Abstract
Applications of seaweeds require the supply of uniform biomass, yet performance of Ulva is generally characterised by a wide variation across study sites and seasons, as well as among species or strains. This study aimed to determine the variation in growth, biochemical composition and nutrient uptake of five Ulva strains collected in the Eastern Scheldt estuary (the Netherlands) and cultivated under standardised outdoor conditions from July to November. Surprisingly, only two strains performed well (maximum 291 ± 95 and 570 ± 122 kg FW ha−1 day−1 for VEE and YER strain), one strain showed slow growth (maximum 155 ± 91 kg FW ha−1 day−1 for SCH strain), and the last two strains (JAC and KOM) did not grow at all. Chemical composition was only determined for the well-performing strains. For most of the parameters, strong seasonality was observed; growth, crude fat (0.4–1.3%), ash (16–25%) and starch (3–11%) decreased, whereas fibres (37–45%) and proteins (7–22% crude; 6–18% true) increased. To evaluate the bio-remediation potential, nutrient uptake of YER, VEE and SCH strains was determined from in- and outflowing water (453–2027 µmol DIN; 4–101 µmol PO4 kg FW h−1). Highest rates were observed for the SCH strain which was not in accordance with growth nor composition, suggesting that nutrients were not (all) directed towards vegetative growth. This study demonstrates a large variation in performance between different Ulva strains collected in relatively close proximity and thereby highlights the importance to test a variety of local seaweed strains prior to commercial farming.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cosmopolitan green seaweed Ulva (Chlorophyta), also known as sea lettuce, has been proposed as a model species for land-based aquaculture due to its high biomass productivity (Msuya and Neori 2008; Robertson-Andersson et al. 2008; Bruhn et al. 2011; Nikolaisen et al. 2011; Praeger et al. 2019; Revilla-Lovano et al. 2021). Ulva also shows potential for the production of high value compounds, such as, e.g. ulvans. These functional biopolymers are among others used for medical applications (Lahaye and Robic 2007; Chiellini and Morelli 2011). It has been shown that Ulva may serve as a bioenergy crop and a source for the production of bioplastics (Bruhn et al. 2011; Helmes et al. 2018; Qarri and Israel 2020; Dave et al. 2021). Furthermore, Ulva spp. are used in land-based tank or pond systems for bio-remediation of dissolved waste from animal aquaculture (Shpigel et al. 1993; Bolton et al. 2009), and they are proposed as biofilters in eutrophic (nutrient rich) marine ecosystems (Cohen and Neori 1991; Areco et al. 2021).
Independent of the application, a key requirement for the production of seaweeds on a commercial scale is to ensure a continuous supply of uniform biomass, since high variation in productivity and biochemical composition poses difficulties for the processing industry (Hafting et al. 2012). However, a glance into literature shows a wide variation in the reported values for biomass production from land-based cultivated Ulva spp. in temperate/northern conditions, with specific growth rates ranging from 1.6 to 18.7% day−1 (Cohen and Neori 1991; Robertson-Andersson et al. 2008; Bruhn et al. 2011) or − 31 to 679 kg DW ha−1 day−1 (Bruhn et al 2011; Debusk et al. 1986; Groenendijk et al 2016). Similarly, considerable differences exist in the biochemical composition of Ulva spp. For instance, reported crude protein and lipid concentration varies between 10–43% and 0.3–2.7% of the dry weight, respectively (Shpigel et al. 1999; Schuenhoff et al. 2003; Marsham et al. 2007; Robertson-Andersson et al. 2008; Silva et al. 2015). Furthermore, the reported nutrient uptake rates by Ulva show a broad range (reviewed by Nederlof et al. submitted). These high variations in biochemical composition and nutrient uptake capacity depend on the experimental conditions (Cohen and Neori 1991; Msuya and Neori 2008; Martínez et al. 2012), season (Marinho-Soriano et al. 2006; Martínez et al. 2012; Marinho et al. 2015; Manns et al. 2017; Dave et al. 2021) or site-specific conditions such as nutrient or light supply (Lamare and Wing 2001). Furthermore, the biochemical method used to determine, for instance, ‘protein level’ might in fact measure other components containing nitrogen resulting in an overestimation when compared to methods that analyse ‘true protein level’ based on amino acid analysis. At last, the high variation presented can be a result of different Ulva species used within the experiments, but also specific Ulva strains can show differences in growth and content, implying a intraspecific variation in terms of nutrient uptake and consequently in ecosystem interactions (Lawton et al. 2013; Fort et al. 2019).
The aim of this study was to identify variations in biomass production and biochemical composition of Ulva sp. collected at five sites within an estuary (Eastern Scheldt, Netherlands). The Ulva sp. collected at the different sites are here referred to as ‘strains’. All strains were cultivated in a land-based tank system under standardised conditions, and biomass production was monitored over a 5-month period from July to November 2017. The biochemical composition of the two most productive strains was studied over a period of 3 months. Furthermore, nutrient uptake of different strains was compared in order to identify the most suitable strains for bio-remediation and biofilter purposes.
Material and methods
Collection of Ulva
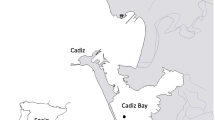
Five different Ulva spp. strains were collected in the Eastern Scheldt estuary (North Sea, Netherlands) in May 2017: Yerseke (YER), Schelphoek (SCH), Jacobahaven (JAC), Kamperland (KAM) and Veerse Meer (VEE). An overview of the sampling sites is given in Fig. 1. Approximately 0.5 to 1 kg of fresh seaweed material was collected at each location.
