Abstract
The kelp Ecklonia radiata has become a target for controlled cultivation. However, to date there are no standardised protocols for the hatchery stage of this species that result in high rates of germination, gametophyte development and transition to sporophytes. Therefore, the objective of this study was to quantify the effect of photoperiod, light intensity, temperature, nutrient media and use of GeO2 on the key hatchery processes of germination, gametophyte development and transition to sporophytes in controlled laboratory experiments. Germination of E. radiata was high (≥ 85%) throughout the study, regardless of treatments. Temperature had a major effect on the length of gametophytes, which increased with increasing temperature. The formation of sporophytes was favoured when individuals were maintained under 17 °C continuously, while reduced by approximately 30% when using F/2 compared to PES nutrient media. Overall, the recommended conditions for the hatchery stage of E. radiata are to maintain cultures under a 12 h L:12 h D photoperiod at 17 °C as this resulted in higher germination rates, good gametophyte development and higher transition to sporophytes compared to other treatments. Moreover, the use of GeO2 has to be limited to no more than 2 days as extended use has detrimental effects on the development of sporophytes. Finally, storage of sorus-bearing fronds of sporophytes up to 4 days after the collection from the field generally increased the number of released zoospores and is a simple mechanism to increase the fertility of brood stock.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The brown seaweed Ecklonia radiata (C. Agardh) J. Agardh (order Laminariales, commonly known as kelps) has become a target for habitat conservation (Layton et al. 2021; Tatsumi et al. 2021), restoration projects (Layton et al. 2021), as well as for commercial seaweed cultivation to provide a wide range of high-value products (Winberg et al. 2011; Wiltshire et al. 2015). All of these applications require the controlled cultivation of E. radiata, including the induction of the release of zoospores, as well as controlled cultivation of early life history stages in a hatchery. Ecklonia radiata has a typical kelp life cycle characterised by a microscopic gametophyte stage which alternates with the macroscopic sporophyte stage (Nelson 2013). Crucially, the transition to the sporophyte stage occurs only under favourable conditions (Nelson 2005; Carney and Edwards 2010; Martins et al. 2017) and the effect of abiotic factors on the development of each of the life stages of E. radiata is poorly understood. Previous studies have identified low rates of transition to sporophytes, with more than half of the seeded surfaces failing to produce sporophytes (Tatsumi and Wright 2016) and transition rates from female gametophyte to sporophyte as low as 10% (Tatsumi and Wright 2016) to 55% (Tatsumi et al. 2022). Therefore, a critical first step towards achieving the controlled cultivation of E. radiata for any application is to develop standardised protocols for the hatchery stage that result in high rates of germination and transition from gametophyte to sporophyte.
A range of factors affect reproduction in kelps and therefore need to be considered for the development of standardised protocols for the cultivation of E. radiata in hatcheries. Kelp species are widespread in temperate regions, which are characterised by strong seasonal variation in daylength, light intensity and temperature (Lüning 1993). In general, growth and reproduction of kelps show clear seasonal patterns (Kain 1979; Novaczek 1984a; Lee and Brinkhuis 1988; Fairhead and Cheshire 2004; Bartsch et al. 2008; Miller et al. 2012; Boderskov et al. 2021) and the reproduction of sporophytes (formation of sorus) is driven by short daylengths (Lüning 1988; Pang and Lüning 2004; Boderskov et al. 2021) and occurs during periods of low or no growth (Kain 1979). Similarly, daylength is the trigger for the reproduction of gametophytes in kelps, although the requirements for the formation of antheridia and oogonia (gametogenesis) are species-specific (Choi et al. 2005; Nelson 2005). While it has been demonstrated that female gametogenesis in Ecklonia requires irradiances above 10–20 µmol photons m−2 s−1 (Bolton and Levitt 1985; Tatsumi and Wright 2016), there is a lack of understanding of how daylength affects gametogenesis. A further key driver affecting the transition of life stages and growth in kelps is temperature (Nelson 2005; Bartsch et al. 2008). Notably, the temperature window for gametogenesis in kelps is narrower than for the germination of zoospores and growth of gametophytes (Lee and Brinkhuis 1988; Müller et al. 2008; Martins et al. 2017; Augyte et al. 2019; Paine et al. 2021). This has also been demonstrated for E. radiata (Novaczek 1984b; Mabin et al. 2013). Additionally, the optimal temperature range for vegetative and reproductive development of E. radiata can differ between ecotypes (Novaczek 1984b; Mohring et al. 2014).
Nutrient medium is another important consideration for the development of standardised protocols for hatchery cultivation of E. radiata. A range of nutrient media have been used in studies of kelp reproduction (Jennings 1967; Zhang et al. 2008; Kerrison et al. 2016; Mabin et al. 2019). F/2 and Provasoli Enriched Seawater (PES) are the most commonly used culture media for E. radiata (Mabin et al. 2013; Mohring et al. 2014; Tatsumi and Wright 2016) and studies using these media have reported successful germination, and growth of gametophytes and sporophytes. However, their particular effects on the growth and transition of each life stage for E. radiata remain unknown and it is important to close this knowledge gap. Similarly, the use of germanium dioxide (GeO2) to control diatom growth during the early stages of kelp cultivation differs substantially between studies. While some studies omit the use of GeO2 entirely (Lee and Brinkhuis 1988; Bartsch et al. 2013; Tatsumi and Wright 2016; Augyte et al. 2019), in other studies the duration of its application varies from the first few days of the cultivation period (Lüning 1981; Novaczek 1984b; Kerrison et al. 2016) to continuous use (Bolton and Levitt 1985; Murúa et al. 2013). Notably, the use of GeO2 can affect the reproduction of gametophytes and the development and growth of sporophytes in kelps (Markham and Hagmeier 1982; Shea and Chopin 2007), including E. radiata (Novaczek 1984b). Therefore, the effects of GeO2 need to be considered for the cultivation of E. radiata as contamination with diatoms can have detrimental effects on growth of E. radiata during the hatchery stage.
Finally, delaying the initiation of zoospore release by storing the sorus-bearing fronds of sporophytes for a few days in the laboratory after collection from a field site allows the time window that zoospores can be obtained to be extended. This is of particular importance when collection in the field is strongly weather-dependent or in a remote location resulting in a limited time window to collect biomass and process it upon return to the laboratory. Studies on E. radiata have initiated zoospore release on the day of collection (Mohring et al. 2013; Suebsanguan et al. 2021) or one day after collection (Tatsumi and Wright 2016; Tatsumi et al. 2022). However, the effect of delaying the initiation of zoospore release on the quantity and viability of released zoospores remains unknown and needs to be considered, including whether initiation of zoospore release can be delayed for more than one day following collection.
The aim of this study was to quantify optimal conditions for the hatchery stage of E. radiata resulting in high germination rates of zoospores and a high transition from gametophytes to sporophytes. The effects of the following fundamental factors for controlled cultivation were tested in laboratory-based experiments: (1) photoperiod in combination with light intensity, (2) temperature, and (3) nutrient medium and the use of GeO2. For each experiment we determined the germination rate after 48 h, the size of gametophytes and the female:male sex ratio after 12 days, and the proportion of female gametophytes transitioned to sporophytes and their size after 30 days. Finally, the effect of storage of sorus-bearing fronds of sporophytes for up to four days after collection was quantified as the number and viability of zoospores. These experiments will provide an optimised baseline method for the cultivation of the early life history stages of E. radiata under highly controlled conditions.
