Abstract
Isochrysis galbana is a microalga that is a potential source of several ‘compounds of interest’ for food and nutraceutical industries. The procurement of these compounds of interest and the generation of bioenergy is a novel and interesting application of microalgae. The present study evaluated the valorization of the microalga I. galbana through the extraction of the carotenoid fucoxanthin and polyunsaturated fatty acids using supercritical fluid extraction (SFE). The spent or extracted microalga I. galbana was valorized through anaerobic digestion (AD) to produce biogas through the integration of the concepts of biorefinery and the circular economy. The biochemical composition of the extracted microalga exhibited a marked decrease in the carbohydrates by approximately ninefold after the SFE, while the proteins and lipids were decreased only to nearly half of their initial contents. The methane (CH4) yields obtained for non-extracted I. galbana and supercritical fluid-extracted I. galbana were 104 ± 5 LSTP CH4 kg–1 VS and 90 ± 2 LSTP CH4 kg–1 VS (VS: volatile solids), respectively, indicating the methane yield loss of only 14.2% when using the extracted microalga. In order to increase methane production, different co-digestion mixtures of I. galbana and olive mill solid waste (OMSW) were evaluated. The methane yield increased to 134 ± 4 and 189 ± 5 mLSTP CH4 g–1 VS for the VS/TKN ratio (w/w) of 20 and 30, respectively (TKN: Total Kjeldahl Nitrogen). These results were encouraging due to the positive synergism established between the co-substrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The algae biorefinery approach has been gaining increasing interest in recent years in both scientific and industrial communities. Microalgae, in particular, reportedly have a great capacity to produce high-value compounds that could be utilized as pharmaceutical compounds, health foods, natural pigments, or biofuels (Chandra et al. 2019).
In the field of bioenergy algal biomass valorization, particularly using the cascade approach, is considered a strategy to significantly reduce the costs involved in producing high-value-added products and minimize the global dependence on fossil fuels by applying the biorefinery concept (Chisti 2007). Biogas, which is released upon the anaerobic digestion (AD) of biodegradable organic matter, is suggested as an important renewable energy source (Zabed et al. 2020). AD is generally conducted by bacteria and archaea via coordinated and interdependent metabolic processes (Zeikus 1980). These microorganisms transform biodegradable organic matter into biogas in the following four major steps: (i) hydrolysis, (ii) acidogenesis, (iii) acetogenesis, and (iv) methanogenesis (Gujer and Zehnder 1983; Passos et al. 2014). The biogas obtained upon AD is a mixture of CH4 (65–70%), CO2 (35–40%), and other constituents (hydrogen sulfide, nitrogen oxides, etc.) that are usually present in less than 1% concentration (Angelidaki and Sanders 2004; Passos et al. 2014). Biogas may be injected into the natural gas grid or used as a fuel in vehicles after undergoing an upgrading process. Biogas may also be applied to generate heat and electricity using cogeneration engines (Roubaud and Favrat 2005; Zabed et al. 2020). AD also generates digestates or effluents that might also contain nutrients and could, therefore, be useful as fertilizers for soils poor in nutrients (Solé-Bundó et al. 2017; Fernández-Rodríguez et al. 2021). Occasionally, the AD of microalgae might be limited due to their morphological characteristics, such as the microalgal cell wall composition, the unbalanced carbon to nitrogen (C/N) ratio, etc. Therefore, different pre-treatment methods have been suggested for the cell wall break down to release the cell contents, including thermal, mechanical, chemical, and biological processes, which are conducted prior to the AD process (Passos et al. 2014).
In the present study the haptophyte microalga Isochrysis galbana was selected as the raw material owing to its application potential for the food industry. This microalga is recognized as a valuable source of polyunsaturated fatty acids (PUFAs), mainly ω-3 fatty acids such as eicosapentaenoic acid (EPA, C20:5 ω-3) and docosahexaenoic acid (DHA, C22:6 ω-3) (Patil et al. 2007; Batista et al. 2013). Isochrysis galbana is also a natural source of vitamins A and E, folic acid, nicotinic acid, and chlorophyll a and c. It is also well-recognized for its specific carotenoid fucoxanthin (Gouveia et al. 2008; Mulders et al. 2013), which is a yellowish pigment (λ max = 420–470 nm) valued for its anti-inflammatory, antioxidant, and antiobesity activities (Kalam et al. 2015). Fucoxanthin is also reported to be useful in preventing chronic diseases such as diabetes mellitus, liver disease, or cancer (Bae et al. 2020). The microalga I. galbana has been particularly utilized to obtain several compounds of interest or bioactive molecules using the supercritical fluid extraction (SFE) method (Ruiz-Domínguez et al. 2020).
SFE is a novel and one of the most efficient green extraction techniques for obtaining compounds of interest from natural sources (Herrero et al. 2010). SFE offers the possibility of modulating variables, such as pressure, temperature, and CO2 flow (as the main extractant), and involves the presence of a polarity modifier, the advantages that render this technique widely applicable in several fields, including food science, natural products, by-product recovery, pharmaceuticals, and environmental sciences (Herrero et al. 2010; Da Silva et al. 2016).
SFE is as effective as a non-conventional pre-treatment, enabling the procurement of compounds of interest and also facilitating the release of intracellular contents and the subsequent AD. Moreover, the main extractant used in SFE is CO2 which might be modified by co-solvents such as ethanol and thereby remove the requirement of using strong solvents, which could have otherwise negatively affected the activity of the microorganisms performing the AD process (Ehimen et al. 2009). Solvents such as hexane, petroleum ether, ethyl acetate, acetone, methanol, etc., are not considered green extractants as most of these are toxic and harmful to human health. Moreover, the use of these solvents is expensive and environmentally hazardous and also requires expensive disposal procedures. However, the ethanol solvent has been approved as GRAS (Generally Recognized As Safe) by the US Food and Drug Administration (FDA 2016) and is, therefore, often employed in the recovery of lipophilic components such as carotenoids, in the pharmaceutical and food industries (Molino et al. 2018).
The present study involved integrating the procurement of the compounds of interest and bioenergy production. Other authors have also attempted such integration using different approaches. For instance, Hogan et al. (2021) used SFE to obtain lipids from the diatom Amphora sp. for nutritional purposes or biodiesel production and also proposed the utilization of the spent microalgae (which was rich in carbohydrates) for bioethanol production. González-González et al. (2018) also reported an integrated approach for lipid extraction (triacylglycerides) for biodiesel production coupled with biogas production using several kinds of microalgae. Such an integrated approach enabled cost reduction by up to 33% during biodiesel production (Harun et al. 2011).Microalgae substrates have high contents of proteins and nitrogen, due to which their C/N ratio is low. Therefore, when microalgae are used as substrates in the AD process, nitrogen is released, and depending on the temperature and pH of the system, it could inhibit the activity of the microorganisms. The C/N ratio of the system may be adjusted by adding carbon-rich substrates, which would balance the composition of the substrate available to the anaerobic microorganisms, thereby facilitating a smooth AD. The addition of co-substrates to the AD system to ensure optimal organic matter composition for an efficient AD process is referred to as ‘co-digestion’ (Mata-Alvarez et al. 2000; Sialve et al. 2009; Mata-Alvarez et al. 2014). In certain cases, anaerobic co-digestion also allows the optimal utilization of the synergy between both the co-substrates, exploiting their complementary composition. Hartmann and Ahring (2005) reported that co-digestion allows balancing the C/N ratio and diluting the effect of the inhibitor compounds present in either of the co-substrates.
The “carbon-rich substrate–microalga” as a co-digestion system was reported to exert synergistic effects and a positive influence on methane yields in certain cases (Ramos-Suárez and Carreras 2014; Gonzalez-Fernandez et al. 2015; Rincón et al. 2018). Ward et al. (2014) reported that the methane yields in co-digestion mixtures were twice as high as those when using the substrates alone. Mahdy et al. (2017) studied five different microalgae in a co-digestion mixture with cattle manure as the carbon-rich substrate to increase the C/N ratio and reported achieving a decrease in the ammonia toxicity.
