Abstract
The in-field protein production of four macroalgae (Gracilariopsis vermiculophylla, Gracilaria gracilis, Gracilariopsis longissima, Ulva australis) and three seagrasses (Cymodocea nodosa, Zostera marina, Zostera noltei) was investigated in four transitional water systems over one year. The protein content in macroalgae ranged from 1.0 to 25.1% and was inversely related with water temperature. The annual protein production was the highest for G. longissima (500 g dw m−2 year−1), followed by G. vermiculophylla (350 g dw m−2 year−1), U. australis (33 g dw m−2 year−1) and G. gracilis (270 g dw m−2 year−1). The most productive months spanned between March and August for G. longissima and between December and May for G. vermiculophylla, reaching 78% and 85% of the annual production, respectively. The protein production was more uniform over the year for the other two macroalgae. The protein production in seagrasses was averagely from 3.4 to 12 times lower than in macroalgae and reached 77.8 g m−2 year−1 in C. nodosa, followed by Z. marina with 55.6 g m−2 year−1 and Z. noltei with 30.6 g m−2 year−1. The peak production between April and August accounted for 63–98% of the annual production. Rhizomes displayed the lowest protein contents (1.2–3.4%), almost half in comparison with leaves (2.3–5.1%) and the lowest protein production with 21.0 g m−2 year−1 for C. nodosa, 6.3 g m−2 year−1 for Z. marina and 9.0 g m−2 year−1 for Z. noltei. Aquatic macrophyte productions proved to be competitive with the main crops currently cultivated on land without competing in terms of land and freshwater destination. The challenge is still open on the front of digestibility and protein extraction but sustainable management and production of macrophytes (especially macroalgae) can significantly contribute to the global protein production in coastal areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Future prospects of climate change and overpopulation pose complex challenges because our food provisioning, production and distribution systems still prove to be unsustainable. This unsustainability is highlighted by the loss and progressive deterioration of essential ecosystem services because of mismanagement and overexploitation (Millennium Ecosystem Assessment 2005). The concerns for an environmental-friendly and sustainable provision and production of high quality proteins for food and forage led to the proposal by non-governmental organizations of an international innovation partnership, the “Protein Challenge 2040”: to explore how it will be possible to balance supply and demand for protein by a growing population (Rosen 2015). Indeed, the optimization of current agri-aquacultural practices, the selection of new plant (Allard 1999) and algal species (Rajauria et al. 2015) and genetic improvement (Nelson 1969; Uzogara 2000) will probably increase the foodstuffs availability and the protein content. However, if we consider the species currently available for the production of biomass and proteins, the attention should be shifted from land to coastal transitional water systems (TWS). The TWS are among the most productive environments (Iglesias-Campos et al. 2015) and, at the same time, some of the most compromised and threatened by pressures, such as pollution, nutrient discharge, climate change, overfishing, habitat conversion and invasive species. Understanding the production and renewability of resources from environments is a necessary condition for a sustainable use. Regarding protein production, the aquatic macrophytes represent the basis of biomass production in TWS food webs (Goldman 1969). Considering the grazing pressure exerted on them, and the loss of about one order of magnitude in terms of energy and biomass in the food chain from primary producers to grazers and then to carnivores (Colinvaux and Barnett 1979), the primary producers represent the most important source of protein in TWS. Consequently, the primary producers are fundamental providers of ecosystem services in terms of biomass and protein production for both provisioning services (for protein harvesting) and regulation and maintenance services (protein supply to the environment itself) (Haines-young and Potschin 2018). Actually, the largest part of protein provisioning in the form of fishery resources in Europe consists of herbivores/filter feeders, predators and scavengers corresponding respectively to second and third level of the trophic food web, with a significant reduction in the protein yield retrieved from TWS in comparison with the potential protein yield associated with primary producers.
In order to quantify the protein production capacity in temperate TWS we have undertaken a study to estimate: the monthly/annual production of proteins of the dominant primary producers in 4 stations of the Venice Lagoon (Italy), together with the physico-chemical parameters that characterize the areas. The species considered for this investigation were: 4 macroalgae—Gracilariopsis vermiculophylla Ohmi, Gracilaria gracilis (Stackhouse) Steentoft, L.M.Irvine & Farnham Gracilariopsis longissima (S.G. Gmelin) Steentoft, L.M. Irvine & Farnham, Ulva australis Areschoug—and 3 seagrasses -Cymodocea nodosa (Ucria) Ascherson, Zostera marina Linnaeus 1753, Zostera noltei Hornemann.
Materials and Methods
Study area
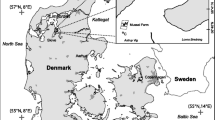
Macroalgae growth was investigated in a shallow water area (average water depth 0.6–0.8 m) of Venice Lagoon (Italy) along the landward, on south-western side of the bridge watershed connecting the Venice historical centre to the mainland (Fig. 1). The station Tresse (TR: 45°26′09.18″N; 12°16′17.76″E) was characterized by high nutrient concentrations conveyed mainly by the Brentella river and high water circulation and renewal ensured by two large canals (Vittorio-Emanuele and Malamocco-Marghera). The growth for all the four macroalgal species was investigated in the same station in which they naturally grew often mixed together or in patches at a short distance from each other.