Materials and methods
Study species
Ecklonia radiata (C. Agardh) J. Agardh is common and abundant along the temperate rocky coasts of Australia and New Zealand and is frequently the dominant sub-tidal canopy forming species in these habitats (Kirkman 1981; Novaczek 1981; Nelson 2013). Ecklonia radiata alternates between a microscopic gametophyte stage and a macroscopic sporophyte stage. Reproductive sporophytes develop reproductive tissue (termed sorus, plural: sori), which releases zoospores. These subsequently settle and germinate into filamentous male and female gametophytes (Fig. 1). These microscopic gametophytes are dioceious and show sexual dimorphism with male gametophytes being generally narrower and more branched compared to female gametophytes (Mabin et al. 2013). Female gametophytes produce non-motile oogonia, each containing an egg, which produce a zygote after being fertilized by the motile antherozoids produced by the male gametophyte. The zygote develops into a young sporophyte (Fig. 1) and individuals can reach an age of 10 years (Novaczek 1981).
Life history stages of E. radiata. (a) Germinated zoospores (circled) 48 h post seeding characterised by a dumbbell shape. Scale bar = 200 µm. (b) Female (F) and male (M) gametophytes after 12 days of cultivation under 17 °C and a photoperiod of 12 h L:12 h D. Female and male gametophytes are distinguishable after approximately 10 days of cultivation by differences in size and morphology. Female gametophytes are comprised of larger cells and the filaments are less branched than males. Scale bar = 200 µm. (c) Female gametophyte with extruded eggs (arrow) typically observed after 20 days of cultivation. A sporophyte develops within 24 h of fertilisation of an egg marked by the first visible transverse cell division of the zygote (circled). Scale bar = 200 µm. (d) Development of elongated sporophytes around 25 days of cultivation. Scale bar = 200 µm. (e) Young sporophytes with longitudal separation develop around 25 days of cultivation. Sporophytes form rhizoids at the basal portion of the blade. Scale bar = 500 µm
Sample collection and zoospore release
Fertile fronds of sporophytes with visible sori (distinct dark and raised patches) were collected by hand from subtidal areas south of Mount Maunganui, Tauranga, Aotearoa New Zealand (37° 38' S 176° 10’ E) for each experiment and returned in a mesh bag exposed to air to the University of Waikato Coastal Marine Field Station, Tauranga, within 45 min of collection (collection permit SP742-3). Fronds were maintained in 10 L buckets with filtered seawater for 1–4 days with gentle aeration. To initiate the release of zoospores, sori were excised from each frond and then cleaned by gently scraping, rinsing with autoclaved filtered seawater (AFSW) and wiping down the surface with lint free tissues to remove contaminants. Subsequently, sori were disinfected in a NaOCl bath (200 ppm, ambient temperature ~ 20 °C) for 2 min following Forbord et al. (2018) and then placed on a plastic tray and left uncovered at ambient temperature for 60 min to desiccate. Sori were then placed in a beaker filled with AFSW and occasionally stirred. After 30 min, the resultant zoospore solution was strained using a 120 µm filter followed by a 37 µm filter to remove sori and organic debris respectively. The spore density of the zoospore solution was determined using a haemocytometer and subsequently adjusted to 2,500 spores mL−1 in AFSW. Provasoli (PES) nutrient medium was added to allow for non-limiting conditions throughout the cultivation period (Mohring et al. 2013) unless stated otherwise. GeO2 was used for the first 2 days to eliminate diatoms (Novaczek 1984b; Redmond et al. 2014). A stock solution containing 250 mg GeO2 L−1 was prepared and added at a concentration of 2 mL L−1 to the culture media following Redmond et al. (2014), resulting in a concentration of 0.5 mg GeO2 L−1 in the culture medium. Water in the culture dishes was changed weekly.
Experiment 1: Effect of photoperiod and light intensity on zoospore germination, gametophyte development and transition to sporophyte
To determine the effect of photoperiod and light intensity on zoospore germination, development of gametophytes and transition to sporophytes, a total of 140 Petri dishes (90 × 20 mm, LabServ, LBS60016) were seeded with zoospores obtained from sori collected in October 2021 as described above. Each Petri dish contained a glass microscope slide, which was pre-cleaned using Decon-90 and 10% hydrochloric acid (v/v) according to Kerrison et al. (2016) and 20 mL of the spore suspension was added to each Petri dish, equating to 50,000 spores per dish. The dishes were maintained in environmental control cabinets (Bio-strategy; MLR-352) at 17 °C under the following three photoperiods for the first 13 days of cultivation: 8 h L:16 h D (short day), 12 h L:12 h D (normal day), and 16 h L:8 h D (long day). The light intensity for all photoperiods during this period was 35 µmol photons m−2 s−1 and was measured using a light sensor (LI-192, LI-COR, USA) connected to a light sensor logger (LI-1500; LI-COR, USA). After 48 h post seeding, the numbers of germinated zoospores (characterised by a germination tube and/or dumbbell shape) and non-germinated zoospores were quantified in 10 haphazardly selected FOV using an inverted microscope (Olympus CKX53) to determine the proportion (%) of settled zoospores in half the dishes (n = 30 for 8 h L:16 h D, n = 20 for 12 h L:12 h D and 16 h L:8 h D). Subsequently, the spore suspension in all dishes was gently poured off and replaced with nutrient enriched (PES) AFSW. Culture water was changed weekly and the position of the dishes rearranged after each water change. All dishes were then returned to the cabinets and maintained under the same conditions. After 12 days post seeding, the same dishes were used to quantify the female:male sex ratio of gametophytes in 5 haphazardly selected fields of view (FOV), as well as the size (length) of 5 haphazardly selected female and male individuals using an inverted microscope (Olympus CKX53) and the Olympus CellSens Entry Software (Version 2.3). Dishes used for these measurements were discarded afterwards to exclude any confounding effects on sporophyte development. After 13 days post seeding, the remaining 70 dishes were either maintained at their previous photoperiod and light intensity or allocated to their previous photoperiod under increased light intensity, or a new photoperiod under the same or increased light intensity (Fig. 2a). Dishes maintained under the short photoperiod of 8 L:16 h D for the initial culture period were transferred to the following photoperiods after 13 days post seeding: 8 h L:16 h D, 12 h L:12 h D, and 16 h L:8 h D. The light intensities for those photoperiods were 35 and 55 µmol photons m−2 s−1 (Fig. 2a). Dishes maintained under 12 h L:12 h D and 16 h L:8 h D were transferred to the following photoperiods: 12 h L:12 h D, and 16 h L:8 h D with the light intensities for each photoperiod being 35 and 55 µmol photons m−2 s−1 (Fig. 2a). Each treatment had 5 replicates (n = 5), resulting in 70 dishes. After 30 days post seeding the proportion of female gametophytes that transitioned to sporophytes was determined in 8 haphazardly selected FOV and the length of the 8 biggest sporophytes was determined for each dish. Sporophytes were classified as such with the first visible cell division of a zygote.
(a) Experimental design for experiment 2 testing the effects of photoperiod and light intensity showing photoperiods (L:D) for the initial 13 days of cultivation (day 0–13) and onwards (day 13–30). Light intensity was 35 µmol photons m−2 s−1 for the initial 13 days and was maintained or increased to 55 µmol photons m−2 s−1 after 13 days of cultivation. (b) Experimental design for experiment 3 testing the effect of temperature. Temperatures were either maintained at their initial temperature over 30 days or allocated to a new temperature after 13 days of cultivation
The experiment used three culture cabinets for the first 13 days, with each culture cabinet set at one of the tested photoperiods (8 h L:16 h D, 12 h L:12 h D, and 16 h L:8 h D) at a light intensity of 35 µmol photons m−2 s−1. After 13 days only two cabinets were used with one set at 35 µmol photons m−2 s−1 and one set at 55 µmol photons m−2 s−1. Both were set at the longest photoperiod of 16 h L:8 h D and shorter photoperiods (12 h L:12 h D and 8 h L:16 h D) were achieved by covering and uncovering dishes with customised folded blackout covers.