Fernández-Rodríguez et al. (2019) reported a methane yield of 461 mLSTP CH4 g–1 VSadded (VS: volatile solids) for a co-digestion mixture of 75% OMSW–25% Scenedesmus quadricauda (C/N ratio = 25.3); the yield was 104% higher than that achieved using S. quadricauda (C/N ratio = 5.6) alone. Zhang et al. (2019) achieved a methane yield of 226 mL CH4 g−1 VS for the co-digestion mixture comprising 75% potato processing waste and 25% Chlorella vulgaris (C/N ratio = 22.7). This yield was 35.33% higher than that obtained using C. vulgaris alone as the substrate (167 mLSTP CH4 g–1 VS). These reports served as the founding evidence that inspired the complete exploitation of the benefits offered by microalga I. galbana by integrating the recovery of major bioactive molecules from this alga (Ruiz-Domínguez et al. 2020) and the generation of bioenergy.
The energy potential of the microalgal residues remaining after extraction with supercritical fluids was assessed using biochemical methane potential (BMP) tests and then compared with the energy potential of the complete non-extracted I. galbana. Three different substrates were compared in these tests: microalga I. galbana, spent microalga or supercritical fluid-extracted I. galbana, and a co-digestion mixture of OMSW with the non-extracted I. galbana. This approach of recovering high-value bioactive molecules (mainly fucoxanthin and PUFAs) from I. galbana combined with the bio-methanization of the exhausted microalga allows for a double valorization. Moreover, the methane produced could be utilized further, thereby reducing the costs associated with SFE, ultimately contributing to a circular economy. The present study, to the best of our knowledge, is a pioneer in providing information on the effects of SFE on natural biomass, such as I. galbana, for facilitating biogas production and the application potential of this microalga in the bioenergy industry.
Materials and Methods
Microalga I. galbana and spent microalga I. galbana
Isochrysis galbana was received as dry biomass through a kind donation from the Mexican Company “Microalgas Oleas de México S.A.” (Guadalajara, Mexico). The alga had been cultured in F/2 medium in 20 L bottles as described by (Guillard 1975). The culture conditions were controlled: 20 ± 2 ℃, constant illumination at 80 µmol photons m−2 s−1 from fluorescent lamps, and constant aeration of 0.1 v/v/min without CO2 supply. The biomass was harvested in the exponential growth phase of the culture (after 12–15 days), freeze-dried, packed in vacuum-sealed plastic bags, and stored in the dark until use. The green extractions were prepared using a Speed Helix supercritical extractor (Applied Separation, USA). Each extraction involved the use of 2 g of the freeze-dried biomass of I. galbana along with polypropylene wool and glass beads (Φ = 1 mm), all of which were placed inside a 24 mL stainless-steel extraction cell. In a previous work (Ruiz-Domínguez et al. 2020), a Box-Behnken design was used to obtain the optimal extraction duration and recovery of the bioactive compounds present in I. galbana, which minimized the operational costs of SFE (Ruiz-Domínguez et al. 2020). Similar to the above study (Ruiz-Domínguez et al. 2020), the variables of pressure (20, 30, and 40 MPa), temperature (40, 50, and 60 ℃), and co-solvent (0%, 4%, and, 8% ethanol) were evaluated at a CO2 flow rate of 7.2 g min–1 for 120 min (Ruiz-Domínguez et al. 2020). Afterward, the non-extracted I. galbana and the spent I. galbana that remained after performing the SFE at 30 MPa and 50 ℃, using 4% of ethanol and a CO2 flow rate of 7.2 g min–1 for 120 min, were subjected to AD studies. Table 1 presents the main characteristics of the spent I. galbana obtained after the SFE conducted under the above conditions and those of the complete I. galbana not subjected to any extraction.
Olive mill solid waste (OMSW)
The Olive Mill Solid Waste (OMSW) generated in a two-phase olive oil elaboration process in the Experimental Olive Oil Mill Factory located at ‘Instituto de la Grasa (CSIC)’, Seville (Spain), was collected and sifted through a 2-mm mesh to remove the olive stone pieces. Thereafter, the OMSW was diluted to a proportion of 45.6 g of OMSW per L in distilled water. The main characteristics of OMSW collected and used in the present study are listed in Table 1. The OMSW (with and without stones) exhibited a pH value of around 4.5 and a potential biodegradability of 139 mg O2 g–1 VS·h (expressed as the specific oxygen uptake rate; SOUR value).
Inoculum
The inoculum used in the experiments was a baker´s yeast vinasse obtained from the municipal water company of the water of Córdoba (EMACSA), La Golondrina plant, located in Córdoba, Spain. The main characteristics of the inoculum are presented in Table 2.
Biochemical Methane Potential (BMP)
The AD of the microalgae was analyzed using biochemical methane potential (BMP) assays. The BMP assays were conducted in batch reactors with a volume of 1200 mL. The reactors were agitated continuously at 200 rpm using magnetic bars inside a thermostatic water bath (LAUDA RTM 20) at a mesophilic temperature (35 ± 2 ℃). In each reactor, 5.7 g of VS of inoculum was used, maintaining an inoculum-to-substrate ratio of 4 (based on VS content) in an effective volume of 1000 mL.
Afterward, the reactors were sealed and the headspace of each flask was flushed with nitrogen to displace oxygen and facilitate anaerobic conditions. The volume of methane produced was measured using a 1-L Boyle-Mariotte reservoir connected to each reactor. The CO2 released from the produced biogas was detected using the 6 N NaOH solution, while the remaining gas was assumed to be methane. The released methane gas removed an equivalent volume of water from the gasometers, which was finally measured using a test tube. All the methane volumes and yields were measured under the standard temperature and pressure conditions (STP) of 0 ℃ and 1 atm.
In order to activate the biomass prior to the experiments, the digesters were fed with a mixture of glucose (50 g L–1), sodium acetate (25 g L–1), and lactic acid (21 mL L–1) [GAL solution] for 7 days. The GAL solution was characterized by a total chemical oxygen demand (TCOD) concentration of 86,900 ± 75 [mg O2 L–1] and a pH value of 7.05 ± 0.01. This initial step was followed by experiments with the microalgae. The digesters were fed with I. galbana (two digesters, two biological replicates) and spent I. galbana (two digesters, two biological replicates) in batch mode. Each load experiment was conducted using a minimum of two replicates for each of the four digesters.
Co-digestion between OMSW and I. galbana
Two different proportions of OMSW and I. galbana were evaluated. The two co-digestion mixtures contained 91.52% and 53.86% of microalga in the mixture (on a dry mass basis), respectively, which had a VS to total Kjeldahl nitrogen (TKN) ratios (w/w) of 20 and 30, corresponding to the C/N ratios (w/w) of 9 and 18, respectively.
The equipment and procedures used in the BMP tests were also employed for the two co-digestion mixtures (20 and 30). Again, two digesters were used for each co-digestion mixture, and each assay was conducted in duplicate for each digester. In order to avoid the dilution of the reactor content, the volume of the OMSW and I. galbana mixture corresponding to the VS/TKN ratio under assessment was removed initially from the reactor content, and the remaining extracted volume was divided into two fractions – the first fraction was used for characterization and the second fraction was used for retaining the biosolids in the reactor. The second fraction was centrifuged at 2000 rpm for 5 min, followed by removing the supernatant and returning the biosolids to the bioreactor. The inoculum-to-substrate ratio of 4 was used by adding 5.7 g VS of inoculum to the reactor.
Analytical methods
The protein content was analyzed colorimetrically using the Lowry method (Lowry et al. 1951) with BSA (bovine serum albumin) solutions (0.0 − 2.0 mg mL–1) for plotting the standard curve. The determined protein contents were expressed as the percentage of total proteins with respect to their dry biomass (%, w/w). The average of the values obtained from the triplicate analyses (n = 3, ± standard deviation (SD)) was used as the final value. Total carbohydrate in the samples was determined using the method of Dubois et al. (1956) and Geresh et al. (2002) with modifications. In total carbohydrate determination, a series of glucose solutions (0.0 − 1.0 mg mL–1) were used as the standard solutions, and the biomass was subjected to acid hydrolysis for cell rupture. Briefly, 20 mg of dry biomass was incubated with 5 mL of 2.5 M HCl at 100 ℃ for 3 h (Spectroquant Thermoreactor TR 320, Merck, Germany), followed by the neutralization of the mixture using 5 mL of 2.5 M NaOH to obtain the microalgal extract. Finally, aliquots of 278 µL of the extract, 167 µL of phenol solution (5%, w/v), and 1000 µL of concentrated sulfuric acid were mixed and incubated at room temperature for 30 min. The absorbance of these samples was measured at 483 nm using a microplate reader (Synergy HTX Multi-Mode microplate reader, software Gen5 2.0, BioTek Instruments, USA). The total carbohydrate content was expressed as a percentage of total carbohydrates with respect to the dry biomass (%, w/w). The average of the values obtained in the triplicate analyses (n = 3, ± SD) was used as the final value. Total lipids were determined using the method of Axelsson and Gentili (2014). The dry biomass of both I. galbana and extracted I. galbana (~ 20 mg) was separately resuspended into a 2:1 (v/v) chloroform:methanol mixture followed by manual vigorously shaking of the tube for a few seconds or until the biomass was dispersed into the solvent system. Finally, 0.73% (w/v) aq. NaCl solution was added to the above suspension to produce a 2:1:0.8 system of chloroform:methanol:water (v/v/v). The content of total lipids was then calculated gravimetrically based on triplicate analyses (n = 3, ± SD). Prior evaporation was performed with N2 on a Flexivap Work Station (Glas-Col 109A YH-1, USA). The total lipid content was expressed as a percentage of lipids in total biomass (%, w/w).