Ulva cultivation
The collected seaweed material was transferred to PVC cultivations tanks (one tank per strain, 400 L, 90 × 110 cm surface area) at the facilities of Wageningen Marine Research in Yerseke and reared to a biomass of 1.25 kg. Then, 250 g of fresh algal material was transferred to each cultivation tank (N = 5 per strain). Replicates of the different strains were distributed randomly among the tanks. Four tanks without seaweeds served as a control for measuring environmental parameters. All tanks were placed outdoor and were connected through a flow-through system with a continuous supply of sea water from a 10–15-m deep entry point in the Eastern Scheldt. The sea water was distributed via four higher situated header tanks using tubes (Ø 15 mm), and no nutrients were added. Water exchange in the tanks was kept constant at approximately 117 L h−1. An aeration system consisting of PVC tubes was established at the bottom of each tank to maintain vertical water movement. Water flux was checked daily and adjusted if necessary, in order to maintain an equal nutrient flux in all tanks. Nets were placed at the outflow of each tank to keep the algal material in the tanks. The tanks were cleaned every 2 weeks.
Environmental parameters
Temperature (°C) and irradiance (lux) were monitored in the four control tanks without seaweeds by data loggers (HOBO, Onset Computer Corporation, US) with continuous measurements in 15-min intervals. Lux values were converted to µmol photons m−2 s−1 using the conversion factor of 0.0185 for sunlight conditions (Moheimani et al 2013). For each parameter, daily average values were calculated.
Biomass
Biomass production of the Ulva strains was determined biweekly over a 5-month period from July to November. The entire seaweed material of each tank was collected and centrifuged at 2800 rpm until the effluent of water from the outlet stopped. Fresh weight (FW) was determined immediately after centrifugation. All instruments were cleaned thoroughly between sampling the different strains in order to minimise the risk of cross-contamination of genetic material. After weighing, 250 g were restocked in the tanks, whereas the rest of the biomass was harvested. The dry weight (DW) was determined for subsamples of each strain by drying the material in an oven at 70 °C for 24 h.
Ulva growth is presented as productivity (kg FW ha−1 day−1) and was calculated using Formula 1, with Wt being the biomass after t days (kg), Wre-stocked being the restocking weight (kg), A being the tank surface area (ha) and Δt being the temporal difference between measurements in days (d):
The specific growth rate (SGR) was determined using Formula 2, with Wt1 and Wt2 being the fresh weight at time point 1 and 2, respectively, and Δt being the temporal difference between measurements in days:
Biochemical composition
Biochemical composition of Ulva strains was determined throughout the growing season at three time points, in August, September and October. Since only the strains YER and VEE were growing well during these months, the analysis was restricted to these two strains.
After drying, the seaweed samples were homogenised by grinding to a powder using a ball mill. Subsamples for C and N content (N = 5 per treatment and sampling date) were analysed according to the DUMAS principle using an isotope-ratio mass spectrometry (IRMS) element analyser. Nutritional composition, such as (crude) protein, fat, starch and amino acids, was analysed using standard analytical methods (N = 2 pooled samples per treatment and sampling date). Crude fat (by acid hydrolysation, ANAL-10497), ash (heating at 550 °C and using gravimetry, ANAL-10028 Q), starch (amyloglucosidase, AGS, method, ANAL-10030 Q), sugars (mono- and disaccharides and reducing sugars conform to the methods NEN 3571 and EU152/2009) and dietary fibres (in accordance to AOAC 991.43, ANAL-10436 Q) were all analysed using standard and validated analysis methods.
For amino acid analysis, 150 mg of powdered sample was added to 500 mL of 70 mM phosphate buffer (pH 7.0), containing 1 mM dithiothreitol (DTT). Three microliter of 2 mM norleucine was added to the extract as an internal standard. The sample was mixed for 3 min, and 2.5 mL of MCW (methanol, chloroform and water; 12/3/5, v/v/v) was added. The sample was mixed again, and 500 mL of distilled deionised water was added. After centrifugation at 3000 × g for 25 min, the water phase was transferred to a new glass tube. A volume of 2.3 mL of water was added to the pellet and homogenised for 3 min. After centrifugation at 3000 × g for 25 min, the water phase was added to the previous tube. The same procedure was repeated once more. All water phases were combined and centrifuged at 3000 × g for 25 min to give a clear solution. The water solution was transferred to a new tube and freeze-dried overnight. The freeze-dried material was dissolved in 1 mL of water and centrifuged at 12,000 × g for 30 min to remove insoluble substances, and the extracted sample was transferred to a 1.5 mL tube. For the hydrolysis of total amino acids, 200 mL of the phosphate buffer extracted sample was transferred to a new tube, and 5 mL 0.2 N NaOH containing 1% (v/v) of dithiodipropionic acid, 40 mL of phenol 1.25% (v/v) and 245 mL of 12 N HCl were added to the extract. The sample was incubated at 110 °C under nitrogen for 24 h, and then the amino acids liberated by hydrolysis were extracted using MCW as described before. Amino acid analysis was performed with a BioChrom 20 (Amersham Pharmacia Biotech). One hundred fifty microliter of 0.2 M lithium citrate buffer (pH 2.2) was added to 150 mL of the sample, and 40 mL of the mixture was loaded onto the ion-exchange column (Ultrapac 8 resin lithium form, I = 200 mm, d = 4–6 mm). A stepwise elution by five lithium citrate buffers (pH 2.8, 3.0, 3.15, 3.5, 3.55) was employed, and the amino acids were detected with ninhydrin reagent, and the concentration was expressed as mg g−1 dry weight (DW).