Experiment 2: Effect of temperature on zoospore germination, gametophyte development and transition to sporophyte
To quantify the effect of temperature on zoospore germination, development of gametophytes and transition to sporophytes, a total of 108 pre-cleaned glass slides were seeded with zoospores obtained from sori collected in November 2021 and placed in Petri dishes as described above. The dishes were maintained under temperatures of 14, 17 and 20 °C, which are representative of water temperatures near Tauranga in winter, spring/autumn and summer, respectively (Chappell 2013). Germination was quantified in half the dishes (n = 18 for each treatment) after 48 h as described above, followed by a water change with nutrient enriched (PES) AFSW as described above. Culture water was changed weekly and the position of the dishes rearranged after each water change. All dishes were then returned to the cabinets and maintained under the same conditions. After 12 days, the same dishes were used to quantify the sex ratio of gametophytes in 5 haphazardly selected FOV, as well as the size (length) of 5 haphazardly selected female and male gametophytes using an inverted microscope (Olympus CKX53) and the Olympus CellSens Entry Software (Version 2.3). Dishes used for these measurements were discarded afterwards to exclude any confounding effects on the sporophyte development. The remaining 54 dishes were either maintained at their previous temperatures or allocated to a new temperature (14, 17 and 20 °C) on day 13 (Fig. 2b) with 6 replicates for each treatment (n = 6) to determine whether a change in temperature affects the transition from gametophytes to sporophytes. After 30 days, the number of female gametophytes transitioned to sporophyte was determined in 8 haphazardly selected FOV and the length of the 8 biggest sporophytes was determined for each dish. For this experiment, the number of female gametophytes transitioned to sporophyte was also determined on day 37 to allow for the fact that lower temperatures might slow the development of both gametophytes and sporophytes. The experiment used three culture cabinets (Bio-strategy; MLR-352) with each culture cabinet set at one of the tested temperatures and a photoperiod of 12 h L:12 h D (35 µmol photons m−2 s−1).
Experiment 3: Effect of nutrient medium and use of germanium dioxide on zoospore germination, gametophyte development and transition to sporophyte
To determine the effect of nutrient medium and the use of germanium dioxide (GeO2) on zoospore germination, development of gametophytes and transition to sporophytes, both factors were fully crossed. PES and F/2 were used as culture media and the following durations of GeO2 exposure were tested for each culture media: 0, 2, 9, and 30 days with 6 replicates for each treatment (n = 6) resulting in 48 dishes in total. All Petri dishes contained a pre-cleaned glass microscope slide and were seeded with zoospores obtained from sori collected in October 2021 as described above. Petri dishes were maintained in a culture cabinet (Bio-strategy; MLR-352) at 17 °C under a photoperiod of 12 h L:12 h D (35 µmol photons m−2 s−1). Germination (day 2), the sex ratio of female and male gametophytes (day 12), and the size (length) of female and male individuals (day 12) were quantified as described above. After 30 days, the proportion of female gametophytes that transitioned to sporophytes was determined in 8 haphazardly selected FOV and the length of the 8 biggest sporophytes was determined for each sample.
Experiment 4: Delaying the initiation of zoospore release
To quantify the effect of delaying the initiation of zoospore release for up to four days following collection on the number and viability of released zoospores, fronds of E. radiata sporophytes with visible sori were collected on three separate occasions in September and October 2021. Upon return to the laboratory, fronds were weighed and 12 randomly selected fronds were allocated to each of four 5 L plastic buckets filled with 4 L filtered seawater. Each bucket was gently aerated through a ceramic air stone and placed in a temperature and light controlled room (18 °C, 12 h L:12 h D, ≤ 6 µmol photons m−2 s−1). The total fresh weight of biomass collected on each occasion was similar (approximately 85 g fresh weight). The release of zoospores was initiated after 1, 2, 3, and 4 days following collection, with each bucket randomly allocated to one of these treatments on the day of collection. As a control, spore release was also initiated on the day of collection (day 0) using 12 randomly selected fronds. The water in the buckets was changed after two days and water samples were taken from each bucket daily to check for the presence of zoospores. The number of zoospores released while sorus-bearing fronds were stored in buckets for 1 to 4 days was calculated by adding the numbers of zoospores found in the daily water samples for each bucket. The number of zoospores released during storage was then calculated as a proportion of the total number of zoospores released (e.g., during storage and the initiation process) to determine whether storing the sorus-bearing fronds resulted in a significant loss of zoospores prior to the initiation process. It was anticipated that this would ensure that if low numbers of zoospores were obtained during the delayed initiation process it would not be due to a high number of zoospores released during storage. Zoospores are expected to settle and adhere to the bucket within 24 h and therefore the number of zoospores found in daily water samples were expected to have been released within this time period. The total number of released zoospores was calculated by adding the number of zoospores released during storage and during the initiation process.
To account for differences in sori weight across samples and allow for comparability across days and collections, the amount of AFSW used after the desiccation step to obtain zoospores was standardised to 150 g sori in 1 L of AFSW (e.g. 15 g sori were placed in 100 mL AFSW). The density of zoospores was determined after 30 min and adjusted to 2,500 spores/mL as described above. Petri dishes (90 × 20 mm; LabServ LBS60016) were filled with 20 mL of the diluted spore suspension (n = 5) and subsequently placed in a culture cabinet (Bio-strategy; MLR-352) at 17 °C under a 12 h L:12 h D photoperiod (15–35 µmol photons m−2 s−1). To quantify the viability of the spores, germination was determined after 48 h in 8 haphazardly selected FOVs in each dish using an inverted microscope (Olympus CKX53). Experiments were conducted with three independent collections of E. radiata with each collection being a replicate (n = 3).
Statistical analysis
Experiment 1: A one-factor PERMANOVA was used to quantify the differences in germination (48 h), length of female and male gametophytes and the female:male sex ratio (day 12) between the three photoperiods (fixed factor) in which seeded slides were maintained for the first 13 days of the experiment. A three-factor PERMANOVA was used to quantify differences in the transition from gametophyte to sporophyte, and the length of sporophytes on day 30 at each of the photoperiod treatments, which comprised of a photoperiod treatment during the initial 13 days of cultivation (fixed factor) and final stages of cultivation (day 13–10; fixed factor), under constant or increasing light intensity over the 30 day experiment (fixed factor).
Experiment 2: A one-factor PERMANOVA was used to quantify the effect of temperature (fixed factor) on the germination (48 h), length of female and male gametophytes and the female:male sex ratio (day 12) for the first 13 days of the experiment. A two-factor PERMANOVA was used to determine the effect of the temperature during the initial (day 0–13; fixed factor) and final stages of cultivation (day 13–30; fixed factor) on the transition to sporophytes and their length after 30 days of cultivation.
Experiment 3: A two-factor PERMANOVA was used to determine the effect of nutrient medium (fixed factor) and exposure period of GeO2 (fixed factor) on the germination (48 h), length of female and male gametophytes and the female:male sex ratio (day 12), and the transition from female gametophytes to sporophytes and their length (day 30). Due to the absence or low number of sporophytes on the slides exposed to GeO2 for ≥ 9 days, only data from the 0 and 2 days exposure to GeO2 were used to quantify differences in the length of sporophytes.
Experiment 4: The effect of delaying the initiation of zoospore release by storing sori over up to 4 days on the number of released zoospores and germination was assessed, with days of storage as fixed factor and collection as random factor.
The PERMANOVA analyses were performed using PRIMER 7 and PERMANOVA + . All PERMANOVA tests presented here used Euclidean similarity matrices and P-values were calculated using permutations of residuals under a reduced model with 9,999 random permutations for multi-factorial PERMANOVAs and unrestricted permutations of raw data for one-factor PERMANOVAs. If there was a significant difference, pair-wise a posteriori comparisons were made among significant groups. Differences were considered significant of P < 0.05, non-significant results are not reported.