The fatty acid composition was determined using a gas chromatograph (Shimadzu 2010, Japan) equipped with a flame ionization detector (FID) and a split/splitless injector. Samples (1 µL) were injected into a capillary column (RESTEK; 30 m × 0.32 mm i.d., 0.25 μm film thickness) at the injector temperature of 250 ℃ in the split mode with a split ratio of 20:1. Nitrogen was used as the carrier gas at a constant flow rate of 1.25 mL min−1. The oven temperature was maintained at 80 ℃ for 5 min, then increased at the rate of 4 ℃ min−1 to 165 ℃, which was maintained for 2 min, followed by further increasing the temperature at the rate of 2 ℃ min−1 to 180 ℃, which was maintained for 5 min. The temperature was further increased at a rate of 2 ℃ min−1 to 200 ℃, which was maintained for 2 min, followed by an increase in temperature at the rate of 4 ℃ min−1 to 230 ℃, which was maintained for 2 min. The final increase at the rate of 2 ℃ min−1 to 250 ℃ was maintained for 2 min. The detector temperature was 280 ℃. Individual FAMEs were identified by comparing their retention times with those of the mixed FAME standards (FAME Mix C4-C24, Supelco Analytical) and quantified by comparing their peak area with those of the mixed FAME standards and the internal standard (tripentadecanoin ~ 10 ppm per sample, Nu-Check Pre, Inc., USA).
Fucoxanthin content in I. galbana biomass was determined using a fucoxanthin standard (SigmaAldrich, 0–50 ppm) on a UV–Vis spectrophotometer (Shimadzu UV–1280, Japan). Since fucoxanthin is the main carotenoid pigment reported in I. galbana, the absorption spectrum of total carotenoids was measured at the maximum absorption wavelength (447.4 nm) selected for fucoxanthin (Kim et al. 2012; Gilbert-López et al. 2015). Equation (1) was used for calculating the total carotenoids content based on the measured absorbance.
where, FX = the fucoxanthin content (in mg g−1 biomass), A447.4 = the absorbance of the sample at λmax, 8.66 = the specific slope of the standard curve, DF = dilution factor of the solvent, V = solvent volume used (in mL), and M_biomass = the mass of I. galbana used (in mg). The determination for each sample was conducted in triplicate (n = 3).
The parameters pH, total alkalinity (T-Alk), volatile fatty acids (VFA), conductivity, total solids (TS), mineral solids or ash, VS, TCOD, and soluble chemical oxygen demand (SCOD) were determined using the standard methods reported by APHA (2005).
A TOC-VCSH carbon analyzer (Shimadzu) was employed to determine the total organic carbon (TOC) using a method based on the catalytic oxidation of all the C atoms present in the sample into CO2. The released CO2 was detected using infrared spectrophotometry. Metal content in samples was determined through flame photometry (APHA et al. 2005) using a Perkin-Elmer A Analyst 300 atomic absorption spectrophotometer. TKN, ammonia nitrogen (un-ionized ammonia and ammonium), oxidable organic carbon (CCOX), and phosphorus content (P-P2O5) were chemically analyzed using the methodology proposed by The US Department of Agriculture and the US Composting Council (2002). The C/N ratio was calculated from the determined CCOX and TKN values. Volatile fatty acid (VFA) contents (acetic, propionic, butyric, isobutyric, valeric, and isovaleric acids) were determined using a Hewlett- Packard HP-5890 gas chromatograph equipped with a 15 m × 5.3 × 10–4 m (i.d.) Nukol-silica semi-capillary column and a flame ionization detector (FID).
The formula proposed by Østergaard (Østergaard 1985) was used for calculating the concentration of free ammonia as follows:
where, [TNH3] denotes the total ammonia concentration, T(K) denotes the temperature (in degree Kelvin), and [NH3] denotes the concentration of free ammonia.
Theoretical methane yields were calculated using the modified Symons and Buswell equation (Symons and Buswell 1933; Sialve et al. 2009) provided below:
Software and Statistical analysis
Sigma-Plot software version 11.0 was employed to plot the graphs and fit the experimental data to a linear regression using a confidence interval of 95%.
Unless otherwise indicated, the presented data represent the means of three replicates in each analytical measurement and two biological reactors, totaling six data values. The result data are expressed as means ± SD, assuming that the variability was higher than the analytical variability.
Using Sigma-Plot software version 11.0, the significance of the differences in the methane yields was determined based on a One-Way Analysis of Variance (ANOVA). Parametric analysis was conducted for the posthoc multiple comparison analysis using Tukey’s test for methane yield values or Dunn’s test for methane yield evolution values. A P-value of < 0.05 was considered statistically significant.
Results
Variation of I. galbana composition with supercritical fluid extraction
In the present study, the SFE was mainly performed to extract the major carotenoid pigment in the evaluated microalga, i.e., fucoxanthin, and the PUFAs. An average of 7.5 mg of fucoxanthin g−1 biomass (32%, w/w) was extracted from I. galbana. The fatty acid composition mainly comprised linoleic acid (C18:2) as the highest component, followed by linolenic acid (C18:3), myristic acid (C14:0), and palmitic acid (C16:0) (Ruiz-Domínguez et al. 2020).
Table 3 summarizes the biochemical composition, including the contents of proteins, carbohydrates, and lipids in I. galbana prior to and after (spent I. galbana) the SFE. Proteins were the main components, followed by lipids, while carbohydrates were present in the lowest amounts in both non-extracted and extracted I. galbana. Therefore, proteins and lipids (46.95 ± 1.01% and 38.05 ± 0.72%, respectively) and, to a lesser extent, carbohydrates (9.80 ± 0.44%) were the main biochemical components detected in the I. galbana analyzed in the present study.
Table 3 also presents the values obtained for the other strains of I. galbana reported in the literature, which exhibited a biochemical composition similar to the species analyzed in the present study.
In addition, Table 3 presents the biochemical composition of the supercritical fluid-extracted I. galbana after the antioxidant compounds of interest had been extracted. The biochemical composition of the extracted microalga comprised markedly decreased levels of carbohydrates to approximately ninefold lower values. The levels of proteins and lipids were nearly half the respective initial contents (23.07 ± 1.81% and 23.42 ± 0.12%, respectively, w/w).
Methane yields achieved with microalga I. galbana and supercritical fluid-extracted I. galbana.
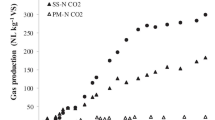
Figure 1 presents the methane yields (in LSTP CH4 kg−1 VS) obtained in the BMP assays for the non-extracted complete I. galbana and the I. galbana extracted using supercritical fluids. The methane yields achieved after 5 days (120 h) were 104 ± 5 LSTP CH4 kg–1 VS and 90 ± 2 LSTP CH4 kg–1 VS for the non-extracted I. galbana and the supercritical fluid-extracted I. galbana, respectively. The methane yield achieved with the complete microalga was higher than that achieved with the extracted microalga. The extraction of the pigment fucoxanthin and the PUFASs through SFE resulted in a reduction of 14.2% in the achieved methane yield.
Cumulative methane yields obtained for microalga Isochrysis galbana and for the supercritical fluid-extracted microalga I. galbana (STP: standard temperature and pressure conditions). The presented data represent the means of two biological reactors (n = 2) for I. galbana and two biological reactors (n = 2) for supercritical fluid extracted I galbana. Error bars denote standard deviation
Characteristics of the effluents obtained after AD
Table 4 presents the different parameters of the effluents measured using BMP assays conducted on non-extracted I. galbana and extracted microalga.