Seagrass growth was investigated in three stations characterized by monospecific dominance and high water renewal: two along the seaward, in proximity of the water inlets of Malamocco and Chioggia and one midway in proximity of the large Perognola canal in an internal shallow water area. From North to South these were: Santa Maria del Mare (SMM: 45°19′51.0″N; 12°18′44.5″E) for C. nodosa, Petta di Bò (BO: 45°16′16.5″N; 12°15′03.2″E) for Z. noltei and Ca’ Roman (CR: 45°15′15.5″N; 12°17′34.0″E) for Z. marina.
Physico-chemical parameters
The main physico-chemical parameters of water column were measured in the stations at each sampling campaign as previously reported in Sfriso et al. (2017), and water temperature (T° w), pH (pHw), redox potential (Ehw), dissolved oxygen (DO), salinity (Sal), suspended filtered particulate matter (FPM), total chlorophyll-a (Chl-a) and nutrient concentrations—nitrites (NO2−), nitrates (NO3−), ammonium (NH4+), reactive phosphorus (RP) and reactive silica (Si) -.
Seagrass production
Seagrass growth was estimated as in Short and Duarte (2001) and Sfriso and Ghetti (1998) along one year. Six biomass samples (20 × 25 cm for Z. marina and C. nodosa and 10 × 25 cm for Z. noltei) were sampled each month. In laboratory, seagrasses were washed and gently scraped in order to remove sediment, epiphytes and both leaves and rhizomes were separately weighed for the estimation of biomasses expressed as g fw m−2. After drying each part, we weighed and converted data in g dw m−2. Every month one sample was collected for the analysis of the protein content and the net gains in biomass per month (converted to dw and m−2) were multiplied by protein percentage to estimate the protein production.
Macroalgal production
The macroalgae growth was measured in field as in Sfriso and Sfriso (2017) along one year. Briefly, a wet weight of 200 g of G. vermiculophylla, G. gracilis, G. longissima, U. australis was placed in cubical cages (25 × 25x25 cm, mesh size 1 cm), one species per cage, taking into account the infield mean biomass of approx. 3 kg fw m−2 (fw: fresh weight). The cages were placed on the bottom of the station and the algal biomass was weighted at intervals of 7–11 days. At each sampling campaign biomasses were drained by a salad spinner, weighted, then the initial 200 g were restored (removing or adding sample to the cage) so that the production rate could be comparable. Data were converted to g dw m−2 (dw: dry weight) after drying each part. The net gains in biomass per month (g dw m−2) were multiplied by protein percentage to calculate protein production.
Protein analysis
Samples of macroalgae and seagrasses were completely dried, ground and stored in the dark. Samples were dried in oven at 50 °C for 72 h before analyses. The ratios dry weight/fresh weight (dw/fw) were recorded. Total proteins were extracted in duplicate by NaOH 0.5 M at 100 °C for 15 min and were determined spectrophotometrically at 595 nm by Bradford assay (Bradford 1976), using bovine serum albumin for the calibration curve. The analytical error was within 4%.
Statistical analysis
Principal Component Analysis (PCA) was performed separately on monthly values of each species of seagrasses and macroalgae using “FactoMineR” and “factoextra” packages on R software version 4.0.3. PCA of seagrass data analysed the multivariate patterns of the matrix of 36 cases (monthly values for the three stations) in response to the independent variables (water temperature, pHw, Ehw, DO, Salinity, FPM, Chlorophyll-a, and nutrient concentrations -nitrite, nitrate, ammonium, silicate-). PCA of macroalgae data analysed the patterns of the matrix of 12 cases (monthly values for Tresse station) in response to the same parameters.
Results
Environmental factors
The geometric mean for all parameters applied to the annual mean values of environmental parameters of the stations ranked the stations from the one with averagely higher to the one with lower trophic conditions (Supplementary material Table S1). The TR station displayed the highest mean temperature, DO, pH, and trophy: with DIN values significantly higher ranging from 2.11 to 54.7 µg L−1 and Chl-a from 0.93 to 10.3 µg L−1. Among the seagrass stations, the trophic conditions recorded at BO (colonized by Z. noltei) were the highest, especially concerning the mean RP values (0.95 µg L−1 – almost three times higher than in the other two stations), turbidity (FPM), reactive silica, salinity, pH, Eh and temperature. The station CR (colonized by Z. marina) displayed instead the highest DIN concentrations among the seagrass stations (6.93 µg L−1) but presented average scores for the other parameters. Eventually, SMM (colonized by C. nodosa) was characterized by the lowest mean scores and trophic level, but presented highest salinity, DO and Eh, confirming itself to be the most marine-like station among all.