Results
Experiment 1: Effect of photoperiod and light intensity on zoospore germination, gametophyte development and transition to sporophyte
Overall, zoospore germination was high (> 93%) and consistent among each treatment. There were significant (one-factor PERMANOVA: F(2,67) = 7.93, P < 0.001) but small differences in germination rates between the photoperiod treatments (Table 1). Germination rates were significantly lower under the short-day photoperiod of 8 L:16 D compared to longer day photoperiods of 12 L:12 D and 18 L:6 D (pair-wise a posteriori comparisons; P < 0.001).
In general, the length of female gametophytes was larger than male gametophytes on day 12 across all of the photoperiods (Table 1). Photoperiod had a significant effect on the length of both female (F(2,67) = 16.85, P < 0.001) and male gametophytes (F(2,67) = 16.85, P < 0.001) with their lengths being significantly different across all photoperiods (pair-wise a posteriori comparisons; P ≤ 0.003). Both female and male gametophytes had the largest length under the normal-day photoperiod of 12 L:12 D (Table 1). Photoperiod had no effect on the sex ratio of gametophytes on day 12 post seeding (Table 1).
The transition from female gametophytes to sporophytes after 30 days of cultivation was neither affected by initial photoperiod (day 0–13 post seeding; three-factor PERMANOVA: F(2, 56) = 0.133, P = 0.88), final photoperiod (day 13–30 post seeding: F(2,56) = 0.641, P = 0.54), nor light intensity (F(1,56) = 0.358, P = 0.55) (Fig. 3a). The mean transition rate ranged from 11.5 ± 2.0% to 33.3 ± 17.9%. Notably, the transition rate was highly variable among replicates within each treatment (Fig. 3a), with the biggest variation in transition rate among replicates within a treatment ranging from 0 to 100%. Only two of the 70 replicates had no sporophytes formed after 30 days.
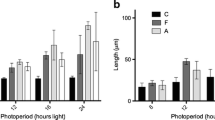
Effects of light intensity and photoperiod in experiment 1. (a) Mean (n = 5; ± S.E.) transition from female gametophyte to sporophyte (%) after 30 days. (b) Mean (n = 5; ± S.E.) length (µm) of sporophytes after 30 days. Samples were maintained under different photoperiods (L:D) for the initial 13 days of cultivation (day 0–13, indicated above the graphs and dashed lines) and onwards (day 13–30; see axis label). Light intensity was maintained at 35 µmol photons m−2 s−1 for 30 days of cultivation or increased to 55 µmol photons m−2 s−1 after 13 days of cultivation
The average length of sporophytes after 30 days of cultivation ranged from 87.9 ± 19.5 µm to 409.5 ± 120.0 µm (Fig. 3b). The length of sporophytes after 30 days post seeding was affected by the initial photoperiod during the first 13 days of cultivation during the gametophyte stage (3-factor PERMANOVA: F(2,54) = 8.380, P = 0.001), while neither photoperiod (F(2,54) = 0.853, P = 0.43) nor light intensity (F(1,54) = 0.418, P = 0.52) from 13 days of cultivation onwards had an effect. In general, sporophytes were bigger when maintained under a photoperiod of 16 L:8 D for the initial 13 days of cultivation, regardless of the photoperiod or light intensity samples were maintained from 13 days of cultivation onwards. These differences were significant when comparing the initial short- and long-day photoperiods (pair-wise a posteriori comparisons; P < 0.001).
Experiment 2: Effect of temperature on zoospore germination, gametophyte development and transition to sporophyte
Temperature significantly affected zoospore germination (one- factor PERMANOVA: F(2,51) = 5.73, P = 0.006), although differences across treatments were minor (Table 2). Significantly more zoospores germinated at 17 °C (pair-wise a posteriori comparisons; P ≤ 0.035) than under colder (14 °C) and warmer temperature conditions (20 °C).
Temperature significantly affected the lengths of both female and male gametophytes, which were similar within each temperature treatment (Table 2). Warmer temperatures resulted in larger lengths for both male and female gametophytes. The length of gametophytes approximately doubled from 14 to 17 °C, and increased by approximately 50% from 17 to 20 °C. The sex ratio of gametophytes was not affected by temperature and was 1.0 under all temperature conditions (Table 2).
The transition from female gametophytes to sporophytes was generally low and ranged from 2.7 ± 1.3% (14 °C for 30 days) to 12.8 ± 3.9% (17 °C for 30 days) after 30 days of cultivation and from 5.0 ± 2.1% (14 °C for 37 days) to 10.4 ± 2.9% (Day 0–13: 20 °C; day 13–37: 14 °C) after 37 days of cultivation (Fig. 4a). Notably, temperature had no effect on the transition to sporophytes and there was no clear trend. However, the length of sporophytes (Fig. 4b) was significantly affected by temperature during the initial (day 0–13 post seeding; two-factor PERMANOVA: F(2,29) = 3.53, P = 0.042) and final stages of cultivation (day 13–30 post seeding; F(2,29) = 3.93, P = 0.032). Sporophytes were generally smaller when cultivated under 14 °C during the initial cultivation period (day 0–13 post seeding) compared to 17 °C (pair-wise a posteriori comparisons; P < 0.024), and when cultivated under 14 °C compared to 20 °C during the final cultivation period (day 13–30 post seeding) (pair-wise a posteriori comparisons; P < 0.006). After 30 days of cultivation, 15 of the 54 replicates had no sporophytes formed and this number reduced to 10 after 37 days of cultivation.
Effects of temperature in experiment 2. (a) Mean (n = 6; ± S.E.) transition from female gametophyte to sporophyte (%) after 30 and 37 days. (b) Mean (n = 6; ± S.E.) length (µm) of sporophytes after 30 days. Samples were maintained under different temperatures (°C) for the initial (day 0–13) and final stages (day 13–37) days of cultivation
Experiment 3: Effect of nutrient medium and use of germanium dioxide on zoospore germination, gametophyte development and transition to sporophyte
Zoospore germination was not affected by nutrient medium (F(1,40) = 0.131, P = 0.72) or the use of GeO2 (F(3,40) = 0.871, P = 0.47) and ranged from 93.3 ± 1.4% (F/2 with continuous use of GeO2) to 96.4 ± 1.1% (F/2, no Ge02).
The type of nutrient medium affected the length of female gametophytes on day 12 (two-way PERMANOVA: F(1,40) = 10.465, P = 0.002), but the use of GeO2 had no clear effect (F(3,40) = 1.021, P = 0.392) (Fig. 5a). Female gametophytes were significantly larger when cultivated in F/2 compared to PES (pair-wise a posteriori comparisons; P = 0.003). Notably, the length of female gametophytes was more variable in F/2 (100.4 ± 3.4 µm to 120.4 ± 6.6 µm) than in PES (98.2 ± 5.7 µm to 101.9 ± 3.3 µm) (Fig. 5a). The length of male gametophytes was affected by nutrient medium and the use of GeO2, with a significant interaction between these factors (two-way PERMANOVA: F(3,40) = 27.85, P = 0.025). The length of male gametophytes followed the same trend as female gametophytes and was generally larger and more variable in F/2 (97.4 ± 2.4 µm to 126.8 ± 6.3 µm) compared to PES (96.0 ± 4.9 µm to 105.2 ± 2.1 µm) (Fig. 5a). The female:male sex ratio of gametophytes was similar between treatments (Fig. 5b) and ranged from 0.9 ± 0.1 (F/2, use of GeO2 for 2 days) to 1.2 ± 0.1 (PES, use of GeO2 for 2 days). There was a significant interactive effect between nutrient medium and use of GeO2 on the sex ratio (two-factor ANOVA: F(3,40) = 4.357, P = 0.009) driven by a higher proportion of male gametophytes in the F/2 treatment where GeO2 was used for 2 days.