Protein content is presented in two different ways. Crude protein content was obtained by using the traditional nitrogen-to-protein conversion factor of 6.25 of the nitrogen content as determined using the Dumas nitrogen analysis method. This is called the ‘crude protein’ content. Additionally, ‘true protein’ content was calculated as the sum of all the protein amino acid derivative content as analysed using the BioChrom amino acid analysis method after hydrolysis of all proteins.
Nutrient uptake rates
Nutrient uptake rates of the Ulva strains were determined at three time points in August, September and October. The analysis was restricted to three strains: the YER and VEE strains which were growing well during this time and the SCH strain which was growing moderately. To reduce confounding effects, such as nutrient uptake by fouling species, nutrient measurements were performed 1 day after the maintenance cleaning (see the Ulva cultivation section). Nutrient uptake was also determined for the control tanks to correct for any potential nutrient uptake by other organisms and/or phytoplankton present.
The nutrient uptake rate was determined using the flow-through method after pilot experiments confirmed a sufficient mixture of the water column in the tanks. On each sampling date, water samples were collected 2 h post-sunrise from the header tanks (i.e. input to experimental tanks) and from the outflow of each experimental tank. Flow rates of each tank were measured simultaneously. Water samples were filtered (Whatman GF/C 47 μm) and stored at − 20 °C until they were analysed for concentration (µmol L−1) of PO43−, NH4+, NO3− and NO2 using an autoanalyser. Dissolved inorganic nitrogen (DIN) was calculated as the sum of NH4+, NO3− and NO2. The difference between nutrient concentrations in the in- and outflow of each tank and the flow rates was used to calculate nutrient uptake rates. Subsequently, these values were corrected for fluxes in the control tank and standardised to 1 kg FW of Ulva biomass.
Statistical analysis
Biomass and SGR were analysed with repeated measures ANOVA (RM ANOVA). Data were checked for normality and sphericity. In cases where sphericity was violated, the Greenhouse–Geisser correction was applied. C:N data were analysed with dependent t tests, after the data were checked for normality. Cumulative harvest data were analysed using the non-parametric Kruskal–Wallis test due to heterogeneity of variances. Differences between treatment groups were analysed with multiple comparison post hoc tests (Tukey’s tests) and considered significant at p < 0.05. No statistical analysis was performed for protein content and the biochemical composition due to low sample size. SPSS was used for all statistical analyses (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, version 22.0, USA).
Results
Environmental parameters
Daily average irradiance and temperature were similar in all four control tanks (Fig. 2). Average daily irradiance ranged from 532 µmol photons m−2 s−1 in July to 2 µmol photons m−2 s−1 in November. This resembles a daily irradiance sum of 46.0 mol photons m−2 day−1 in July to 0.17 mol photons m−2 day−1 in November. Temperature decreased over the cultivation period from a maximum of 25 °C in July to minimum values of 9 °C in November.
The higher oxygen concentrations in the Ulva tanks did not lead to an increase in the pH which was stable across tanks and seasons (Table 1). Dissolved inorganic nitrogen concentrations in the ambient water increased throughout the season, while phosphate concentrations remained relatively stable.
Biomass
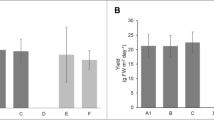
Extrapolation of the results obtained in this study lead to an estimated average total harvested yield for the five strains of 13.4 ± 13.3 t FW ha−1 (Fig. 3). The total yield obtained from the five different strains varied significantly (Fig. 3) (Kruskal–Wallis, p < 0.01). The highest yield was achieved using the YER strain (33.1 ± 8 t FW ha−1), followed by the VEE strain (24.4 ± 6 t FW ha−1). The yield obtained by the SCH strain was significantly lower (4.9 ± 1.1 t FW ha−1), but the strain was cultivated until the end of October. The cultivation of the JAC and KAM strains, on the other hand, was stopped in early August as they were not growing any longer.
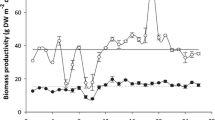
Productivity of the five strains also varied over time. Overall, there was a significant decrease throughout the cultivation season (Fig. 4) (RM ANOVA, p < 0.01). The YER strain showed the highest productivity and SGR, reaching an average of 568 ± 122 kg FW ha−1 day−1 and 9.2 ± 0.2% day−1 in July, respectively (Fig. 4). Growth of the YER strain decreased continuously from the second week of July to the end October. The VEE strain showed a continuous productivity of 200 to 300 kg FW ha−1 day−1 between July and August, but productivity decreased from the beginning of September onward. VEE and YER showed comparable production from September to November, when the final harvest of these two strains took place. Productivity of the strain SCH was at its maximum in the second week of July, but growth rates decreased significantly in August and even reached negative values before the last harvest in the end of October, meaning that the restocked tissue degraded in the tank. The strains KAM and JAC did not grow well in the tanks, even at the start of the experiment. In August, they reached average growth rates of 0.2 ± 0.9 and 1.1 ± 1.3% day−1, respectively, and their cultivation was terminated after week 31 (Fig. 3).