The pH of the post-AD residual effluents of the complete I. galbana and the supercritical fluid-extracted microalga increased with the increase in the load assayed (Fig. 2a). The pH values of the non-extracted and supercritical fluid-extracted microalga after the second load were 8.57 ± 0.02 and 8.37 ± 0.01, respectively. In the BMP the pH was left free. Figure 2a illustrates a significant increase in the pH, reaching up to the range that is unsuitable for AD. These pH values could significantly affect the ammonia toxicity in the system, thereby requiring regulation of the pH in the system for a semi-continuous operation mode once the process is scaled. Operating the reactors with consecutive loads allowed the detection of such effects.
pH (a) and conductivity (b) achieved in the effluents obtained after BMP assays (first load and second load) for microalga Isochrysis galbana and for the supercritical fluid-extracted microalga I. galbana. The presented data represent the means of three replicates in each analytical measurement and two biological reactors (n = 6). Error bars denote standard deviation
Figure 2b illustrates the variation in conductivity with the load studied, the first load, and the second load. The conductivity of the inoculum used for the BMP test was 18 mS cm–1, which became almost constant after the BMP tests at approximately 18 mS cm–1 for both the loads studied, while a slight increase was observed for the non-extracted microalga (Fig. 2b, Table 4).
The nitrogen content in the effluents obtained after AD was higher in the case of non-extracted I. galbana, with the values reaching 1975 ± 75 and 2110 ± 45 mg L–1 for the first and second loads, respectively (Fig. 3a). The TKN levels in the effluents obtained after the AD of supercritical fluid-extracted were lower than those in the effluents obtained after the AD of non-extracted microalga, with the values of 1785 ± 10 and 1852 ± 10 mg L–1 for the first and second loads, respectively (Fig. 3a).
Total Kjeldahl Nitrogen (TKN) (a) and ammonia nitrogen (N-NH4+) (b) achieved in the effluents obtained after BMP assays (first load and second load) for microalga Isochrysis galbana and for the supercritical fluid-extracted microalga I. galbana. The presented data represent the means of three replicates in each analytical measurement and two biological reactors (n = 6). Error bars denote standard deviation
The ammonia nitrogen (N-NH4+) level reflects the levels of ammonium ion plus free ammonia in the system. These levels were observed to be increased in all the reactors at the end of the application of first and second loads (Fig. 3b). The N-NH4+ values were high in the effluents obtained after the AD of spent or extracted I. galbana, with the values of approximately 1283 ± 10 and 1356 ± 10 mg L–1 N-NH4+ for the first and second loads, respectively. The N-NH4+ values were further high for the complete I. galbana, with values reaching 1496 ± 75 and 1563 ± 50 mg L–1 N-NH4+ for the first and second loads respectively. The initial ammonia nitrogen content in the inoculum was 1055 ± 6 mg L–1 N-NH4+. The values of free nitrogen observed are discussed further in detail in Sect. 4.3.
Figure 4a and b present the T-Alk and VFA values for the effluents obtained after the AD of complete I. galbana and in the effluents obtained after the AD of supercritical fluid-extracted microalga, respectively. Extremely high T-Alk values of 10,150 ± 50 mg CaCO3 L–1 and 11,258 ± 50 mg CaCO3 L–1 were observed for the first and the second loads assayed, respectively, for complete non-extracted microalga, while the corresponding values for the supercritical fluid-extracted microalga were 10,258 ± 50 and 11,190 ± 50 mg CaCO3 L–1 for the first and second loads, respectively. These values could be owed to the increasing values of ammonia nitrogen.
Total alkalinity (a) and volatile fatty acids (VFA) (b) achieved in the effluents obtained after BMP assays (first load and second load) for microalga Isochrysis galbana and for the supercritical fluid-extracted I. galbana. The presented data represent the means of three replicates in each analytical measurement and two biological reactors (n = 6). Error bars denote standard deviation
Co-digestion mixtures of OMSW and I. galbana and the BMP test
OMSW and I. galbana with VS/TKN values of 44.3 ± 2.2 and 12.6 ± 0.1, respectively (C/N ratio 6.18 and 5.1, respectively) were mixed, to obtain two OMSW–I. galbana mixtures with respective VS/TKN ratios of 20 and 30 (C/N ratio 9.0 and 18.0, respectively). Subsequently, the BMP tests for the two mixtures, and the individual substrates, OMSW alone and I. galbana alone, were carried out with inoculum-to-substrate ratio of 4 (5.7 g of VS of inoculum as described above. Figure 5 presents the methane yields achieved for the four substrates, two co-digestion OMSW–I. galbana mixtures, OMSW alone, and I. galbana alone (without any co-digestion). The methane yields for OMSW and I. galbana were 278 ± 5 and 98 ± 5 mLSTP CH4 g–1 VS, respectively, while the values for the OMSW–I. galbana mixtures with the VS/TKN ratios of 20 and 30 were 134 ± 4 and 189 ± 5 mLSTP CH4 g–1 VS, respectively, after 5 days of operation. The C/N ratio of the I. galbana increased from an initial value of 5.1 up to the values of 9.0 (VS/TKN = 20) and 18.0 (VS/TKN = 30) upon the addition of a carbon-rich co-substrate OMSW, which allowed for an increase in the methane yields, which reached values of up to 36.7% and 92.9%, respectively (Fig. 6), compared to the use of microalga alone as the substrate.
Cumulative methane yields obtained for co-digestion of microalga Isochrysis galbana with olive mill solid waste (OMSW) at different volatile solids/total Kjeldahl nitrogen (VS/TKN) ratios and for OMSW and non-extracted I. galbana (STP: standard temperature and pressure conditions). The presented data represent the means of two biological reactors (n = 2) and error bars denote standard deviation. There is a statistically significant difference (P = < 0.001)
Influence of the Volatile solids/Total Kjeldahl Nitrogen (VS/TKN) ratios for the substrates studied also expressed as C/N ratio: Olive mill solid waste (VS/TKN = 44, C/N = 30.8), microalga Isochrysis galbana (VS/TKN = 12.6, C/N = 5.1) and anaerobic co-digestion mixtures of I. galbana with olive mill solid waste (VS/TKN of 20 and 30, C/N = 9.0 and 18.0, respectively), on the methane yields. The presented data represent the means of two biological reactors (n = 2) and error bars denote standard deviation. There is a statistically significant difference (P = 0.016)
Table 5 presents the main characteristics of the initial substrate and the effluents obtained after the AD of OMSW alone, I. galbana alone, and the OMSW–I. galbana co-digestion mixtures with the VS/TKN ratios of 20 and 30. The data presented in Table 5 includes the values of pH, conductivity, heavy metals, VFA, etc., measured initially and after the second load assayed in the BMP test.
When the VS/TKN ratio increased from 12.6 to 44.3 upon increasing the OMSW percentage in the co-digestion mixture, the conductivity of the inoculum in the digestates reached 14.59 mS cm–1, which was slightly decreased compared to the initial value of 17.44 (Table 5). This could be a result of the reduction in the amount of saline microalgae in the co-digestion mixture.
The VS removal ranged between 55 and 60% for all cases. The values obtained for I. galbana (C/N = 5.1), co-digestion mixture with the VS/TKN ratio of 20 (C/N = 9.0), co-digestion mixture with the VS/TKN ratio of 30 (C/N = 18.0), and OMSW (C/N = 30.8) were 55.0 ± 7.75%, 55.60 ± 4.95%, 57.9 ± 17.50%, and 60.00 ± 8.00%, respectively.
Discussion
Effect of supercritical fluid extraction on the composition of I. galbana
The evaluation of the effect of SFE on I. galbana provided insights into the changes produced in the biochemical composition of this microalga.
The composition of I. galbana determined in the present study was similar to that reported for I. galbana by Zabed et al. (2017) (Table 3). Niccolai et al. (2019) reported that the T-Isochrysis lutea F&M-M36 strain had protein and lipid contents of 42.9 ± 0.42% and 27.9 ± 3.25%, respectively, which were also closer to the values obtained in the present study. The I. galbana composition reported by Sayegh et al. (2007) was least similar to the strain analyzed in the present study in terms of its carbohydrate content, which was over threefold higher than the value observed in the present study (30%–45%) (Table 3).