Proteins in macroalgae
Macroalgae showed fluctuating protein percentages throughout the year (Fig. 2a) ranging from 1.0 ± 0.0 to 25.1 ± 0.7%. The average annual protein percentage was similar for all Gracilariales with an overall mean value of 11.1 ± 5.7% and peak percentages recorded in February and mid-summer (18.2 ± 0.5% for G. longissima). The green seaweed U. australis displayed the least protein content with a slightly lower average annual score of 7.3 ± 3.2% and the maximum protein percentage, never exceeded 13.0 ± 0.4%. A marked seasonal trend was noted for Gracilariales with a higher mean protein content (17.1 ± 5.4%) during cold winter months (Dec-Feb) in comparison with the mean value for the rest of the year (9.1 ± 4.3%). Conversely, U. australis displayed two marked protein content peaks in spring and autumn at mild temperatures (Feb/Nov).
a Protein percentage in macroalgae (U. australis, G. gracilis, G. longissima, G. vermiculophylla) during one year. The standard deviation was within ± 3.6%. b Monthly protein production of macroalgae (U. australis, G. gracilis, G. longissima, G. vermiculophylla) from January to December. The error bars show the average coefficient of variation (n = 12)
Inconsistent with the annual protein percentages, the annual protein productions (which are linked not only to the protein content but also to the algal growth) appeared to be very different. The annual protein production for macroalgae was the highest for G. longissima (500 g dw m−2 year−1), followed by G. vermiculophylla (350 g dw m−2 year−1), U. australis (330 g dw m−2 year−1) and G. gracilis (270 g dw m−2 year−1). In terms of protein production, the most productive months spanned from March to August for G. longissima, reaching 78% of the annual production, and from December to May for G. vermiculophylla, reaching 85% of the annual production. The protein production was instead quite uniform over the year for the other two macroalgae (Fig. 2b). With a view oriented to the harvest optimization, these represent the ideal months for the provision of the aforementioned species.
Proteins in seagrasses
The protein percentages and the seagrass productions were subdivided between leaves and rhizomes due to the different ecological role and fate. The leaves are exposed to grazing, changing their lengths according to the different growing periods and the oldest one detaches periodically every 15–90 days during the growing period being often transported by the currents in the open sea and on the nearby shores. Conversely, the rhizomes represent a more stable biomass buried in the sediment. The leaves presented an averagely higher protein content in comparison with rhizomes (1.7 times higher scores), ranging between 2.3 ± 0.0 and 5.1 ± 0.2%. However, in comparison with the protein content of macroalgae, seagrasses displayed a much lower and steady protein fraction both in leaves and rhizomes (Fig. 3a-4a). Protein production was also a lot lower in seagrasses in comparison with macroalgae. In order to appreciate the seasonal variations of protein content and production in seagrass leaves (Fig. 3a,b) and roots (Fig. 4a,b) the scale of the graphs was set 6 times lower in comparison with the macroalgae graphs of Fig. 2a,b. Also considering the sum of leaf and rhizome production for each species the seagrass production was averagely 3.4–12 times lower than in macroalgae. The annual protein production in seagrass leaves was highest in C. nodosa (77.8 g m−2 year−1), followed by Z. marina (55.6 g m−2 year−1) and Z. noltei (30.6 g m−2 year−1) with a peak between April and August accounting for the 98%, 63% and 84% of the annual production, respectively. Rhizomes, not only displayed the lowest protein contents, but also the lowest production ranging from 21.0 g m−2 year−1 for C. nodosa, to 9.0 g m−2 year−1for Z. noltei and 6.3 g m−2 year−1 for Z. marina, without significant seasonal changes (Fig. 4b).
a Protein percentage in seagrass leaves (C. nodosa, Z. noltei, Z. marina) over one year. The standard deviation was within ± 0.5%. b Monthly protein production (g dw m−2) of seagrass (C. nodosa, Z. noltei, Z. marina) leaves from January to December. The error bars show the average coefficient of variation (n = 12)
a Protein percentage in seagrass rhizomes (C. nodosa, Z. noltei, Z. marina) over one year. The standard deviation was within ± 0.5%. b Monthly protein production of seagrass (C. nodosa, Z. noltei, Z. marina) rhizomes from January to December. The error bars show the average coefficient of variation (n = 12)
Multivariate analysis
PCA analysis was carried out separately for macroalgae (Fig. 5a) and seagrasses (Fig. 5b) on the biochemical variables and the average monthly values of the environmental parameters, consisting in two (seagrasses)/three (macroalgae) samples per month. Figure 5a displayed a PCA biplot which explained the 58.9% of the total variance. The macroalgal protein contents grouped on the right side of the biplot displaying positive association with DIN, RP, salinity and FPM. In contrast, they were inversely related to water temperatures and Chl-a concentrations. The PCA in Fig. 5b displays instead a biplot which explains the 37.7% of the total variance. The percentages of proteins both in leaves and rhizomes grouped on the right side of the biplot and were associated to dissolved oxygen, pHw and Ehw. On the other side, water temperatures, turbidity (FPM) and nutrient concentrations displayed an inverse relation with the protein content. In the case of macroalgae only temperature and phytoplanktonic blooms lowered the protein content, on the other hand the seagrass protein content was also lowered by high nutrient concentrations.