Effects of nutrient media and GeO2 in experiment 3. (a) Mean (n = 6; ± S.E.) length (µm) of female and male gametophytes after 12 days. (b) Mean (n = 6; ± S.E.) female:male sex ratio of gametophytes after 12 days. (c) Mean (n = 6; ± S.E.) transition from female gametophyte to sporophyte (%) after 30 days
The transition from female gametophytes to sporophytes was affected by nutrient medium and the use of GeO2, with a significant interaction effect between these factors (two-factor PERMANOVA: F(3,40) = 4.475, P = 0.007). Transitions were lower overall in F/2 (≤ 26%) compared to PES (≤ 38%) (Fig. 5c). The use of GeO2 for more than 2 days had a marked inhibitory impact on the transition from gametophyte to sporophyte. When GeO2 was used for ≥ 9 days transitions were ≤ 0.6% for F/2, and no successful transition was recorded for PES. Exposure to GeO2 for 2 days resulted in a nearly two-third decrease in transition in F/2, while the transition was not affected in PES. Notably, the use of GeO2 for ≥ 9 days resulted in the successful elimination of diatoms in all dishes after 30 days of cultivation, while these were present in all samples with shorter exposure periods to GeO2.
Sporophytes were significantly larger in F/2 than in PES after 30 days of cultivation (two-factor PERMANOVA: F(1,20) = 4.679, P = 0.044). The mean length of sporophytes without GeO2 exposure was 297.1 ± 39.8 µm and 173.0 ± 29.9 µm for F/2 and PES, respectively. When individuals were exposed to GeO2 for 2 days, sporophytes had a mean length of 207.6 ± 53.5 µm in F/2 and a mean length of 150.3 ± 41.1 µm in PES.
Experiment 4: Delaying the initiation of spore release
The number of released zoospores on the day of collection (day 0) was similar between collections and ranged from 23,125 spores mL−1 (collection 1) to 37,500 spores mL−1 (collection 2). The number of released zoospores was not significantly affected by delaying the initiation of spore release (two-factor PERMANOVA: F(4,8) = 0.871, P = 0.476). However, it was highly variable, with more than an order of a magnitude difference between treatments and collections ranging from 22,500 spores mL−1 (delay of 3 days, collection 2) to 1,561,875 spores mL−1 (delay of 3 days, collection 3). To identify the change in reproductive output over time, the number of released zoospores was plotted as fold change compared to the release on day 0 (Fig. 6). In general, a higher number of zoospores were released when the spore release was delayed up to four days compared to day 0 with 1.3 to 64.1-fold increases (Fig. 6). Only one data point (delay of 3 days, collection 2) resulted in a fold decrease less than 1.
The released spores were viable with the average germination rates across collections ≥ 85% after 48 h, ranging from 85.8 ± 8.0% (day 0) to 97.3 ± 0.1% (delay of 3 days). The number of days zoospore initiation was delayed had no effect on germination rates, but there were significant differences between collections. This effect was not consistent among collections, as evidenced by a significant interactive effect between collection x delay of initiation (two-factor PERMANOVA: F(8,60) = 19.954, P < 0.001).
Each of the daily collected water samples from the buckets used for storing the sorus-bearing fronds contained zoospores, accounting for 20–30% of the total number of released zoospores. Notably, the majority of zoospores were released during the initiation process, which accounted for 70–80% of the total number of zoospores released.
Discussion
Germination
This study provides a foundation for understanding the factors affecting germination, gametophyte development and transition to sporophytes, and has identified the best treatment combination for the successful cultivation of early life stages of E. radiata under hatchery conditions. High rates of germination are fundamental to establish the controlled cultivation of any seaweed species as this is the first critical process of the hatchery stage. Germination was high in E. radiata with ≥ 85% in all the experiments run in this study. This falls well into the reported range of other kelp species (Müller et al. 2008; Augyte et al. 2019), although germination rates of E. radiata were generally in the higher range compared to Macrocystis pyrifera (39–66%; (Leal et al. 2014)), Saccharina latissima (syn. Laminaria saccharina; 0–75%; (Lee and Brinkhuis 1988)), Alaria esculenta (~ 60%; (Zacher et al. 2019)), and Laminaria digitata (~ 55–70%; Bartsch et al. 2013; Zacher et al. 2019)). Both photoperiod and temperature significantly affected germination of E. radiata with short daylength (8:16) and winter (14 °C) and summer (20 °C) temperatures resulting in significantly reduced germination rates. However, germination was still high (≥ 93%) under those conditions and only slightly lower (< 3%) than in other tested photoperiod and temperature treatments. This is in stark contrast to other studies, where differences in germination were more pronounced between photoperiod (Kerrison et al. 2016) and temperature treatments (Lee and Brinkhuis 1988; Müller et al. 2008; Bartsch et al. 2013; Augyte et al. 2019). For example, the germination percentage of S. latissima nearly doubled when zoospores were maintained in the dark for 48 h compared to a 12 h L:12 h D photoperiod (Kerrison et al. 2016). Notably, such differences in germination percentage between photoperiods were not found for E. radiata in the present study (experiment 1), even when the treatment of 48 h darkness was tested in a pilot study (see supplementary data). Moreover, previous studies have shown that temperatures representative of those occurring at the locations where study species were collected could cause a twofold change in germination (Lee and Brinkhuis 1988; Müller et al. 2008; Bartsch et al. 2013; Augyte et al. 2019) or higher (Izquierdo et al. 2002). Overall, E. radiata maintained high germination percentages under all tested conditions, including photoperiod, light intensity (see supplementary data), temperature, nutrient media, and exposure to GeO2. This ability to successfully germinate under a broad range of environmental conditions may be a competitive advantage of E. radiata over other seaweed species allowing for the successful colonization of available substrates within and outside of kelp forests, regardless of the season and the associated differences in temperature, light intensity and light period. Crucially, high germination percentages under non-specific conditions fulfils the first critical process for the successful hatchery production of E. radiata.
Gametophyte stage
The second critical hatchery process for the successful controlled cultivation of E. radiata is the growth and development of gametophytes. Temperature, photoperiod, and nutrient medium had a significant effect on the growth of gametophytes, with temperature being the factor which resulted in the most pronounced size differences across treatments. Notably, the length of gametophytes increased with increasing temperature and was more than 3-times larger (~ 140 µm in length) under summer temperature conditions (20 °C) compared to winter temperature conditions (14 °C). This summer temperature falls into the optimal range of gametophyte growth reported for E. maxima (17.5—20°C; Bolton and Levitt 1985) and other ecotypes of E. radiata in Tasmania (16.5—22 °C; (Mabin et al. 2013)), Western Australia (18—23 °C; Mohring et al. 2014) and New Zealand (12—20 °C; Novaczek 1984b).
Differences in gametophyte length were less pronounced across photoperiod treatments compared to temperature treatments. Interestingly, the largest gametophytes were found under the normal day photoperiod (12 h L:12 h D; ~ 80 µm in length) in experiment 1, while the short (8:16) and long day photoperiods (16 h L: 8 h D) resulted in approximately 20% and 10% smaller gametophytes, respectively. This finding was unexpected, as algal growth is driven by photoperiod and light intensity (total daily dose of light). For example, increasing light periods resulted in increased vegetative growth of gametophytes in L. digtata at light intensities of 10–15 µmol photons m−2 s−1 (Martins et al. 2017; Ratcliff et al. 2017), and in Undaria pinnafidata at light intensities of 17–60 µmol photons m−2 s−1 (Choi et al. 2005). In general, vegetative growth of gametophytes is improved at lower light intensities (5–20 µmol photons m−2 s−1), although growth still occurs at high intensities up to 200 µmol photons m−2 s−1 (Bolton and Levitt 1985; Lee and Brinkhuis 1988; Augyte et al. 2019). The light saturation threshold for gametophyte growth in kelps is 10 – 20 µmol photons m−2 s−1 (Lüning and Neushul 1978; Lüning 1980; Izquierdo et al. 2002) and around 20 µmol photons m−2 s−1 for E. maxima (Bolton and Levitt 1985). Therefore, extended light periods with light intensities above the light saturation threshold have the potential to increase stress and thus may have resulted in reduced growth in gametophytes of E. radiata in the present study (35 µmol photons m−2 s−1).