Fortunately, the production of biogas from microalgae relies on its biochemical composition and the production conditions (Mussgnug et al. 2010; Zabed et al. 2020). The highest theoretical yield of methane obtained from microalgae is from the lipids (1.014 L CH4 g–1 VS), while the theoretical yields from carbohydrates and proteins of microalgae are 0.415 L CH4 g–1 VS and 0.851 L CH4 g–1 VS, respectively (Sialve et al. 2009). On the contrary, it was reported that the percentages of the order of 1.5% fat in the substrate produce a lag phase in methane production, inhibiting the methane production in archaea (Usman et al. 2020).
BMP of I. galbana and the supercritical residues
The productivity and yield of biogas from a given substrate are directly related to the VS and the biochemical composition of the biomass digested. In the case of microalgae, the productivity and yield of biogas could be impacted mainly by two factors: cell wall characteristics of the microalgal genus and a low C/N ratio (Rincón 2020).
In particular, the genus Isochrysis is an interesting option when considering the biorefinery concept as these marine microalgae have no cell wall and are rich in the bioactive compound fucoxanthin and fatty acids (Sun et al. 2018).
Moreover, the spent microalgae remaining after the extraction of intracellular compounds contain huge quantities of cellular material that could be utilized in AD processes (Ramos-Suárez and Carreras 2014). Intracellular metabolite extraction using SFE could be considered a kind of pre-treatment. SFE could, therefore, facilitate the AD process, assisting the bacteria that conduct the initial steps of AD in accessing the organic matter of the microalga, thereby increasing the microalgal biodegradability.
The methane yields obtained in the present study for non-extracted I. galbana and the supercritical fluid-extracted I. galbana were 104 ± 5 LSTP CH4 kg–1 VS and 90 ± 2 LSTP CH4 kg–1 VS, respectively. Although these methane yields were not high, these were within the previously reported range of 90–540 L CH4 kg–1 VS for other microalgae (Sialve et al. 2009).
The theoretical methane yields from I. galbana and extracted I. galbana were calculated using the adapted equation reported by Symons and Buswell (1933) (Sialve et al. 2009). The theoretical methane yields obtained were 175 mLSTP CH4 g–1 VS and 123 mLSTP CH4 g–1 VS for I. galbana (C6.18N1S0.02H1.34O7.47) and extracted I. galbana (C5.95N1S0.03H1.47O8.21), respectively.
The comparative analysis of the experimentally achieved methane yields and the theoretical methane yields revealed that only 59.4% and 73% of the theoretical yields were achieved from non-extracted and supercritical fluid-extracted I. galbana, respectively. However, it is noteworthy that the theoretical values are calculated without considering the possible inhibitions, cell maintenance, or anabolism while these factors are involved during the experiments.
Kinnunen et al. (2014) worked with Nannocloropsis sp. in batch conditions and reported a methane yield of 194 L CH4 kg–1 VS and 482 L CH4 kg–1 VS for the dried and wet extract residues, respectively, obtained from biodiesel extraction using methanol and hexane. Other studies have reported similar methane yields for oil-extracted microalgae. For instance, Ehimen et al. (2009) reported methane yield values between 234 and 283 L CH4 kg–1 VS for dried, frozen, and oil-extracted Chlorella spp. using conventional and in situ-catalyzed transesterification methods. Keymer et al. (2013) reported methane yields of 240 L CH4 kg–1 VS for dried and lipid-extracted Scenedesmus spp. and a value of 380 L CH4 kg–1 VS for the same microalga when dried, lipid-extracted, and thermally hydrolyzed.
These results are similar to the results obtained in the present study, although not entirely comparable, as these studies involved different microalgal species and extraction methods other than SFE.
The digestates or effluents obtained after the AD of I. galbana and spent I. galbana remaining after SFE may be applied as a nutrient supplement to soils poor in nutrients. Solé-Bundó et al. (2017) reported that all the microalgal digestates in their work were suitable for use as soil fertilizers owing to their residual nitrogen (organic and ammonium nitrogen) and organic matter contents and low heavy metal content that was below the levels recommended by the European legislation. In the present study as well, the concentrations of heavy metals in the effluents obtained after the AD of I. galbana and that in the spent I. galbana were also quite low (Table 4). The anaerobic effluents of the AD process may also be utilized as media for the growth of fresh microalgae as these effluents retain various nutrients, such as carbon, nitrogen, phosphorous, etc. Certain authors have also reported the viability of such growth media for different microalgal strains including Nannochloropsis sp. (Mayers et al. 2017), C. vulgaris (Sforza et al. 2017), Muriellopsis sp., and Pseudokirchneriella subcapitata (Morales-Amaral et al. 2015).
The biogas produced from spent I. galbana could be utilized for energy co-generation to reduce the energy required for SFE and/or for the growth of fresh microalgal biomass (I. galbana), enabling circularity to the overall process. However, the methane yield of even the non-extracted I. galbana was not high, which led to the speculation that a co-substrate could be added to the AD system to improve the overall substrate composition and, in certain cases, to allow for a positive synergy between the two co-substrates (Rincón et al. 2018), which would ultimately increase the biogas production. The co-substrate selected for the subsequent experiments was the OMSW, a vegetal by-product from the two-phase olive oil extraction process. OMSW has a high carbon content and has, therefore, been used previously with different microalgae, achieving positive outcomes, as described in Sect. 3.4.
Characteristics of the effluents obtained after AD
The pH values for the effluents obtained after the second load and those obtained from the non-extracted and supercritical fluid-extracted microalgae are presented in Fig. 2a. While most methanogens are active in a pH range of 6.5–7.5, the optimal activity is exhibited at close-to-neutral pH values, such as 7.0 and 7.2. Ammonia and VFA are almost completely dissociated in the anaerobic reactors operated in the pH range of 6.0–7.5, because of which the pH buffer action is negligible. The carbonic system and the strong alkalis present in the system control the pH in this pH range (Capri and Marais 1975).
The pH values observed in the present study were 8.57 and 8.37 in the final effluents after the second load and from the non-extracted and supercritical fluid-extracted microalgae, respectively. These values are considerably high and could, therefore, destabilize the system. Therefore, at these values the influence of pH on chemical equilibrium must be considered. In certain systems the shift toward the formation of a particular chemical compound might exert inhibitory effects on the AD process. This is true in the case of the ammonium–ammonia equilibrium, as described below.
Figure 2b illustrates the variation in the conductivity with the load studied, first load, and second load. The conductivity was almost constant after reaching the value of 18 mS cm–1, indicating that the salinity of both extracted and unextracted I. galbana microalgae does not influence the respective AD processes because the conductivity remained almost constant prior to and after the BMP test.
Figure 3a presents the TKN or total nitrogen in each load added. The nitrogen content in the effluents obtained after AD was higher for the non-extracted I. galbana, while lower values were observed for the extracted microalga, which was consistent with the reduction of proteins in I. galbana after SFE, as presented in Table 3. Therefore, the protein content in the supercritical fluid-extracted I. galbana decreased from 46.95 ± 1.01% to 23.07 ± 1.81% (w/w).
Figure 3b illustrates the evolution of ammonia nitrogen in the system. The high protein content and the low C/N ratio of microalgae (Yen and Brune 2007; Mahdy et al. 2017) produce ammonium and free ammonia during the AD process. These two forms of nitrogen, ammonium and free ammonia, are in equilibrium. The free ammonia, which has high toxicity, is present in higher amounts in the AD systems with higher pH and temperatures, within the threshold of 150 mg N-NH3 L–1 (Chen et al. 2008).
The N-NH4+ values in the effluents obtained after AD (1283 ± 10 and 1356 ± 10 mg L–1 N-NH4+ for the first and second loads, respectively, of supercritical fluid-extracted I. galbana, and 1496 ± 75 and 1563 ± 50 mg L–1 N-NH4+ for the first and second loads, respectively, of I. galbana) were close to the values previously reported inhibiting anaerobic systems. Wang et al. (2016) reported 22.3% lower methane yields at the concentration of 1000 mg L–1 of N-NH4+. (Liu et al. 2015) reported N-NH4+ values of around 1000 mg L–1 as the inhibition threshold for expanded granular sludge bed reactors. McCarty (1964) reported that ammonia nitrogen concentrations of over 3000 mg NH4+-N L–1 were inhibitory at any pH in AD reactors. Moreover, AD systems could reportedly become unstable when the ammonia nitrogen values reach the range of 1500–3000 mg N-NH4+ L–1 (Angelidaki and Ahring 1993), while the tolerance to ammonia concentrations could be higher, up to the range of 1500–7000 mg N-NH4+ L–1, in an adapted process (Hejnfelt and Angelidaki 2009). These wide ranges of toxicity and inhibition for N-NH4+ could be due to factors such as the type of substrate, inoculum, pH, temperature, etc. which must be accounted for in the AD systems (Chen et al. 2008).