a PCA biplot for macroalgae. b PCA biplot for seagrasses. Legend: Sal = Salinity; DIN = Dissolved Inorganic Nitrogen; pHw = water pH; Ehw = water Eh; Chl-a = total chlorophyll-a; T°w = water temperature; Si = reactive silica; FPM = filtered particulate matter; % Rhizomes/leaves = protein percentage in seagrass rhizomes/leaves; DO = dissolved oxygen; RP = reative phosphorus; % Ulva/G. grac./G. long./G. verm. = protein percentage in U. australis/G. gracilis/G. longissima/G. vermiculophylla
Discussion
Macroalgae and seagrasses are recognized to be important ecosystem service providers in TWS (Mtwana Nordlund et al. 2016) and are the main primary biomass producers in coastal areas. Among the numerous ecosystem services provided there is the production of proteins whose nutritional contribution is fundamental to the sustenance of grazers and the subsequent levels of the trophic chain (Scott et al. 2018). Macroalgae are a resource that is often cultivated and managed with a view oriented to biomass provisioning. Conversely, numerous actions and regulations have been pursued for the protection and recovery of seagrass environments (see the funded EU initiatives: Life SEAFOREST, Life SEPOSSO, Life SERESTO, Life TRANSFER and many others) in order to restore the regulation and maintenance ecosystem services these species provide (Mtwana Nordlund et al. 2016). Therefore, direct seagrass harvesting for provisioning services would weaken the seagrass meadows protection and recovery actions and it is not a management option. Seagrass meadows, however, play a fundamental ecological role, the proteins produced and stored in the leaves not only contribute to the natural foraging of the local fauna but at the end of the life cycle, the leaves detach and float in the form of mats in the sea, eventually beaching on the coasts, contributing also to the foraging of open sea species and of beach fauna such as invertebrates, birds and mammals (Heck et al. 2008). Although, the direct use of seagrasses should be discouraged in order to favor seagrass meadows protection, these represent an important supply of detritus (Heck et al. 2008) and proteins for coastal marine ecosystems. The protein production of the segrassess produces an indirect return (in terms of protein provisioning) through the foraging of valuable fish and bird species (Heck et al. 2008). On the other hand, macroalgae are often a plentiful resource in TWS and large quantities of this resource (up to 20 kg fw m−2), if not exploited, are regarded as a problem when “green and black tides” occur due to seaweed overgrowth and degradation (Sfriso and Facca 2007). This is an issue still far from being solved (Bastianini et al. 2013; Sfriso 2015; Menesguen 2018) and is connected to the lack of knowledge and techniques on the use of this biomass. The growth rate of this resource is fairly higher than that of plants (both aquatic and terrestrial) with mean relative growth rates of 10–20% (up to 33% day−1; Sfriso and Sfriso 2017), which are lower than microalgae growth rates (up to 50% day−1;Duong et al. 2015; Latiffi et al. 2017) but, keeping a constant field biomass of 2–3 kg fw m−2, Gracilariaceae provide a biomass yield up to 4.7 kg dw m−2 year−1, reaching 5.7 kg dw m−2 year−1 when cultivated in controlled conditions (Table 1). Additionally, if U. rigida growth in tank is considered, biomass productions ranging from 4.4 to 14.7 kg dw m−2 year−1could be obtained. This production is comparable with the autotrophic growth of microalgae in raceway open ponds from 3.2 to 18.2 kg dw m−2 year−1. In Table 2 the protein production per area (that depends on both biomass productivity and protein content) of the main food crops was compared with the protein production from macroalgae and microalgae. The animal annual protein production (actually still the main global protein source) was the lowest with fractions of gram to few grams per square meter. For commercial crops, a higher protein production was reported in comparison to animals, ranging from 0.023 to 0.250 kg dw m−2 year−1 with the highest yields reported for legumes. The protein productions found for seagrasses in this study (0.040–0.099 kg dw m−2 year−1) fell in the range reported for crops (Table 2) with 77–89% of the production associated to the leaves, that are the most ecologically relevant part of these species. The highest protein scores among the vascularized plants have been reported for Lemnoidae, commonly referred as duckweed, a small free-floating family of freshwater plants, with high protein content and an areal protein production (0.2–2.3 kg dw m−2 year−1) comparable to that of micro- and macroalgae.
Macroalgae protein productions in this study ranged 0.270–0.500 kg dw m−2 year−1 falling in the protein production range estimated for Gracilariaceae and Ulvaceae. In terms of provisioning services, considering the recommended daily intake of good-quality protein of 0.83 g (kg of body weight)−1 day−1 (EFSA 2012) and the average weight of an adult of 75 kg (Walpole et al. 2012), the annual protein demand is approx. 23 Kg year−1 capita−1. In order to sustain the annual protein demand for one person (assuming 100% protein digestibility) it should be necessary approx. 40–80 m2 capita−1 of macroalgae or 200–600 m2 capita−1 of seagrass for protein production (with the production range recorded in the Venice Lagoon for macroalgae of 0.270–0.500 kg dw m−2 year−1 and for seagrasses of 0.040–0.099 kg dw m−2 year−1).