In addition to temperature and photoperiod, nutrient medium affected the length of gametophytes. Growth of gametophytes was promoted in F/2 and resulted in approximately 10% larger gametophytes than in PES after 12 days, likely to be driven by compositional differences in these culture media. Similarly, F/2 facilitated rapid growth of gametophytes of L. digitata for the initial 21 days of cultivation, while growth in PES was significantly slower (Ratcliff et al. 2017). However, after 25 days of cultivation gametophytes in F/2 and PES had similar lengths (Ratcliff et al. 2017). It is therefore likely, that the minor differences in gametophyte length of E. radiata between F/2 and PES quantified on day 12 would diminish over time. As a consequence, either nutrient medium can be used for the vegetative cultivation of gametophytes of E. radiata and similar growth is expected.
Importantly, the sex ratio remained 1 throughout the present study, equating to equal numbers of female and male gametophytes regardless of temperature, photoperiod, nutrient medium or use of GeO2. Previous studies have found that the sex ratio of gametophytes is temperature-dependent in S. latissima (Lee and Brinkhuis 1988), Lessonia variegata (Nelson 2005), Laminaria ochroleuca (Izquierdo et al. 2002), and A. esculenta (Park et al. 2017). Shifts in the sex ratio are driven by a higher survival of one sex over another and differences in the tolerance limit between female and male gametophytes have been determined for temperature, as well as light conditions (Lee and Brinkhuis 1988; tom Dieck 1993; Augyte et al. 2019). However, given that the tested temperature range and light conditions in the present study were within the naturally occurring range of E. radiata, the finding that the sex ratio remained unchanged was not surprising.
Sporophyte stage
The final critical process of the hatchery stage is the successful transition from female gametophytes to sporophytes, which only occurs under favourable conditions (Nelson 2005; Carney and Edwards 2010; Martins et al. 2017). While gametophytes of E. radiata had high survival rates, the transition rates were ≤ 38% in all experiments, and considerably lower than those reported for other kelp species, including S. latissima (~ 80–100%; Lee and Brinkhuis 1988; Park et al. 2017; Boderskov et al. 2022)), L. digitata (~ 90%; (Müller et al. 2008)), and A. esculenta (~ 100%; (Park et al. 2017)) under favourable conditions. However, low transition rates have also been shown for E. radiata in previous studies. For example, Tatsumi and Wright (Tatsumi and Wright 2016) obtained an average of 5 female gametophytes mm−2 and 0.5 sporophytes mm−2 in a laboratory experiment after 14 and 30 days respectively, equating to a transition rate of 10%. Another study quantified the transition rate of E. radiata during June (austral winter) and November (austral summer) and found that the transition from female gametophyte to sporophyte was higher in winter, with 30–55% transition compared to 19–36% transition in summer (Tatsumi et al. 2022). Notably, cultures were maintained under 12 °C in June and under 17 °C in November to represent ambient winter and summer water temperatures respectively in that study (Tatsumi et al. 2022), suggesting that seasonality as well as a temperature may be important. An effect of both seasonality and temperature on gametogenesis and transition to sporophyte has been previously demonstrated for kelp species (Lee and Brinkhuis 1988; Tala et al. 2004; Murúa et al. 2013). In particular, temperature has been identified as the key driver for reproduction in gametophytes of other kelp species with transition to sporophytes being delayed, reduced or absent for temperatures outside of the optimal range (Lee and Brinkhuis 1988; Izquierdo et al. 2002; Müller et al. 2008; Park et al. 2017; Mabin et al. 2019). Although temperature had no significant effect on the transition to sporophytes on E. radiata in the present study, a different picture emerges when we simplify the results and solely shift the focus to those three treatments where the cultivation temperature remained unchanged throughout experiment 2. On this occasion, transition rates of E. radiata were highest during continuous spring/autumn temperatures after 30 days (17 °C; 12.8 ± 3.9%) and more than halved during continuous summer (20 °C; 6.8 ± 2.8%) and winter temperatures (14 °C; 2.7 ± 1.3%). This is a clear indication that the transition to sporophytes is favoured around 17 °C and agrees with previous studies in which the transition to sporophytes was favoured at the lower temperature range of optimal gametophyte growth (Lee and Brinkhuis 1988; Martins et al. 2017). Notably, sporophytes exhibit a much narrower range of survival temperature than gametophytes (Lee and Brinkhuis 1988) and the restriction of gametogenesis and transition to sporophytes outside of temperatures favourable for sporophyte growth considerably enhances their survival. Moreover, the temperature favoured by E. radiata in the present study was similar to the ambient temperature at the collection site. Zoospores for the present study were obtained form sori collected during austral spring and there may be a significant seasonality effect on the processes of gametogenesis and transition to sporophyte, and optimal temperatures and photoperiods may differ between seasons.
An important result of this study was the demonstrated detrimental effect of GeO2 on the development of sporophytes of E. radiata. In general, brown algae are sensitive to GeO2 as Ge interferes with essential cell processes (Tarakhovskaya et al. 2012). Although the use of GeO2 for ≥ 9 days generally resulted in the successful elimination of diatoms, the transition to sporophytes was inhibited. Therefore, the use of GeO2 has to be strictly limited to ≤ 2 days under hatchery conditions when cultures are grown from zoospores. Our findings are in contrast to previous studies, where gametophytes successfully transitioned to sporophytes when exposed to GeO2 for longer periods (Izquierdo et al. 2002; Kerrison et al. 2016) and even when used continuously (Bolton and Levitt 1985; Murúa et al. 2013). Moreover, a previous study tested the effect of the duration of GeO2 exposure on the growth of S. latissima, where the use of GeO2 for the first 9 days of cultivation (from zoospores) resulted in better cover and growth of sporophytes compared to shorter or longer exposure periods (Kerrison et al. 2016). Notably, the concentration of GeO2 was similar between studies with 0.5 mg GeO2 L−1 culture medium in the present study (2 mL L−1 of 250 mg GeO2 L−1 stock solution) and 0.56 mg GeO2 L−1 culture medium (0.125 mL of 4.48 g GeO2 L−1 stock solution) used by Kerrison et al. (Kerrison et al. 2016). This falls well within the concentration (0.45 – 2.26 mg GeO2 L−1 culture medium) recommended by Shea and Chopin (Shea and Chopin 2007) to prevent the growth of diatoms and eliminate their detrimental effect on the early life stages of kelps (Shea and Chopin 2007). However, that study applied GeO2 at day 8, when gametophytes were already developed and the sensitivity to GeO2 is likely to be higher for zoospores and developing gametophytes at the start of the cultivation period. Moreover, lower concentrations of GeO2 (0.045 – 0.179 mg GeO2 L−1 culture medium) have been recommended to inhibit diatom growth in cultures of brown and green seaweeds (Markham and Hagmeier 1982). Whilst further work is required to determine suitable protocols for the elimination of diatoms during the hatchery stage of E. radiata, lower concentrations of GeO2 applied at a later cultivation stage is most likely to be successful without negatively impacting the growth, development and reproduction of E. radiata.
Overcoming low transition rates is the key for the successful cultivation of this target species and there are several avenues to improve the transition rates to sporophytes that were recorded here. Firstly, water motion by gentle aeration is likely to improve gametogenesis and the transition to sporophytes as demonstrated for Lessonia trabeculata (Tala et al. 2004), Pterygophora californica and M. pyrifera (Reed et al. 1991). Moreover, aeration is commonly used during the hatchery stage of kelps (Edwards and Watson 2011; Redmond et al. 2014; Rolin et al. 2014) as water movement increases the nutrient uptake (Barr et al. 2008), resulting in improved growth of sporophytes (Yoneshigue-Valentin 1990). Secondly, lower nutrient concentrations might improve the transition rates as high concentrations of nitrogen have been shown to inhibit the transition (Yarish et al. 1990; Boderskov et al. 2022). Full strength PES as used here has an N concentration of > 800 µM N, which is well above the recommendation (100–150 µM N) for optimal transition rates of S. latissima (Boderskov et al. 2022). Similarly, concentrations of 100 µM N severely reduced the formation of sporophytes in Laminaria longicruris (Yarish et al. 1990).