In the present study, free ammonia was also calculated using the Østergaard (1985) formula. Free ammonia was determined to be at the level of 5.3% (55.7 mg L–1) of the total ammonia nitrogen in the case of anaerobic inoculum when the digestate of I. galbana grew from the first to the second load. A similar trend was observed for the supercritical fluid-extracted microalga. The free ammonia for the first and second loads, when the non-extracted I. galbana grew from 247.2 to 457.2 mg L–1, were 16.5% and 29.3% of the total ammonia nitrogen, respectively. These values were always under that found inhibitory for the AD process. Free ammonia at concentrations over 3000 mg L–1 have been reported to be inhibitory by certain authors, although this depends on the system and it is, therefore, possible to find systems working with free ammonia in the concentration range of 4000–9000 mg L–1 (Montalvo Martínez 2000). The extracted I. galbana exhibited lower values of free ammonia, in general, compared to those observed for the non-extracted microalga, with the values for the former reaching 280.6 mg L–1 from 145.4 mg L–1, accounting for 20.7% and 11.3% of the total ammonia nitrogen, respectively, in the reactors, thereby being non-inhibitory for the AD process.
The increase in the T-Alk value, presented in Fig. 4a, could be due to the increase in the ammonia concentration during the BMP test. The initial increase in the ammonia concentration might supply additional alkalinity (Zhang et al. 2016), thereby contributing to the stability and buffering capacity of the digesters. However, at high concentrations, this increase in ammonia could inhibit the process of methanogenesis.
Alkalinity in AD reactors may range between 1500 and 5000 mg CaCO3 L–1. An appropriate level of alkalinity in the AD process allows coping with pH fluctuations. The alkalinity values above 5000 mg CaCO3 L–1 are reported to have little effect on the AD process, although these are also not too risky (Rittmann and McCarty 2001).
In the present study, the VFA values (Fig. 4b) decreased to 160 ± 10 and 97 ± 5 mg C L–1 for I. galbana and extracted I. galbana (residues following supercritical fluid extraction), respectively, by the end of the second load.
Effects of the co-digestion mixture on AD
The methane yields obtained for the OMSW–I. galbana mixtures with the VS/TKN ratios of 20 and 30 were higher than those obtained for the I. galbana used as the sole substrate, indicating a certain level of positive synergy between the co-substrates. This increase in the methane yield could be due to the lesser formation of inhibitory ammonia during the digestion, which affected the activity of the acidogenic bacteria and the archaea (Ward et al. 2014) and led to the accumulation of VFA. This behavior could also be due to the decreased salinity of the substrates fed to the reactors. Similarly, the TKN and N-NH4+ values for the effluents obtained after the digestion of I. galbana used as the sole substrate were 1852 ± 10 mg L–1 and 1283 ± 10 mg L–1, respectively, while the values for the OMSW–I. galbana mixtures with the VS/TKN ratio of 20 were 1496 ± 50 and 1146 ± 25 mg L–1, respectively. The OMSW–I. galbana mixture with the VS/TKN ratio of 30 presented the best methane yield among the two co-digestion mixtures assayed, with the values for the two mixtures being 1459 ± 55 and 880 ± 30 mg L–1, respectively. As visible in Table 5, the TKN value decreased to 1459 mg L–1 for the OMSW–I. galbana co-digestion mixture with the VS/TKN ratio of 30, which was a reduction of 21.22% compared to the complete I. galbana. Moreover, the ammonia concentration decreased from the value of 1283 mg L–1 for complete I. galbana to 880 mg L–1 for the OMSW–I. galbana co-digestion mixture with the VS/TKN ratio of 30. Herrmann et al. (2016) reached analogous conclusions, stating that the reduction in the inhibitory ammonia due to the reduction in the nitrogen-rich feedstock was one of the main factors affecting the yields of the digestion processes. In the present study, VFA also increased as the proportion of microalga fed to the reactor increased. Therefore, in the final effluents, the VFA concentrations were 475, 404, 281, and 40 mg CH3COOH L–1 (Table 5) for I. galbana used as the sole substrate, the OMSW–I. galbana mixture with the VS/TKN ratio of 30, the OMSW–I. galbana mixture with the VS/TKN ratio of 20, and OMSW used as the sole substrate, respectively.
Fernández-Rodríguez et al. (2019) reported a methane yield of 461 mLSTP CH4 g–1 VSadded for the C/N ratio of 25.3 (corresponding to the 75% OMSW–25% Scenedesmus quadricauda co-digestion mixture). This methane yield was 104% higher than that obtained when using S. quadricauda (C/N ratio 5.6) alone. Herrmann et al. (2016) studied the co-digestion of Arthrosphira platensis and carbon-rich co-substrates, such as beet silage, brown seaweed Laminaria digitata, and barley straw, and reported no synergistic effects when operating in batch mode, while a synergy was observed when working in continuous mode.
Figure 6 illustrates the linear relationship between the VS/TKN ratio and the methane yields (r2 = 0.9931). The increase in the VS/TKN ratio up to the values of 20 and 30 with the addition of OMSW to the co-digestion mixtures increased the methane yields obtained for I. galbana (VS/TKN 12.6) digested anaerobically as the sole substrate. The values of 134 and 189 mLSTP CH4 g–1 VS were obtained for the VS/TKN ratios of 20 and 30 (respective C/N ratios of 9.0 and 18.0), respectively, compared to the value of 98 mLSTP CH4 g–1 VS obtained for the microalga used as the sole substrate (C/N ratio of 5.1).
Figure 7a illustrates the increment in the VS, which is defined as the difference between the VS in the effluents after AD and the initial VS, with the VS/TKN ratio. The increment in the VS was correlated with the VS/TKN ratio (r2 = 0.9768) and ranged between 400 and 450 mg L–1. Figure 7b depicts the plot of the methane yields obtained at different VS/TKN ratios assayed versus the increase in the VS after AD. The increment in the VS decreased with the increase in the VS/TKN ratio of the substrates. The lowest VS increment of 400 mg L–1 was observed when using OMSW as the sole substrate, while the highest value of 450 mg L–1 was obtained for the microalga I. galbana used as the sole substrate. The correlation between the two variables was high, with R2 = 0.99875.
Variation of volatile solids (VS) incremented (a) with the Volatile solids/Total Kjeldahl Nitrogen (VS/TKN) ratio and variation of the methane yield (b) with the increment in VS for the substrates studied: Olive mill solid waste (VS/TKN = 44), microalga Isochrysis galbana (VS/TKN = 12.6) and anaerobic co-digestion mixtures of microalga I. galbana with olive mill solid waste (VS/TKN = 20 and 30). VS incremented was calculated using the means of three replicates in each analytical measurement and two biological reactors (n = 6). Methane yields represent the means of two biological reactors (n = 2). Error bars denote standard deviation in all the cases
Moreover, the highest methane yield of 278 mLSTP CH4 g–1 VS was obtained for OMSW (VS/TKN = 44.3, C/N = 30.8) and was related to the lowest increment in the VS. The highest VS increment of 450 mg L–1 corresponded to the I. galbana used alone (VS/TKN = 12.6, C/N = 5.1). The increase in the VS/TKN ratio observed for the co-digestion mixtures favored the increase of the methane yield and the decrease in the VS increment. This was because the VS were consumed (Fig. 7a and b) during the biomethanization process.
The TKN incremented (Fig. 8a) increased with the VS/TKN ratio. The highest value of 467 mg L–1 was observed for OMSW used as the sole substrate (VS/TKN = 44.3, C/N = 30.8), and this value gradually lowered as the VS/TKN ratio decreased, with the lowest value obtained for microalga I. galbana (VS/TKN = 12.6, C/N = 5.1). The opposite trend was observed for the T-Alk value (Fig. 8b), which decreased when the VS/TKN ratio increased as a consequence of the increase in the VFA. The T-Alk incremented were always over the 1500 mg CaCO3 L−1 recommended to keep the stability of anaerobic systems regardless of the digested substrate.