Eventually, the highest estimated protein production in the range 0.4–10 kg dw m−2 year−1 was reported for microalgae. Because of this extraordinary growth many efforts have been invested in the development of technologies for microalgae cultivation (i.e. whose growth outclasses the crops production by an order of magnitude of productivity; Hintz and Heitman 1967) promising the resolution of the predicted so called “protein gap” (Becker 2007). However, these efforts have not yet paid off with a sustainable production of microalgae and proteins of algal origin. The high production costs, technical difficulties (such as the maintenance of axenic cultures and a negative energy balance for many microalgae production plants; Lam and Lee 2012) make sustainable micro-algal protein production a challenge still open (Becker 2007). Macroalgae (and Lemnoideae) are instead exempt from many of the difficulties bound to cultivation and harvesting that apply to microalgae, sharing similar productivity with them, albeit to a slightly lesser degree. Macroalgae harvesting and aquaculture production is dominated by relatively few commercial species for direct consumption or phycocolloid production: Saccharina japonica (Areschoung) C.E. Lane, C. Mayes et al., Undaria pinnatifida (Harvey) Suringar, Pyropia spp., Kappaphycus alvarezii (Doty) L.M. Liao, Eucheuma striatum F. Schmitz and Gracilaria/Gracilariopsis spp. (Kim et al. 2017). However, macroalgae are seldom considered for their protein content (Indegaard and Minsaas 1991) which is variable with species and seasonality and can range from 5 to 47% dw (Černá 2011), with an amino acid composition similar to that reported for the proteins of eggs and leguminous plants(Fleurence 1999) and an estimated digestibility for example for Gracilaria sp. of 42% compared to the proteins of milk (Marrion et al. 2005). The integration of macroalgae in human and animal diets proved already positive results for the high protein content and the richness of essential amino acids, which in the case of Gracilariaceae is comparable to that of chicken eggs (Norziah and Ching 2000; Makkar et al. 2016). The use of algae as fodder has been successfully implemented (Hintz and Heitman 1967; Ventura and Castañón 1998; Hansen et al. 2003; El-Deek and Brikaa 2009) and a FAO report (Hasan and Chakrabarti 2009) underlined how the growth performance of fish farmed and fed with diets based on 10–20% of macroalgae provided similar results to commercial feed. However, the feed formulations with percentages higher than 20% in term of algal biomass (corresponding to a protein contribute of no more than 3%) led to a progressive decrease in fish growth due to the high content of ash, non-digestible complex carbohydrates (Hasan and Chakrabarti 2009) and thick cell walls hindering the digestion progress. In order to increase algal digestibility and protein assimilation, pre-treatments of biomass with enzymes for hydrocolloid and cellulose hydrolysis have been tested (Fleurence et al. 1995; Marrion et al. 2005), increasing the extraction of proteins by 3 times from Gracilaria verrucosa (subsequently distinguished in the species G. longissima, G. gracilis and G. longa Gargiulo, De Masi, Tripodi). Additionally, food grade protein isolates have been successfully produced for both microalgae (Soto-Sierra et al. 2018) and macroalgae (Gracilaria sp. and Ulva sp.) by alkali treatment (Kazir et al. 2019) with protein contents ranging from 70 to 86%, considerable concentrations in all essential amino acids and good digestibility.
Special attention must be paid to contamination especially by heavy metals and metalloids in macroalgae when harvested in field, as the high trophy areas where macroalgae thrive can be affected by the presence of inorganic pollutants that get easily bioaccumulated. For the Venice Lagoon, in which the study was carried out, metal contamination in macroalgae (Ulva sp. and Gracilaria sp.) was reported with: As concentration in the ranges 2–32 µg g−1 dw (Caliceti et al. 2002) and 1.6–9.9 µg g−1 dw (Juhmani et al. 2021); Cd concentration in the ranges 0.13–0.27 (Sfriso et al. 1995) and < 0.1–0.7 µg g−1 dw (Caliceti et al. 2002); Hg concentration in the range 0.1–0.3 µg g−1 dw (Juhmani et al. 2021) and Pb concentration in the ranges 0.3–2.7 µg g−1 dw (Juhmani et al. 2021), 1.1–7.2 µg g−1 dw (Sfriso et al. 1995) and 0.7–20.6 µg g−1 dw (Caliceti et al. 2002). Taking into consideration the upper limits established by European regulations for toxic and bioaccumulative metals in macroalgae and derivatives: inorganic As 3.0 µg g−1 dw (CEVA - Centre Etudes et de Valorisation des Algues 2019) for “commercialized algae”; Cd 3.0 µg g−1 dw (European Commission 2006, 2008) for “marine algae and derivatives”; Hg 0.5 µg g−1 dw (European Parliament 2002; European Commission 2006) for “fishery products” and “feedingstuffs” from marine organisms; Pb 3.0 µg g−1 dw (European Commission 2008) for “food supplements” and Pb 5 µg g−1 (CEVA - Centre Etudes et de Valorisation des Algues 2019) for “commercialized algae”; special attention should be paid in Venice for Pb and As contamination in macroalgae. However this contamination has proved to be highly localized in the near proximity of the industrial Area of Porto Marghera and at the estuary of the Osellino River. Moreover, especially Pb contamination was reported to be dependent on algae age with an increased metal bioaccumulation in older algae (Sfriso et al. 1995). In a view oriented to maximizing production, a continuous harvest of young and fast-growing thalli is encouraged during the most productive periods thus limiting the prolonged growth and aging of the thalli and the consequent accumulation of metals within them.
Special attention should also be paid for the accumulated iodine in macroalgae, especially in the case of direct ingestion of unprocessed seaweed. In regard to the macroalgal species investigated, concentrations ranging 14–94 µg g−1 were reported for Gracilariales and 12–79 µg g−1 for Ulvales (Solimabi and Das 1977; Milinovic et al. 2021). With consideration of the tolerable upper intake levels for iodine of the European Scientific Committee on Food (600 µg day−1 Zimmermann and Trumbo 2013) and of the US Institute of Medicine (1100 µg day−1; Zimmermann and Trumbo 2013) and the above mentioned macroalgae iodine concentration ranges, the intake of macroalgae per capita should not exceed 6.4–92 g dw day−1 (from 5 to 6 times higher as fw). This would approximately correspond to a daily protein intake ranging from 0.5 to 10 g day−1. Even if food grade protein isolates are produced, iodine should be monitored as protein-bound iodine was reported to be an important fraction of total iodine content in brown seaweeds and can get easily extracted especially by alkali treatment (Hou et al. 2000).
