiol 6:934
Wierzchos J, Casero MC, Artieda O, Ascaso C (2018) Endolithic microbial habitats as refuges for life in polyextreme environment of the Atacama Desert. Curr Opin Microbiol 43:124–131
Wu X, Joyce E, Mason T (2011) Effect of ultrasound on cyanobacteria in water. Harmful Algae 10:738–743
Zeng X, Guo X, Su G, Danquah MK, Chen XD, Lin L, Lu Y (2016) Harvesting of microalgal biomass. In: Bux F, Chisti Y (eds) Algae Biotechnology. Green Energy and Technology. Springer, Cham, pp 77–89
Zijffers JWF, Schippers KJ, Zheng K, Janssen M, Tramper J, Wijffels RH (2010) Maximum photosynthetic yield of green microalgae in photobioreactors. Mar Biotechnol 12:708–718
Acknowledgements
CV and JW are thankful for financial support by PGC2018-094076-B-I00 grant from MCIU/AEI (Spain) and FEDER (UE). The authors would like to thank to M.C. Casero (MNCN, CSIC) for cyanobacteria isolation from gypsum rocks, and V. Beltrán (University of Huelva) for her technical assistance.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Robles, M., Garbayo, I., Wierzchos, J. et al. Effect of low-frequency ultrasound on disaggregation, growth and viability of an extremotolerant cyanobacterium. J Appl Phycol 34, 2895–2904 (2022). https://doi.org/10.1007/s10811-022-02831-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02831-x