Delaying the initiation of zoospore release
Storage of sorus-bearing fronds of sporophytes up to 4 days after the collection from the field generally increased the number of released zoospores without affecting the viability of released zoospores. Notably, delaying the initiation of spore release allows the time window that zoospores can be obtained to be extended, e.g. in in situations where the sampling site is located some distance away from the hatchery, or if weather and/or tidal windows preclude field collection and induction of zoospore release being undertaken on the same day. Moreover, delaying the initiation of zoospore release is a simple mechanism to increase the fertility of brood stock.
Conclusions
In conclusion, critical parameters for the hatchery stage have been identified which resulted in high rates of germination, vegetative growth and successful transition to sporophytes. We recommend to maintain the early life stages of E. radiata under a 12 h L:12 h D photoperiod at 17 °C as this resulted in higher germination rates, good gametophyte development and higher transition to sporophytes compared to other treatments. Moreover, maintaining cultures at constant light periods and temperatures allows for the simultaneous cultivation of different life stages, minimising the required space for a hatchery. While germination rates remained high and gametophytes grew well under various conditions, the transition from gametophyte to sporophyte was low in E. radiata and requires improvement to enable the efficient use of the resource inputs in the hatchery process. However, low transition rates may be an artefact of running experiments in static Petri dishes and are likely to improve when applying our findings to larger-scale hatchery conditions, where seeded ropes are maintained in aerated tanks with higher water exchange rates and lower nutrient concentrations.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Augyte S, Yarish C, Neefus CD (2019) Thermal and light impacts on the early growth stages of the kelp Saccharina angustissima (Laminariales, Phaeophyceae). Algae 34:153–162
Barr NG, Kloeppel A, Rees TAV, Scherer C, Taylor RB, Wenzel A (2008) Wave surge increases rates of growth and nutrient uptake in the green seaweed Ulva pertusa maintained at low bulk flow velocities. Aquat Biol 3:179–186
Bartsch I, Vogt J, Pehlke C, Hanelt D (2013) Prevailing sea surface temperatures inhibit summer reproduction of the kelp Laminaria digitata at Helgoland (North Sea). J Phycol 49:1061–1073
Bartsch I, Wiencke C, Bischof K, Buchholz CM, Buck BH, Eggert A, Feuerpfeil P, Hanelt D, Jacobsen S, Karez R, Karsten U, Molis M, Roleda MY, Schubert H, Schumann R, Valentin K, Weinberger F, Wiese J (2008) The genus Laminaria sensu lato: recent insights and developments. Eur J Phycol 43:1–86
Boderskov T, Rasmussen MB, Bruhn A (2021) Obtaining spores for the production of Saccharina latissima: seasonal limitations in nature, and induction of sporogenesis in darkness. J Appl Phycol 33:1035–1046
Boderskov T, Rasmussen MB, Cassard CH, Svensgaard J, Enevoldsen LN, Bruhn A al (2022) Comparing effects of nutrient sources approved for organic seaweed production on hatchery stage development of sugar kelp, Saccharina latissima. Algal Res 61:102602
Bolton JJ, Levitt GJ (1985) Light and temperature requirements for growth and reproduction in gametophytes of Ecklonia maxima (Alariaceae: Laminariales). Mar Biol 87:131–135
Carney LT, Edwards MS (2010) Role of nutrient fluctuations and delayed development in gametophyte reproduction by Macrocystis pyrifera (Phaeophyceae) in Southern California. J Phycol 46:987–996
Chappell PR (2013) The climate and weather of the Bay of Plenty—3rd edition (NIWA Science and Technology Series, Number 62). Wellington, New Zealand
Choi HG, Kim SY, Lee SJ, Park EJ, Nam KW (2005) Effects of daylength, irradiance and settlement density on the growth and reproduction of Undaria pinnatifida gametophytes. J Appl Phycol 17:423–430
Edwards M, Watson L (2011) Aquaculture explained No 26. Cultivating Laminaria digitata. Bord Iascaigh Mhara (Irish Sea Fisheries Board), Dublin 72
Fairhead VA, Cheshire AC (2004) Rates of primary productivity and growth in Ecklonia radiata measured at different depths, over an annual cycle, at West Island, South Australia. Mar Biol 145:41–50
Forbord S, Braaten Steinhovden K, Kjølbo Rød K, Handå A, Skjermo J (2018) Cultivation protocol for Saccharina latissima. In: Charrier B, Wichard T, Reddy CRK (eds) Protocols for macroalgae research. Taylor & Francis, Boca Raton, pp 37–59
Izquierdo LJ, Pérez-Ruzafa IM, Gallardo T (2002) Effect of temperature and photon fluence rate on gametophytes and young sporophytes of Laminaria ochroleuca Pylaie. Helgol Mar Res 55:285–292
Jennings R (1967) The development of the gametophyte and the young sporophyte of Ecklonia radiata (C.Ag.) J.Ag (Laminariales). J Roy Soc W A 50:93–96
Kain JM (1979) A view of the genus Laminaria. Ocean Mar Biol Ann Rev 17:101–161
Kerrison PD, Stanley MS, Kelly M, MacLeod A, Black KD, Hughes AD (2016) Optimising the settlement and hatchery culture of Saccharina latissima (Phaeophyta) by manipulation of growth medium and substrate surface condition. J Appl Phycol 28:1181–1191
Kirkman H (1981) The first year in the life history and the survival of the juvenile marine macrophyte, Ecklonia radiata (Turn.) J Agardh. J Exp Mar Bio Ecol 55:243–254
Layton C, Cameron MJ, Shelamoff V, Tatsumi M, Wright JT, Johnson CR (2021) A successful method of transplanting adult Ecklonia radiata kelp, and relevance to other habitat-forming macroalgae. Restor Ecol 29:1–5
Leal PP, Hurd CL, Roleda MY (2014) Meiospores produced in sori of nonsporophyllous laminae of Macrocystis pyrifera (Laminariales, Phaeophyceae) may enhance reproductive output. J Phycol 50:400–405
Lee JA, Brinkhuis BH (1988) Seasonal light and temperature interaction effects on development of Laminaria saccharina (Phaeophyta) gametophytes and juvenile sporophytes. J Phycol 24:181–191
Lüning K (1981) Egg release in gametophytes of Laminaria saccharina: Induction by darkness and inhibition by blue light and U.V. Br Phycol J 16:379–393
Lüning K (1993) Environmental and internal control of seasonal growth in seaweeds. Hydrobiologia 260–261:1–14
Lüning K (1988) Photoperiodic control of sorus formation in the brown alga Laminaria saccharina. Mar Ecol Prog Ser 45:137–144
Lüning K (1980) Critical levels of light and temperature regulating the gametogenesis of three Laminaria species (Phaeophyceae). J Phycol 16:1–15
Lüning K, Neushul M (1978) Light and temperature demands for growth and reproduction of laminarian gametophytes in southern and central California. Mar Biol 45:297–309
Mabin CJT, Gribben PE, Fischer A, Wright JT (2013) Variation in the morphology, reproduction and development of the habitat-forming kelp Ecklonia radiata with changing temperature and nutrients. Mar Ecol Prog Ser 483:117–131
Mabin CJT, Johnson CR, Wright JT (2019) Family-level variation in early life-cycle traits of kelp. J Phycol 55:380–392
Markham JW, Hagmeier E (1982) Observations on the effects of germanium dioxide on the growth of macro-algae and diatoms. Phycologia 21:125–130