The sustainability and the technological improvements in the harvesting of macroalgae and in the processes aimed at increasing accessibility to the protein fraction, digestibility and excess iodine removal are fundamental and will be decisive for a future sustainable and efficient use of these resources. Eventually, this study presents an invitation to update national regulations in order to include the invasive alga G. vermiculophylla in the list of usable species in order not to preclude the possibility of using this important coastal resource which can be harvested not only for its protein content but also as forage and for the production of agar–agar (Sousa et al. 2010; Abreu et al. 2011; Sfriso et al. 2017).
Conclusions
Seagrasses and macroalgae showed important protein productions in TWS, competitive with those of the main crops actually harvested on land. Macroalgae in field displayed a protein production from 3.4 to 12 times higher than seagrasses with a higher average protein content in Gracilariales, highly variable with seasonality and inversely correlated with temperature. Seagrasses displayed a steady protein content, 1.7 times higher in leaves than in rhizomes, and a production centered in late spring-early summer. Many areas deeply impacted by the effects of trophy and of difficult ecological requalification are unlikely to see colonization with seagrass beds in the coming years. In this perspective, the destination of these areas for the cultivation or careful collection of macroalgae can foster the local production in terms of proteins, without competing in terms of land and freshwater destination with other crops, while at the same time subtracting excess of dissolved nitrogen and phosphates from the water column.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
25 August 2022
Missing Open Access funding information has been added in the Funding Note.
References
Abreu MH, Pereira R, Yarish C, Buschmann AH, Sousa-Pinto I (2011) IMTA with Gracilaria vermiculophylla: Productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 312:77–87
Allard RW (1999) Principles of plant breeding. Wiley & Sons, New York
Bastianini M, Bernardi-Aubry F, Acri F, Braga F, Facca C, Sfriso A, Finotto S (2013) The Redentore fish die-off in the Lagoon of Venice: an integrated view. In: ATTI Gruppo di Algologia, Riunione Scientifica Annuale. p 32
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25:207–210
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Caliceti M, Argese E, Sfriso A, Pavoni B (2002) Heavy metal contamination in the seaweeds of the Venice lagoon. Chemosphere 47:443–454
Černá M (2011) Seaweed proteins and amino acids as nutraceuticals. Elsevier, Amsterdam
CEVA - Centre Etudes et de Valorisation des Algues (2019) Edible seaweed and microalgae-Regulatory status in France and Europe. Pleubian, France. pp 15
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Colinvaux PA, Barnett BD (1979) Lindeman and the ecological efficiency of wolves. Am Nat 114:707–718. Accessed 4 Feb 2021
del Río MJ, Ramazanov Z, García-Reina G (1996) Ulva rigida (Ulvales, Chlorophyta) tank culture as biofilters for dissolved inorganic nitrogen from fishpond effluents. Hydrobiologia 326:61–66
Duong VT, Thomas-Hall SR, Schenk PM (2015) Growth and lipid accumulation of microalgae from fluctuating brackish and sea water locations in South East Queensland—Australia. Front Plant Sci 6:359
Edding M, Macchiavello J, Black H (1987) Culture of Gracilaria sp. in outdoor tanks: productivity. Hydrobiologia 151:369–373
EFSA (2012) EFSA sets population reference intakes for protein. https://www.efsa.europa.eu/en/press/news/120209. Accessed 24 May 2021
El-Deek AA, Brikaa MA (2009) Nutritional and biological evaluation of marine seaweed as a feedstuff and as a pellet binder in poultry diet. Int J Poult Sci 8:875–881
European Commission (2008) REG (CE) N. 629/2008
European Commission (2006) REG (CE) N. 1881/2006
European Parliament (2002) DIRECTIVE 2002/32/EC
Fabregas J, Herrero C (1985) Marine microalgae as a potential source of single cell protein (SCP). Appl Microbiol Biotechnol 23:110–113
Fleurence J (1999) Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci Technol 10:25–28
Fleurence J, Massiani L, Guyader O, Mabeau S (1995) Use of enzymatic cell wall degradation for improvement of protein extraction from Chondrus crispus, Gracilaria verrucosa and Palmaria palmata. J Appl Phycol 7:393