Chemical composition
C:N content of the two tested strains VEE and YER decreased significantly from 19.1 ± 2.3 in August to 8.5 ± 0.3 in October (Fig. 5) (dependent t test, p < 0.01). No significant difference was found between the two strains (Fig. 5). C:N content of the SCH strain in August was not significantly different from the other two strains (Fig. 5).
Most of the analysed compounds of the two Ulva strains VEE and YER showed strong seasonality (Fig. 6). Crude fat content of the Ulva samples was relatively low (between 0.4 and 1.3% of the dry weight). The crude fat content of the VEE strain decreased during the season, whereas there was an increase in crude fat content in the YER strain from September to October (Fig. 6A), although the levels are too low to show significant difference between the two strains. The variation in crude fat content between replicates of the same strain was high in both the VEE and the YER strain (Fig. 6A), which again might be influenced by the very low fat concentrations in the Ulva samples.
Fibre content, on the other hand, was high and varied between 35 and 45% of the dry weight. The dietary fibre content increased slightly in both strains from August to October (Fig. 6B) and was higher in the VEE strain than in the YER strain, reaching a maximal value of 45% in October. Ash content in both strains decreased from August to October (Fig. 6C). While there was no difference in ash content between the strains in August, it decreased more rapidly in the VEE strains, leading to a difference of approximately 4% in October (Fig. 6C). Starch content of both strains also decreased over the season (Fig. 6D). The starch content was higher in the VEE strain, and there was a difference of more than 3% between the two samples of the VEE strain. Sugar content could not be determined as the values were below the detection level (0.6%).
The crude protein content and true protein analysed were similar for the two tested strains (Table 2). Calculating the ‘crude protein’ content based on the nitrogen content resulted in an overestimation of up to 20% as compared to the ‘true protein content’ calculated from the sum of all protein AA amino acids (Table 2). Through time, there was a good correlation between the two methods, and both true and crude protein contents increased threefold during the season from August to October, reaching maximum values of 22.0% for crude and 18.3% for true proteins in the VEE strain (Table 2). The amino acid composition of the proteins stayed constant during the 3 months, with aspartic and glutamic acids being the most abundant amino acids.
Nutrient uptake
Significant differences occurred in the nutrient uptake rates of the three strains VEE, YER and SCH (Fig. 7A). All strains showed a seasonal variation in DIN uptake rates (Fig. 7A). Surprisingly, productivity of the SCH strain was lower than of the VEE and YER strain, whereas DIN uptake by the SCH strain was higher than by the other strains in August and September. In the SCH and YER strains, the highest DIN uptake was observed in September (Fig. 7A). DIN uptake rates by the VEE strain also increased between August and September and stayed at a constant level of 1132 ± 88 µmol kg FW−1 h−1 in October (Fig. 7A). While DIN uptake was positively correlated to productivity (kg FW ha−1 day−1) in the VEE and YER strain (R2 = 0.75 and R2 = 0.57, respectively), this was not the case for the SCH strain (R2 = 0.01). Overall, DIN uptake by the SCH strain differed significantly from the VEE and YER strain (RM ANOVA p < 0.01).
Similar observations were made regarding the phosphate uptake rate. The SCH strain showed a high seasonal variation, as it decreased from 100.6 ± 57 µmol kg FW−1 h−1 in August to 4.4 ± 17 µmol kg FW−1 h−1 in October (Fig. 7B). While phosphate uptake in this strain was three times higher than in the VEE strain during August, by October, it had decreased significantly and was lower than in the other two strains. In the VEE and YER strains, there was no seasonal variation in phosphate uptake rates, and continuous values of 31.0 ± 3 µmol kg FW−1 h−1 and 45.9 ± 2 µmol kg FW−1 h−1 were observed, respectively (Fig. 7B). Overall, there was a significant difference in phosphate uptake rates between the SCH strains and the two other strains (Fig. 7B) (RM ANOVA, p < 0.01).
Discussion
Ulva shows a great potential for land-based aquaculture due to the opportunistic capacity to grow very fast under suitable environmental conditions (Nikolaisen et al. 2011). In this study, we demonstrate that the biomass production, biochemical content and nutrient assimilation of Ulva sp. vary depending on both the selected strain and on seasonality, having important consequences regarding Ulva aquaculture.
Productivity
The five Ulva spp. strains collected at different sites in the Eastern Scheldt estuary were cultivated in land-based tanks located at our experimental facility near the YER collection site. Here, the Ulva strains were cultivated under controlled uniform conditions and thus exposed to the same environmental parameters during the time of the experiment. Although the strains were collected in the same estuary, the different Ulva spp. populations may be adapted to particular local environmental conditions at the collection sites. When the seaweed material was transferred to the tanks, some strains performed better under the new environmental cultivation conditions than others. The observed differences in biomass production were especially surprising for the KAM and VEE strain, as they had been collected only few kilometres apart. The productivity of the VEE strain in July was approximately five times higher than the productivity of the KAM strain.