Variation of Total Kjeldahl Nitrogen (TKN) (a) and variation of the total alkalinity (b) with the Volatile Solids/Total Kjeldahl Nitrogen (VS/TKN) ratio for the substrates studied: Olive mill solid waste (VS/TKN = 44), microalga Isochrysis galbana (VS/TKN = 12.6) and anaerobic co-digestion mixtures of microalga I. galbana with olive mill solid waste (VS/TKN = 20 and 30). TKN and Alkalinity incremented were calculated using the means of three replicates in each analytical measurement and two biological reactors (n = 6). Error bars denote standard deviation in all the cases
The value of the slope of the line of adjustment to the pairs of values (VS/TKN ratio, TKN incremented) (Fig. 8a) increased at the coefficient of proportionality of 6.19 mg L–1. When the alkalinity declined (Fig. 8b), the proportionality between the VS/TKN ratio and the variation in the alkalinity was observed. The negative slope value of 54.60 mg L–1 was obtained as the VS/TKN ratio increased by one unit, which indicated a lower alkalinity maintenance capacity. However, according to Table 5, the effluents of all loads presented extremely high alkalinity values, because of which this issue was not considered a limiting problem for the digestion of the substrates.
Conclusions
The present study used a combination of supercritical fluid extraction with anaerobic digestion of the supercritical fluid-extracted microalga Isochrysis galbana for achieving the double benefit of obtaining the compounds of interest, fucoxanthin and PUFASs, and generating bioenergy in the form of biogas. The use of a green technology-based method such as supercritical fluid extraction allowed obtaining the extracted microalga without requiring any solvents, as is required in other extraction methods. Moreover, the obtained extracted microalga had a good quality and could therefore be used in a bioprocess conducted by microorganisms such as anaerobic digestion. Furthermore, methane yields of 104 ± 5 LSTP CH4 kg–1 VS and 90 ± 2 LSTP CH4 kg–1 VS were achieved for non-extracted I. galbana and extracted microalga I. galbana, respectively. The produced methane could be utilized to decrease the energy required for conducting the supercritical fluid extraction, for maintaining mesophilic temperatures during the process of anaerobic digestion, or even for the growth of fresh microalgal biomass, thereby facilitating a circular economy in which all materials are utilized. Moreover, the use of olive mill solid waste as a carbon-rich co-substrate along with Isochrysis galbana in the anaerobic digestion increased the methane yield achieved using I. galbana alone as the substrate. The methane yields of 134 ± 4 and 189 ± 5 LSTP CH4 kg–1 VS were achieved using the OMSW–I. galbana co-digestion mixtures with the VS/TKN ratios of 20 and 30, these values were 36.7% and 92.9% higher, respectively, compared to those obtained when I. galbana was used as the sole substrate. These findings were interesting as a positive synergy between the co-substrates was established based on these results. However, the co-digestion of spent I. galbana and olive mill solid waste warrants further research.
References
Angelidaki I, Ahring B (1993) Thermophilic anaerobic digestion of livestock waste: the effect of ammonia. Appl Microbiol Biotechnol 38:560–564
Angelidaki I, Sanders W (2004) Assessment of the anaerobic biodegradability of macropollutants. Rev Env Sci Bio/Technol 3:117–129
APHA, AWWA, WPCF (2005) Standard Methods for the Examination of Water and Wastewater. 17th Edition edn. American Public Health Association., Washington, DC. USA
Axelsson M, Gentili F (2014) A single-step method for rapid extraction of total lipids from green microalgae. Plos One 9:6
Bae M, Kim M-B, Park Y-K, Lee J-Y (2020) Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim Biophys Acta - Mol Cell Biol Lipids 1865:158618
Batista AP, Gouveia L, Bandarra NM, Franco JM, Raymundo A (2013) Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res 2:164–173
Capri M, Marais G (1975) pH adjustment in anaerobic digestion. Water Res 9:307–313
Chandra R, Iqbal HMN, Vishal G, Lee HS, Nagra S (2019) Algal biorefinery: A sustainable approach to valorize algal-based biomass towards multiple product recovery. Bioresour Technol 278:346–359
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Bioresour Technol 99:4044–4064
Chisti Y (2007) Biodiesel from microalgae. Biotech Adv 25:294–306
Da Silva RP, Rocha-Santos TA, Duarte AC (2016) Supercritical fluid extraction of bioactive compounds. Trends Anal Chem 76:40–51
Dubois M, Gilles KA, Hamilton JK, Pt Rebers, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Ehimen EA, Connaughton S, Sun Z, Carrington GC (2009) Energy recovery from lipid extracted, transesterified and glycerol codigested microalgae biomass. GCB Bioenergy 1:371–381
FDA (2016) Substances generally recognized as safe, Final rule. Fed.Regist. 2016. vol Final Rule. Fed Regist 81
Fernández-Rodríguez M, De la Lama-Calvente D, Jiménez-Rodríguez A, Borja R, Rincón-Llorente B (2019) Anaerobic co-digestion of olive mill solid waste and microalga Scenedesmus quadricauda: effect of different carbon to nitrogen ratios on process performance and kinetics. J Appl Phycol 31:3583–3591
Fernández-Rodríguez MJ, Mancilla-Leytón JM, Jiménez-Rodríguez A, Borja R, Rincón B (2021) Reuse of the digestate obtained from the biomethanization of olive mill solid waste (OMSW) as soil amendment or fertilizer for the cultivation of forage grass (Lolium rigidum var. wimmera). Sci Total Environ 792:148465
Geresh S, Adin I, Yarmolinsky E, Karpasas M (2002) Characterization of the extracellular polysaccharide of Porphyridium sp.: molecular weight determination and rheological properties. Carbohydr Polym 50:183–189
Gilbert-López B, Mendiola JA, Fontecha J, van den Broek LA, Sijtsma L, Cifuentes A, Herrero M, Ibáñez E (2015) Downstream processing of Isochrysis galbana: a step towards microalgal biorefinery. Green Chem 17:4599–4609
Gonzalez-Fernandez C, Sialve B, Molinuevo-Salces B (2015) Anaerobic digestion of microalgal biomass: Challenges, opportunities and research needs. Bioresour Technol 198:896–906
González-González LM, Correa DF, Ryan S, Jensen PD, Pratt S, Schenk PM (2018) Integrated biodiesel and biogas production from microalgae: towards a sustainable closed loop through nutrient recycling. Renew Sustain Energy Rev 82:1137–1148
Gouveia L, Coutinho C, Mendonça E, Batista AP, Sousa I, Bandarra NM, Raymundo A (2008) Functional biscuits with PUFA-ω3 from Isochrysis galbana. J Sci Food Ag 88:891–896
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum Press, New York, pp 29–60
Gujer W, Zehnder AJB (1983) Conversion processes in anaerobic digestion. Water Sci Technol 15:127–167
Hartmann H, Ahring BK (2005) Anaerobic digestion of the organic fraction of municipal solid waste: influence of co-digestion with manure. Water Res 39:1543–1552
Harun R, Davidson M, Doyle M, Gopiraj R, Danquah M, Forde G (2011) Technoeconomic analysis of an integrated microalgae photobioreactor, biodiesel and biogas production facility. Biomass Bioenergy 35:741–747
Hejnfelt A, Angelidaki I (2009) Anaerobic digestion of slaughterhouse by-products. Biomass Bioenergy 33:1046–1054
Herrero M, Mendiola JA, Cifuentes A, Ibáñez E (2010) Supercritical fluid extraction: Recent advances and applications. J Chromatogr A 1217:2495–2511
Herrmann C, Kalita N, Wall D, Xia A, Murphy JD (2016) Optimised biogas production from microalgae through co-digestion with carbon-rich co-substrates. Bioresour Technol 214:328–337
Hogan P, Otero P, Murray P, Saha SK (2021) Effect of biomass pre-treatment on supercritical CO2 extraction of lipids from marine diatom Amphora sp. and its biomass evaluation as bioethanol feedstock. Heliyon 7:e05995
Kalam S, Gul MZ, Singh R, Ankati S (2015) Free radicals: Implications in etiology of chronic diseases and their amelioration through nutraceuticals. Pharmacologia 6:11–20
Keymer P, Ruffell I, Pratt S, Lant P (2013) High pressure thermal hydrolysis as pre-treatment to increase the methane yield during anaerobic digestion of microalgae. Bioresour Technol 131:128–133
Kim SM, Kang S-W, Kwon O-N, Chung D, Pan C-H (2012) Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J Kor Soc Appl Biol Chem 55:477–483