Francavilla M, Franchi M, Monteleone M, Caroppo C (2013) The red seaweed Gracilaria gracilis as a multi products source. Mar Drugs 11:3754–3776
Goldman CR (1969) Primary Productivity in Aquatic Environments. University of California Press, Berkeley
Haines-young R, Potschin M (2018) Common International Classification of Ecosystem Services Guidance on the Application of the Revised Structure. European Environment Agency, Nottingham, UK. Negotiated procedure No EEA/NSS/16/002. www.cices.eu. Accessed 4 Feb 2021
Han P, Lu Q, Fan L, Zhou W (2019) A review on the use of microalgae for sustainable aquaculture. Appl Sci 9:2377
Hansen HR, Hector BL, Feldmann J (2003) A qualitative and quantitative evaluation of the seaweed diet of North Ronaldsay sheep. Anim Feed Sci Technol 105:21–28
Hasan MR, Chakrabarti R (2009) Use of algae and aquatic macrophytes as feed in small-scale aquaculture: a review. FAO Fish Aquac Tech Pap 531. Rome, Italy. 123 p
Heck KL, Carruthers TJB, Duarte CM, Randall HA, Kendrick G, Orth RJ, Williams SW (2008) Trophic transfers from seagrass meadows subsidize diverse marine and terrestrial consumers. Ecosystems 11:1198–1210
Hintz HF, Heitman H (1967) Sewage-grown algae as a protein supplement for swine. Anim Prod 9:135–140
Hong JW, Kim OH, Jo SW, Do JM, Yoon HS (2017) Microalgal biomass productivity and dominant species transition in a Korean mass cultivation system. Algal Res 26:365–370
Hou X, Yan X, Chai C (2000) Chemical species of iodine in some seaweeds II. Iodine-bound biological macromolecules. J Radioanal Nucl Chem 245:461–467
Iglesias-Campos A, Meiner A, Bowen K, Ansong JO (2015) Coastal population and land use changes in Europe: Challenges for a sustainable future. In: Baztan J, Chouinard O, Jorgensen B, Tett P, Vanderlindes J-P, Vasseur L (eds) Costal Zones. Elsevier, Amsterdam, pp 29–49
Indegaard M, Minsaas J (1991) Animal and human nutrition. Seaweed resources in Europe. Uses and Potential. In: Guiry, M.D., Blunden G (ed). Seaweed resources in Europe. Uses and Potential . John Wiley & Sons, Chichester pp 21–64
Juhmani A-S, Sfriso AA, Wahsha M, Buosi A, Tomio Y, Sfriso A (2021) metal bioaccumulation and oxidative stress in Ulva laetevirens in the Venice Lagoon: Early warning biomarker for metal bioaccumulation. Water 13:2626
Kaldy MS (1972) Protein yield of various crops as related to protein value. Econ Bot 26:142–144
Kazir M, Abuhassira Y, Robin A, Nahor O, Luo J, Israel A, Golberg A, Livney YD (2019) Extraction of proteins from two marine macroalgae, Ulva sp. and Gracilaria sp., for food application, and evaluating digestibility, amino acid composition and antioxidant properties of the protein concentrates. Food Hydrocoll 87:194–203
Kim JK, Yarish C, Hwang EK, Park M, Kim Y (2017) Seaweed aquaculture: Cultivation technologies, challenges and its ecosystem services. Algae 32:1–13
Korzen L, Abelson A, Israel A (2016) Growth, protein and carbohydrate contents in Ulva rigida and Gracilaria bursa-pastoris integrated with an offshore fish farm. J Appl Phycol 28:1835–1845
Lam MK, Lee KT (2012) Microalgae biofuels: A critical review of issues, problems and the way forward. Biotechnol Adv 30:673–690
Latiffi NAA, Radin Mohamed RMS, Apandi NM, Tajuddin RM (2017) Preliminary assessment of growth rates on different concentration of microalgae Scenedesmus sp. in industrial meat food processing wastewater. MATEC Web Conf 103:06010
Leng R, Stambolie J, Bell R (1995) Duckweed - a potential high-protein feed resource for domestic animals and fish. Livest Res Rural Dev 7:1–11
López CVG, del Carmen Cerón García M, Fernández FGA, Bustos CS, Chisti Y, Sevilla JMF (2010) Protein measurements of microalgal and cyanobacterial biomass. Bioresour Technol 101:7587–7591
Makkar HPS, Tran G, Heuzé V, Giger-Reverdin S, Lessire M, Lebas F, Ankers P (2016) Seaweeds for livestock diets: A review. Anim Feed Sci Technol 212:1–17
Marrion O, Fleurence J, Schwertz A, Guéant JL, Mamelouk L, Ksouri J, Villaume C (2005) Evaluation of protein in vitro digestibility of Palmaria palmata and Gracilaria verrucosa. J Appl Phycol 17:99–102
McLachlan J, Bird CJ (1986) Gracilaria (Gigartinales, Rhodophyta) and productivity. Aquat Bot 26:27–49
Menesguen A (2018) Les marées vertes 40 clés pour comprendre. Editions Quae, France.