In this study we assumed that the observed variations could be attributed to differences in strains that had been adapted to local conditions at the cultivation sites. However, it cannot be ruled out that strains actually represented different species of Ulva. Currently there are 130 taxonomically accepted species in the genus Ulva (Guiry and Guiry 2019). There is not only a high morphological similarity between Ulva species (Hayden et al. 2003), but they also show morphological variation depending on environmental parameters (Gao et al. 2016) which makes species determination based on morphological characters impossible. Fort et al. (2019) showed that different Ulva species are present in the Eastern Scheldt, with U. australis at the Schelphoek and U. laetevirens further inside of the Eastern Scheldt, suggesting that the strains included in this study may belong to different Ulva species. This suggests that future studies should apply molecular methods, such as barcoding of the plastid markers RbcL or tufA (Heesch et al. 2009; Saunders and Kucera 2010; Fort et al. 2019), in order to identify the Ulva spp. from the Eastern Scheldt used for experimental studies.
Productivity did not only vary between strains, but also showed a strong seasonal variation as seaweed growth is a function of environmental conditions. When Bruhn et al. (2016) cultivated the sugar kelp Saccharina latissima at five different sites in Limfjorden, Denmark, they showed that biomass yield fluctuated by a factor of 10 depending on the environmental conditions that differed strongly between the sites. Light was shown to be the main factor influencing kelp productivity (Bruhn et al. 2016). Overall, the productivity and growth rates of the Ulva strains in the present study were low compared to a previous study performed in the same tank system (Groenendijk et al. 2016) and to other studies on Ulva spp. cultivated in land-based systems (Table 3).
Biochemical composition
Not only the biomass production, but also biochemical composition varied between strains and changed over the cultivation period. A closer look on the biochemical compounds showed that some decreased from August to October (ash, starch), whereas others increased (protein and fibres). Although no statistical analysis could be made due to small sampling size, there also seemed to be differences between the YER and VEE strain. Similarly, Fort et al. (2019) have reported a large variation of metabolic characteristics among Ulva strains. The overall tendencies (increase or decrease), however, were similar in the two strains. The protein content in the Ulva tissue increased threefold during the cultivation period. Based on the results presented here, we recommend that seasonal variation has to be taken into account when seaweed is cultivated for specific biochemical compounds. When high-protein containing biomass is wanted for feed or food purposes, Ulva should therefore be harvested in autumn, although it must be mentioned that the maximum true protein level only reaches 18% on dry weight. Thus, similar to what has been shown for the sugar kelp (Marinho et al. 2015), there seems to be no match between the period of highest productivity and the highest protein content. As previously reported in literature (Mariotti et al. 2008; Angell et al. 2016), the determination of the protein content by analysing the nitrogen content that also measures non-protein nitrogen and applying the nitrogen-to-protein conversion factor of 6.25 was not deemed suitable for the determination of the true protein content of Ulva spp. In the present study, this calculation led to an overestimation of the protein content of up to 20% as compared to the sum of protein amino acids after hydrolysis, and reports in literature show that it can even lead to an overestimation of 42% (Ortiz et al. 2006; Msuya and Neori 2008; Angell et al. 2016). However, the general tendencies, i.e. an increase of protein content over the season, were the same, and we found a good correlation between crude protein analysis and true protein analysis. N content could therefore be used as a proxy for true protein content of Ulva, when adapting the nitrogen-to-protein conversion factor. In case of the VEE strain, the average multiplication factor was 5.08, in case of the YER strain it was 4.91. Thus, a universal multiplication factor of 5, as proposed by Angell et al. (2015) and Bikker et al. (2016) seems justified. When it comes to industrial applications of seaweed biomass, not only the quantity of proteins is of importance but also the quality, i.e. the amino acid composition. Ulva is used in fish meal at certain proportions, and integrated multi-trophic aquaculture (IMTA) produced Ulva has been proven to be suitable to replace 100% of fishmeal for juvenile Sparus aurata, the Gilt-head bream (Shpigel et al. 2017). High-protein U. lactuca strains could replace up to 14.6% of fish feed biomass and 35% of the animal source biomass without any apparent negative effects on fish performance, thereby saving of 10% of costs (Shpigel et al. 2017). Generally, green macro algae have higher protein content than brown macro algae but lower than red macro algae (Castro-Gonzáles et al. 1996; Galland-Irmouli et al. 1999; Dawczynski et al. 2007). Although the protein content showed a seasonal variation, unlike what has been shown for S. latissima (Marinho et al. 2015), amino acid composition did not change significantly over the season. Furthermore, there was no significant difference in amino acid composition between the two tested Ulva strains.
Seasonal variation occurred also in the C:N ratio of the Ulva tissue and decreased over the cultivation time from ratios that suggest nitrogen limitation in August (> 10) (Lapointe et al., 1976) to values where nitrogen is no longer limiting (< 10) in September and October. This is in line with low TAN concentrations in the ambient water in the Eastern Scheldt during summer. Ambient values observed during the nutrient removal measurements were comparable to a monitoring program for the entire bay that reports average ambient TAN concentrations increasing from 4.9 ± 3.1 µmol L−1 in August to 9.7 ± 2.2 µmol L−1 in October between 2010 and 2016 (www.waterbase.nl). Overall, the C:N ratios obtained in this study were lying within the reported range in literature of 7.9 to 24.4 for U. lactuca (Table 3).