Kinnunen H, Koskinen P, Rintala J (2014) Mesophilic and thermophilic anaerobic laboratory-scale digestion of Nannochloropsis microalga residues. Bioresour Technol 155:314–322
Liu Z, Dang Y, Li C, Sun D (2015) Inhibitory effect of high NH4+–N concentration on anaerobic biotreatment of fresh leachate from a municipal solid waste incineration plant. Waste Manage 43:188–195
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mahdy A, Fotidis IA, Mancini E, Ballesteros M, González-Fernández C, Angelidaki I (2017) Ammonia tolerant inocula provide a good base for anaerobic digestion of microalgae in third generation biogas process. Bioresour Technol 225:272–278
Mata-Alvarez J, Macé S, Llabres P (2000) Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour Technol 74:3–16
Mata-Alvarez J, Dosta J, Romero-Güiza M, Fonoll X, Peces M, Astals S (2014) A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew Sust Energy Rev 36:412–427
Mayers JJ, Nilsson AE, Albers E, Flynn KJ (2017) Nutrients from anaerobic digestion effluents for cultivation of the microalga Nannochloropsis sp.—impact on growth, biochemical composition and the potential for cost and environmental impact savings. Algal Res 26:275–286
McCarty PL (1964) Anaerobic waste treatment fundamentals, Part III, Toxic materials and their control. Public Works 95:91–94
Molino A, Mehariya S, Iovine A, Larocca V, Di Sanzo G, Martino M, Casella P, Chianese S, Musmarra D (2018) Extraction of astaxanthin and lutein from microalga Haematococcus pluvialis in the red phase using CO2 supercritical fluid extraction technology with ethanol as co-solvent. Mar Drugs 16:432
Montalvo Martínez SJ (2000) Tratamiento anaerobio de aguas residuales. Generalidades. Monografía 1–36
Morales-Amaral MdM, Gómez-Serrano C, Acién FG, Fernández-Sevilla JM, Molina-Grima E (2015) Production of microalgae using centrate from anaerobic digestion as the nutrient source. Algal Res 9:297–305
Mulders KJ, Weesepoel Y, Lamers PP, Vincken J-P, Martens DE, Wijffels RH (2013) Growth and pigment accumulation in nutrient-depleted Isochrysis aff. galbana T-ISO. J Appl Phycol 25:1421–1430
Mussgnug JH, Klassen V, Schlüter A, Kruse O (2010) Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J Biotech 150:51–56
Niccolai A, Chini Zittelli G, Rodolfi L, Biondi N, Tredici MR (2019) Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res 42:101617
Østergaard N (1985) Biogasproduktion i det thermofile temperaturinterval. STUB Rapport 21
Passos F, Uggetti E, Carrère H, Ferrer I (2014) Pretreatment of microalgae to improve biogas production: A review. Bioresour Technol 172:403–412
Patil V, Källqvist T, Olsen E, Vogt G, Gislerød HR (2007) Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquac Int 15:1–9
Ramos-Suárez JL, Carreras N (2014) Use of microalgae residues for biogas production. Chem Eng J 242:86–95
Rincón B (2020) Biogas from microalgae. In: Jacob-Lopes E, Maroneze MM, Queiroz MI, Zepka LQ (eds) Handbook of Microalgae-Based Processes and Products. Academic Press, NY, pp 311–328
Rincón B, Fernández-Rodríguez MJ, de la Lama-Calvente D, Borja R (2018) The influence of microalgae addition as co-substrate in anaerobic digestion processes. In: Jacob-Lopes E, Zepka LQ, Queiroz MI (eds) Microalgal Biotechnology. IntechOpen, London, pp 59–90
Roubaud A, Favrat D (2005) Improving performances of a lean burn cogeneration biogas engine equipped with combustion prechambers. Fuel 84:2001–2007
Ruiz-Domínguez MC, Cerezal P, Salinas F, Medina E, Renato-Castro G (2020) Application of Box-Behnken design and desirability function for green prospection of bioactive compounds from Isochrysis galbana. Appl Sci 10:2789
Sayegh FAQ, Radi N, Montagnes DJS (2007) Do strain differences in microalgae alter their relative quality as a food for the rotifer Brachionus plicatilis? Aquaculture 273:665–678
Sforza E, Barbera E, Girotto F, Cossu R, Bertucco A (2017) Anaerobic digestion of lipid-extracted microalgae: enhancing nutrient recovery towards a closed loop recycling. Biochem Eng J 121:139–146
Sialve B, Bernet N, Bernard O (2009) Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol Adv 27:409–416
Solé-Bundó M, Cucina M, Folch M, Tàpias J, Gigliotti G, Garfí M, Ferrer I (2017) Assessing the agricultural reuse of the digestate from microalgae anaerobic digestion and co-digestion with sewage sludge. Sci Total Environ 586:1–9
Sun Z, Wei H, Zhou Z-g, Ashokkumar M, Liu J (2018) Screening of Isochrysis strains and utilization of a two-stage outdoor cultivation strategy for algal biomass and lipid production. Appl Biochem Biotech 185:1100–1117
Symons G, Buswell A (1933) The methane fermentation of carbohydrates. J Am Chem Soc 55:2028–2036
The US Department of Agriculture and the US Composting Council (2002) Test Methods for the Examination of Composting and Compost (TMECC). Edaphos International, Houston
Usman M, Salama E-S, Arif M, Jeon B-H, Li X (2020) Determination of the inhibitory concentration level of fat, oil, and grease (FOG) towards bacterial and archaeal communities in anaerobic digestion. Renew Sust Energy Rev 131:110032
Wang H, Zhang Y, Angelidaki I (2016) Ammonia inhibition on hydrogen enriched anaerobic digestion of manure under mesophilic and thermophilic conditions. Water Res 105:314–319
Ward A, Lewis D, Green F (2014) Anaerobic digestion of algae biomass: a review. Algal Res 5:204–214
Yen H-W, Brune DE (2007) Anaerobic co-digestion of algal sludge and waste paper to produce methane. Bioresour Technol 98:130–134
Zabed H, Boyce AN, Sahu J, Faruq G (2017) Evaluation of the quality of dried distiller’s grains with solubles for normal and high sugary corn genotypes during dry–grind ethanol production. J Cleaner Prod 142:4282–4293
Zabed HM, Akter S, Yun J, Zhang G, Zhang Y, Qi X (2020) Biogas from microalgae: Technologies, challenges and opportunities. Renew Sust Energy Rev 117:109503
Zeikus J (1980) Microbial populations in digesters. In: Stafford DA, Wheatley BI, Hughes DE (eds) Anaerobic digestion:[Proceedings of the First International Symposium on Anaerobic Digestion, held at University College, Cardiff, Wales, September 1979]. Applied Science Publishers, London pp 61–89
Zhang J, Wang S, Lang S, Xian P, Xie T (2016) Kinetics of combined thermal pretreatment and anaerobic digestion of waste activated sludge from sugar and pulp industry. Chem Eng J 295:131–138
Zhang Y, Caldwell GS, Zealand AM, Sallis PJ (2019) Anaerobic co-digestion of microalgae Chlorella vulgaris and potato processing waste: effect of mixing ratio, waste type and substrate to inoculum ratio. Biochem Eng J 143:91–100
Acknowledgements
The research group “LAMICBA” at University of Antofagasta, Chile, thanks “Microalgas Oleas de México S.A.” for providing microalgal samples and to Miss Inmaculada Bellido Padillo for her kind and excellent help with the experimental work. This work was supported by the public funds of Chile – Agencia Nacional de Investigación y Desarrollo (ANID): grants FONDECYT-11170017 and FONDEQUIP EQM-160073.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by the public funds of Chile – Agencia Nacional de Investigación y Desarrollo (ANID): grants FONDECYT-11170017 and FONDEQUIP EQM-160073.
Author information
Authors and Affiliations
Contributions
Mari Carmen Ruiz-Domínguez: Conceptualization, resources, funding acquisition, supervision; writing—original draft preparation; Bárbara Rincón: Conceptualization, funding acquisition, supervision, validation, writing-original draft preparation, writing—review and editing final version; Maria de los Ángeles Martín: supervision, validation, writing—review and editing María del Carmen Gutierrez: investigation, writing: review and editing; Francisca Salinas: investigation; Elena Medina: investigation; Pedro Cerezal: Conceptualization, resources, funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
There is no conflict of interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruiz-Domínguez, M.C., Rincón, B., de los Ángeles Martín, M. et al. Microalga Isochrysis galbana biorefinery: Obtaining fucoxanthin and biogas after supercritical fluid extraction. J Appl Phycol 34, 1997–2014 (2022). https://doi.org/10.1007/s10811-022-02768-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02768-1