Martins N, Tanttu H, Pearson GA et al (2017) Interactions of daylength, temperature and nutrients affect thresholds for life stage transitions in the kelp Laminaria digitata (Phaeophyceae). Bot Mar 60:109–121
Miller RJ, Bennett S, Keller AA et al (2012) TiO2 nanoparticles are phototoxic to marine phytoplankton. PLoS ONE 7:e30321
Mohring MB, Kendrick GA, Wernberg T, Rule MJ, Vanderklift MA (2013) Environmental influences on kelp performance across the reproductive period: an ecological trade-off between gametophyte survival and growth? PLoS ONE 8:e65310
Mohring MB, Wernberg T, Wright JT, Connell SD, Russell BD (2014) Biogeographic variation in temperature drives performance of kelp gametophytes during warming. Mar Ecol Prog Ser 513:85–96
Müller R, Wiencke C, Bischof K (2008) Interactive effects of UV radiation and temperature on microstages of Laminariales (Phaeophyceae) from the Arctic and North Sea. Clim Res 37:203–213
Murúa P, Westermeier R, Patiño DJ, Müller DG (2013) Culture studies on early development of Lessonia trabeculata (Phaeophyceae, Laminariales): Seasonality and acclimation to light and temperature. Phycol Res 61:145–153
Nelson W (2013) New Zealand seaweeds - an illustrated guide. Te Papa Press, Wellington
Nelson WA (2005) Life history and growth in culture of the endemic New Zealand kelp Lessonia variegata J. Agardh in response to differing regimes of temperature, photoperiod and light. J Appl Phycol 17:23–28
Novaczek I (1984a) Development and phenology of Ecklonia radiata at two depth in Goat Island Bay, New Zealand. Mar Biol 81:189–197
Novaczek I (1984b) Response to gametophytes of Ecklonia radiata (Laminariales) to temperature in saturating light. Mar Bol 82:241–245
Novaczek I (1981) Stipe growth rings in Ecklonia radiata (C.Ag.) J.Ag. (Laminariales). Br Phycol J 16:363–371
Paine ER, Schmid M, Gaitán-Espitia JD, Castle J, Jameson I, Sanderson JC, Hurd CL (2021) Narrow range of temperature and irradiance supports optimal development of Lessonia corrugata (Ochrophyta) gametophytes: implications for kelp aquaculture and responses to climate change. J Appl Phycol 33:1721–1730
Pang SJ, Lüning K (2004) Breaking seasonal limitation: year-round sporogenesis in the brown alga Laminaria saccharina by blocking the transport of putative sporulation inhibitors. Aquaculture 240:531–541
Park J, Kim JK, Kong J-A, Depuydt S, Brown MT, Han T (2017) Implications of rising temperatures for gametophyte performance of two kelp species from Arctic waters. Bot Mar 60:39–48
Ratcliff JJ, Soler-Vila A, Hanniffy D, Johnson MP, Edwards MD (2017) Optimisation of kelp (Laminaria digitata) gametophyte growth and gametogenesis: effects of photoperiod and culture media. J Appl Phycol 29:1957–1966
Redmond S, Green L, Yarish C, Kim J, Neefus C (2014) New England seaweed culture handbook. Nuresery Systems. Connecticut Sea Grant CTSG‐14‐01. 92 pp
Reed DC, Neushul M, Ebeling AW (1991) Role of settlement density on gametophyte growth and reproduction in the kelps Pterygophora californica and Microcystis pyrifera (Phaephyceae). J Phycol 27:361–366
Rolin C, Inkster R, Laing J, Hedges J, McEvoy L (2014) Seaweed cultivation manual. Shetland Seaweed Growers Project 2014–16. NAFC Marine Centre, University of the Highlands and Islands. 38
Shea R, Chopin T (2007) Effects of germanium dioxide, an inhibitor of diatom growth, on the microscopic laboratory cultivation stage of the kelp, Laminaria saccharina. J Appl Phycol 19:27–32
Suebsanguan S, Strain EMA, Morris RL, Swearer SE (2021) Optimizing the initial cultivation stages of kelp Ecklonia radiata for restoration. Restor Ecol 29:e13388
Tala F, Edding M, Vásquez J (2004) Aspects of the reproductive phenology of Lessonia trabeculata (Laminariales: Phaeophyceae) from three populations in northern Chile. N Z J Mar Freshw Res 38:255–266
Tarakhovskaya ER, Kang E-J, Kim K-Y, Garbary DJ (2012) Effect of GeO2 on embryo development and photosynthesis in Fucus vesiculosus (Phaeophyceae). Algae 27:125–134
Tatsumi M, Layton C, Cameron MJ, Shemaloff V, Johnson CR, Wright JT (2021) Interactive effects of canopy-driven changes in light, scour and water flow on microscopic recruits in kelp. Mar Environ Res 171:105450
Tatsumi M, Mabin CJT, Layton C, Shelamoff V, Cameron MJ, Johnson CR, Wright JT (2022) Density-dependent and seasonal variation in reproductive output and sporophyte production in the kelp, Ecklonia radiata. J Phycol 58:92–104
Tatsumi M, Wright JT (2016) Understory algae and low light reduce recruitment of the habitat-forming kelp Ecklonia radiata. Mar Ecol Prog Ser 552:131–143
tom Dieck I (1993) Temperature tolerance and survival in darkness of kelp gametophytes (Laminariales, Phaeophyta) - ecological and biogeographical implications. Mar Ecol Prog Ser 100:253–264
Wiltshire KH, Tanner JE, Gurgel CFD, Deveney MR (2015) Feasibility study for integrated multitrophic aquaculture in southern Australia. Report to the Fisheries Research & Development Corporation, SARDI: Adelaide, Australia.
Winberg P, Skropeta D, Ullrich A (2011) Seaweed cultivation pilot trials - towards culture systems and marketable products. Rural Industries Research & Development Corporation Technical Report, Canberra, Australia
Yarish C, Penniman CA, Egan B (1990) Growth and reproductive responses of Laminaria longicruris (Laminariales, Phaeophyta) to nutrient enrichment. Hydrobiologia 204–205:505–511
Yoneshigue-Valentin Y (1990) The life cycle of Laminaria abyssalis (Laminariales, Phaeophyta) in culture. Hydrobiologia 204–205:461–466
Zacher K, Bernard M, Daniel Moreno A, Bartsch I (2019) Temperature mediates the outcome of species interactions in early life-history stages of two sympatric kelp species. Mar Biol 166:1–16
Zhang QS, Qu SC, Cong YZ, Luo SJ, Tang XX (2008) High throughput culture and gametogenesis induction of Laminaria japonica gametophyte clones. J Appl Phycol 20:205–211
Acknowledgements
We thank Peter Randrup, Ariane Brandenburg, Logan Forsythe, Christopher Glasson, Alisa Mihaila, Holly Fergusson, Yanika Reiter and Maro Guy for help with manipulation of photoperiods and/or collection of algal biomass from the field. This research is part of the Entrepreneurial Universities Macroalgal Biotechnologies Programme, jointly funded by the University of Waikato and the Tertiary Education Commission.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions Financial support was provided by New Zealand Tertiary Education Commission.
Author information
Authors and Affiliations
Contributions
CP: conception and design, analysis and interpretation of data, drafting of article, statistical expertise, collection and assembly of data. MM: Conception, revisions, final approval of the article, obtaining of funding. RL: Conception and design, analysis and interpretation of data, critical revision of the article for important intellectual content, final approval of the article, statistical expertise, collection and assembly of data.
Corresponding author
Ethics declarations
Competing interests
No conflict, informed consent, human or animal right applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Praeger, C., Magnusson, M. & Lawton, R. Optimising the zoospore release, germination, development of gametophytes and formation of sporophytes of Ecklonia radiata. J Appl Phycol 34, 2535–2549 (2022). https://doi.org/10.1007/s10811-022-02806-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02806-y