Nutrient removal capacity
Generally, Ulva is opportunistic in nutrient uptake and is considered to be able to assimilate nitrogen quickly at high external concentrations in order to fulfil the high nutrient demand that supports its fast growth (Martínez et al. 2012). These bio-remediation characteristics makes them popular for biological waste water treatment in fish culture systems (Shpigel et al. 1993; Bolton et al. 2009; Guttman et al. 2019; Chatzoglou et al. 2020), and they are proposed as biofilters in eutrophic (nutrient rich) marine ecosystems (Cohen and Neori 1991; Gao et al. 2018). Similar to productivity and growth rates, nutrient removal rates were only half of the rates observed in a previous study performed in our experimental system (Groenendijk et al. 2016) and are low in comparison to rates reported in literature (Lubsch & Timmermans 2018; Tremblay-Gratton et al. 2018). Significant differences in nutrient uptake were observed between the three tested strains. Despite the low and even negative growth of the SCH strain, it showed surprisingly high uptake rates of DIN in August and September. Neither was it a result of a potential increased nitrogen content in Ulva tissue, as the SCH strain did not differ from the other strains. When nutrient assimilation was calculated based on the combination of growth and nitrogen content, generally lower rates were observed. Tremblay-Gratton et al. (2018) also report approximately two times lower rates when estimating nutrient removal based on tissue samples in comparison to nutrient depletion in the culture water, which is similar to the VEE and YER strains in August. The deviation was particularly clear for the SCH strain in August (30 times lower rates based on tissue samples) and for the other two strains in October (6–13 times lower rates based on tissue samples). Sporulation is a potential reason that may explain why assimilated nutrients are directed towards reproduction (Oza and Sreenivasa Rao 1977) rather than vegetative growth. However, it remains unclear why this would have occurred with the SCH strain and not with VEE nor YER strains nor why this would be higher later during the season. As nutrient uptake is not always correlated to growth, this indicates that strains react differently to the environment than might be expected based on performance data only. Rates reported here can, however, not directly be used to estimate the true bio-mitigation potential as our approach included point measurements focussing on differences between strains but lack diurnal (Krom et al. 1995), daily and weekly variation.
Implications for commercial application
A key step in development of the Ulva production is to obtain high productivity and stable biochemical composition of biomass consistently at scales and timeframes relevant for commercial production (Mata et al. 2016). Usually, Ulva strains for cultivation are collected close to the farming site (Silva et al. 2015; Korzen et al. 2016). Overall, our results demonstrate that both strain selection and harvesting time are crucial factors when it comes to the use of Ulva for industrial applications. We also suggest that strains interact differently with the environment, which means that the impact of seaweed cultivation on the surrounding ecosystem may depend on the choice of strain. The reported differences between Ulva spp. strains open a wide range of possibilities for genetic selection on growth rates or specific biochemical compounds within eco-type seaweed varieties, which is already done for other seaweed species cultivated in Asia, such as Porphyra spp. and Saccharina japonica (Robinson et al. 2013). We recommend testing a variety of local seaweed strains in the specific cultivation conditions on site before selecting a single wild seaweed variety for commercial farming.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Angell AR, Mata L, de Nys R, Paul NA (2016) The protein content of seaweeds: a universal nitrogen-to-protein conversion factor of five. J Appl Phycol 28:511–524
Areco M, Salomone VN, dos Santos Afonso M (2021) Ulva lactuca: a bioindicator for anthropogenic contamination and its environmental remediation capacity. Mar Env Res 171:105468
Bikker P, van Krimpen MM, van Wikselaar P, van Wikselaar P, Houweling-Tan B, Scaccia N, van Hal JW, Huijgen WJJ, Cone JW, Lopez-Contreras AM (2016) Biorefinery of the green seaweed Ulva lactuca to produce animal feed, chemicals and biofuels. J Appl Phycol 28:3511–3525
Bolton JJ, Robertson-Andersson DV, Shuuluka D, Kandjengo L (2009) Growing Ulva (Chlorophyta) in integrated systems as a commercial crop for abalone feed in South Africa: a SWOT analysis. J Appl Phycol 21:575–583
Bruhn A, Dahl J, Nielsen HB, Nikolaisen L, Rasmussen MB, Markager S, Olesen B, Arias C, Jensen PD (2011) Bioenergy potential of Ulva lactuca: biomass yield, methane production and combustion. Bioresour Technol 102:2595–2604