Mensi F, Nasraoui S, Bouguerra S, Ben-Ghedifa A, Chalghaf M (2020) Effect of lagoon and sea water depth on Gracilaria gracilis growth and biochemical composition in the northeast of Tunisia. Sci Rep 10:10014
Milinovic J, Rodrigues C, Diniz M, Noronha JP (2021) Determination of total iodine content in edible seaweeds: Application of inductively coupled plasma-atomic emission spectroscopy. Algal Res 53:102149
Millennium Ecosystem Assessment (2005) Ecosystems and human well-being : wetlands and water synthesis Synthesis. World Resources Institute, Washington, DC, 66 p
Mtwana Nordlund L, Koch EW, Barbier EB, Creed JC (2016) Seagrass ecosystem services and their variability across genera and geographical regions. PLoS ONE 11:e0163091
Nelson OE (1969) Genetic modification of protein quality in plants. Adv Agron 21:171–194
Norziah MH, Ching CY (2000) Nutritional composition of edible seaweed Gracilaria changgi. Food Chem 68:69–76
Paiva L, Lima E, Neto AI, Marcone M, Baptista J (2017) Nutritional and functional bioactivity value of selected Azorean macroalgae: Ulva compressa, Ulva rigida, Gelidium microdon, and Pterocladiella capillacea. J Food Sci 82:1757–1764
Pimentel D, Dritschilo W, Krummel J, Kutzman J (1975) Energy and land constraints in food protein production. Science 190:754–761
Popp J, Szakaly Z, Polereczki Z, Olah J (2016) Substitution of soybean with alternative sources of protein for feed in the EU. Hungarian Agric Res 25:37
Rajauria G, Cornish L, Ometto F, Msuya FE, Villa R (2015) Identification and selection of algae for food, feed, and fuel applications. In: Tiwari BK, Troy DJ (Eds) Seaweed sustainability. Food and non-food applications . Elsevier, Amsterdam pp 315–345.
Rosen J (2015) The Protein Challenge 2040: Aquaculture’s Role. In: Global Outlook on Aquaculture Leadership Conferece. Vancouver, BC. https://www.globalseafood.org/wp-content/uploads/2016/08/Day3_JessicaRosen_GOAL2015-copy.pdf?x69012. Accessed 4 Feb 2021
Satpati GG, Pal R (2011) Biochemical composition and lipid characterization of marine green alga Ulva rigida-a nutritional approach. J Algal Biomass Utln 2:10–13
Scott AL, York PH, Duncan C, Macreadie PI, Connolly RM, Ellis MT, Jarvis JC, Jinks KI, Marsh H, Rasheed MA (2018) The role of herbivory in structuring tropical seagrass ecosystem service delivery. Front Plant Sci 9:127
Sfriso A, Facca C (2007) Distribution and production of macrophytes and phytoplankton in the lagoon of Venice: comparison of actual and past situation. Hydrobiologia 577:71–85
Sfriso A, Ghetti PF (1998) Seasonal variation in the biomass, morphometric parameters and production of rhizophytes in the lagoon of Venice. Aquat Bot 61:207–223
Sfriso A, Marcomini A, Pavoni B (1994) Gracilaria distribution, production and composition in the Lagoon of Venice. Bioresour Technol 50:165–173
Sfriso A, Marcomini A, Zanette M (1995) Heavy metals in sediments, SPM and phytozoobenthos of the lagoon of Venice. Mar Pollut Bull 30:116–124
Sfriso AA (2015) Macroalgae growth rates and biomass production in eutrophic areas of the Venice Lagoon. Biol Mar Mediterr 22:66–67
Sfriso AA, Gallo M, Baldi F (2017) Seasonal variation and yield of sulfated polysaccharides in seaweeds from the Venice Lagoon. Bot Mar 60:339-349
Sfriso AA, Sfriso A (2017) In situ biomass production of Gracilariaceae and Ulva rigida: the Venice Lagoon as a study case. Bot Mar 60:271-283
Short FT, Duarte CM (2001) Methods for the measurement of seagrass growth and production. In: Short FT, Coles RG (eds) Global Seagrass Research Methods. Elsevier, Amsterdam, pp 155–182
Solimabi, Das B (1977) Distribution of iodine in marine algae of Goa Region. Indian J Mar Sci 6:180–181
Soto-Sierra L, Stoykova P, Nikolov ZL (2018) Extraction and fractionation of microalgae-based protein products. Algal Res 36:175–192
Sousa AMM, Alves VD, Morais S, Delerue-Matos C, Gonçalves MP (2010) Agar extraction from integrated multitrophic aquacultured Gracilaria vermiculophylla: Evaluation of a microwave-assisted process using response surface methodology. Bioresour Technol 101:3258–3267
Uzogara SG (2000) The impact of genetic modification of human foods in the 21st century: A review. Biotechnol Adv 18:179–206
Ventura MR, Castañón JIR (1998) The nutritive value of seaweed (Ulva lactuca) for goats. Small Rumin Res 29:325–327
Wakibia JG, Anderson RJ, Keats DW (2001) Growth rates and agar properties of three gracilarioids in suspended open-water cultivation in St. Helena Bay, South Africa. J Appl Phycol 13:195–207
Walpole SC, Prieto-Merino D, Edwards P, Cleland J, Stevens G, Roberts I (2012) The weight of nations: An estimation of adult human biomass. BMC Public Health 12:439
Zimmermann M, Trumbo PR (2013) Iodine. Adv Nutr 4:262–264
Funding
Open access funding provided by Università degli Studi di Ferrara within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Andrea Augusto Sfriso – Conceptualization, Writing—original draft -, Yari Tomio – Methodology, Writing—review & editing -, Alessandro Buosi -Investigation-, Abdul-Salam Juhmani -Investigation-, Adriano Sfriso -Supervision-, Cristina Munari -Writing—Review & Editing-, Michele Mistri -Supervision-.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Augusto Sfriso, A., Tomio, Y., Buosi, A. et al. Pursuing the protein challenge 2040: macrophytes protein production in temperate transitional water systems. J Appl Phycol 34, 2145–2156 (2022). https://doi.org/10.1007/s10811-022-02757-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02757-4