Bruhn A, Tørring D, Thomsen M, Canal-Vergés P, Nielsen MM, Rasmussen MB, Eybye KL, Larsen MM, Balsby TJS, Petersen JK (2016) Impact of environmental conditions on biomass yield, quality, and bio-mitigation capacity of Saccharina latissima. Aquac Environ Interact 8:619–636
Buisman K (2019) Productivity and content of wild Ulva spp. strains in monoculture and in mussel co-cultivation. MSc thesis, Wageningen University and Research, Wageningen
Castro-Gonzáles MI, Pérez-Gil Romo F, Pérez-estrella S, Carrillo-Domínguez S (1996) Chemical composition of the green alga Ulva lactuca. Cienc Mar 22:205–213
Chatzoglou E, Kechagia P, Tsopelakos A, Miliou H (2020) Co-culture of Ulva sp. and Dicentrarchus labrax in recirculating aquaculture system: effects on growth, retention of nutrients and fatty acid profile. Aquat Living Resour 33:19
Chemodanov A, Jinjikhashvily G, Habiby O, Liberzon A, Israel A, Yakhini Z, Golberg A (2017) Net primary productivity, biofuel production and CO2 emissions reduction potential of Ulva sp. (Chlorophyta) biomass in a coastal area of the Eastern Mediterranean. Energy Convers Manag 148:1497–1507
Chiellini F, Morelli A (2011) Ulvan: a versatile platform of biomaterials from renewable resources. In: Pignatello R (ed) Biomaterials - Physics and chemistry. InTech, Riejeka, p 75–98
Cohen I, Neori A (1991) Ulva lactuca biofilters for marine fishpond effluents. I. Ammonia uptake kinetics and nitrogen content. Bot Mar 34:475–482
Dave N, Varadavenkatesan T, Singh RS, Giri BS, Selvaraj R, Vinayagam R (2021) Evaluation of seasonal variation and the optimization of reducing sugar extraction from Ulva prolifera biomass using thermochemical method. Env Sci Poll Res 28:58857–58871
Dawczynski C, Schubert R, Jahreis G (2007) Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem 103:891–899
Debbarma J, Madhusudana Rao B, Narasimha Murthy L, Mathew S, Venkateshwarlu G, Ravishankar CN (2016) Nutritional profiling of the edible seaweeds Gracilaria edulis, Ulva lactuca and Sargassum sp. Indian J Fish 63:81–87
Fort A, Lebrault M, Allaire M, Esteves-Ferreira AA, McHale M, Lopez F, Fariñas-Franco JM, Alseekh S, Fernie AR, Sulpice R (2019) Extensive variations in diurnal growth patterns and metabolism amongst Ulva spp strains. Plant Physiol 180:109–123
Galland-Irmouli AV, Fleurence J, Lamghari R, Luçon M, Rouxel C, Barbaroux O, Bronowicki JP, Villaume C, Guéant JL (1999) Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse). J Nutr Biochem 10:353–359
Gao G, Clare AS, Rose C, Caldwell GS (2017) Reproductive sterility increases the capacity to exploit the green seaweed Ulva rigida for commercial applications. Algal Res 24:64–71
Gao G, Zhong Z, Zhou X, Xu J (2016) Changes in morphological plasticity of Ulva prolifera under different environmental conditions: a laboratory experiment. Harmful Algae 59:51–58
Gao G, Clare AS, Rose C, Caldwell GS (2018) Ulva rigida in the future ocean: potential for carbon capture, bioremediation and biomethane production. GCB Bioenergy 10:39–51
Ge C, Yu X, Kan M, Qu C (2017) Adaption of Ulva pertusa to multiple-contamination of heavy metals and nutrients: biological mechanism of outbreak of Ulva sp. green tide. Mar Pollut Bull 125:250–253
Groenendijk F, Bikker P, Van Den Burg SWK, Harmsen PFH, Jak RG, Kamermans P, van Krimpen MM, Prins H, Stuiver M, van der Werf AK, Scholl MM, Wald J (2016) North-Sea-Weed-Chain: sustainable seaweed from the North Sea; an exploration of the value chain. IMARES Research Report C055/16
Guttman L, Shpigel M, Ben-Ezra D, Yu J, Shaobo C (2019) Is Ulva sp able to be an efficient biofilter for mariculture effluents? J Appl Phycol 31:2449–2459
Hafting JT, Critchley AT, Cornish ML, Hubley SA, Archibald AF (2012) On-land cultivation of functional seaweed products for human usage. J Appl Phycol 24:385–392
Hayden HS, Blomster J, Maggs CA, Silva PC, Stanhope MJ, Waaland JR (2003) Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. Eur J Phycol 38:277–294
Heesch S, Broom JES, Neill KF, Farr TJ, Dalen JL, Nelson WA (2009) Ulva, Umbraulva and Gemina: genetic survey of New Zealand taxa reveals diversity and introduced species. Eur J Phycol 44:143–154
Helmes RJK, López-Contreras AM, Benoit M, Abreu H, Maguire J, Moejes F, van den Burg SWK (2018) Environmental impacts of experimental production of lactic acid for bioplastics from Ulva spp. Sustainability 10:2468
Ho YB (1990) Ulva lactuca as bioindicator of metal contamination in intertidal waters in Hong Kong. Hydrobiologia 203:73–81
Hofmann LC, Nettleton JC, Neefus CD, Mathieson AC (2010) Cryptic diversity of Ulva (Ulvales, Chlorophyta) in the great bay Estuarine system (Atlantic USA): introduced and indigenous distromatic species. Eur J Phycol 45:230–239
Hurd CL, Harrison PJ, Bischof K, Lobban CS (2014) Seaweed ecology and physiology. Cambridge University Press, Cambridge, UK
Korzen L, Abelson A, Israel A (2016) Growth, protein and carbohydrate contents in Ulva rigida and Gracilaria bursa-pastoris integrated with an offshore fish farm. J Appl Phycol 28:1835–1845
Krom MD, Ellner S, van Rijn J, Neori A (1995) Nitrogen and phosphorus cycling and transformations in a prototype “non-polluting” integrated mariculture system, Eilat, Israel. Mar Ecol Prog Ser 118:25–36
Lahaye M, Robic A (2007) Structure and function properties of ulvan, a polysaccharide from green seaweeds. Biomacromol 8:1765–1774
Lamare MD, Wing SR (2001) Calorific content of New Zealand marine macrophytes. N Z J Mar Freshw Res 35:335–341
Lawton RJ, Mata L, de Nys R, Paul NA (2013) Algal bioremediation of waste waters from land-based aquaculture using Ulva: Selecting target species and strains. PLoS One 8:e77344
Manns D, Nielsen MM, Bruhn A, Saake B, Meyer AS (2017) Compositional variations of brown seaweeds Laminaria digitata and Saccharina latissima in Danish waters. J Appl Phycol 29:1493–1506
Marinho-Soriano E, Fonseca PC, Carneiro MAA, Moreira WSC (2006) Seasonal variation in the chemical composition of two tropical seaweeds. Biosour Technol 97:2402–2406
Marinho GS, Holdt SL, Angelidaki I (2015) Seasonal variations in the amino acid profile and protein nutritional value of Saccharina latissima cultivated in a commercial IMTA system. J Appl Phycol 27:1991–2000
Mariotti F, Tomé D, Mirand PP (2008) Converting nitrogen into protein - beyond 6.25 and Jones’ factors. Crit Rev Food Sci Nutr 48:177–184
Marsham S, Scott GW, Tobin ML (2007) Comparison of nutritive chemistry of a range of temperate seaweeds. Food Chem 100:1331–1336
Martínez B, Pato LS, Rico JM (2012) Nutrient uptake and growth responses of three intertidal macroalgae with perennial, opportunistic and summer-annual strategies. Aquat Bot 96:14–22
Mata L, Magnusson M, Paul NA, de Nys R (2016) The intensive land-based production of the green seaweeds Derbesia tenuissima and Ulva ohnoi: biomass and bioproducts. J Appl Phycol 28:365–375
Moheimani NR, Borowitzka MA, Isdepsky A, Fon Sing S (2013) Standard methods for measuring growth of algae and their composition. In: Borowitzka MA, Moheimani NR (eds) Algae for Biofuels and Energy. Springer, Dordrecht, pp 265–284
Msuya FE, Neori A (2008) Effect of water aeration and nutrient load level on biomass yield, N uptake and protein content of the seaweed Ulva lactuca cultured in seawater tanks. J Appl Phycol 20:1021–1031
Nederlof MAJ, Verdegem MCJ, Smaal AC, Jansen HM (2021) Nutrient retention efficiencies in integrated multi-trophic aquaculture. Rev Aquacult. https://doi.org/10.1111/raq.12645
Neori A, Cohen I, Gordin H (1991) Ulva lactuca biofilters for marine fishpond effluents II. growth rate , yield and C:N ratio. Bot Mar 34:483–489
Neto RT, Marçal C, Queirós AS, Abreu H, Silva AMS, Cardoso SM (2018) Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as functional ingredients. Int J Mol Sci 19:2897
Nikolaisen L, Daugbjerg Jensen P, Svane Bech K, Dahl J, Busk J, Brodsgaard T, Rasmussen MB, Bruhn A, Bjerre AB, Bangso Nielsen H (2011) Energy production from marine biomass (Ulva lactuca). Danish Technological Institute, PSO Project No. 2008–1–0050
NRC (National Research Council) (2013) Nutrient requirements of fish and shrimp. The National Academies Press, Washington, DC
Ortiz J, Romero N, Robert P, Araya J, Lopez-Hernandez J, Bozzo C, Navarrete E, Osorio A, Rios A (2006) Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem 99:98–104
Oza RM, Sreenivasa Rao P (1977) Effect of different culture media on growth and sporulation of laboratory raised germlings of Ulva fasciata Delile. Bot Mar 20:427–432
Pedersen MF, Borum J (1996) Nutrient control of algal growth in estuarine waters. Nutrient limitation and the importance of nitrogen requirements and nitrogen storageamong phytoplankton and species of macroalgae. Mar Ecol Prog Ser 142:261–272
Pereira L (2015) A review of the nutrient composition of selected edible seaweeds. In: Pomin VH (ed) Seaweed: ecology, nutrient composition and medicinal uses. Nova Science Publishers Inc, Coimbra, pp 15–47
Praeger C, Vucko MJ, De Nys R, Cole A (2019) Maximising the productivity of attached cultivation of Ulva tepida in land-based systems. Algal Res 40:101507
Qarri A, Israel A (2020) Seasonal biomass production, fermentable saccharification and potential oil yields in the marine macoralgae Ulva sp. (Chlorophyta). Renew Energy 145:2101–2107
Revilla-Lovano S, Sandoval-Gil JM, Zertuche-González JS, Belando Torrentes MD, Bernardeau-Esteller J, Rangel Mendoza LK, Ferreira-Arrieta Guzmán-Calderón JM, Camacho-Ibar VF, Muniz-Salazar R, Ávilla-López MdC (2021) Physiological responses and productivity of the seaweed Ulva ohnoi (Chlorophyta) under changing cultivation conditions in pilot large land-based ponds. Algal Res 56:102316
Robertson-Andersson DV, Potgieter M, Hansen J, Bolton JJ, Troell M, Anderson RJ, Kalling C, Probyn T (2008) Integrated seaweed cultivation on an abalone farm in South Africa. J Appl Phycol 20:579–595
Robinson N, Winberg P, Kirkendale L (2013) Genetic improvement of macroalgae: status to date and needs for the future. J Appl Phycol 25:703–716
Runcie JW, Ritchie RJ, Larkum AWD (2004) Uptake kinetics and assimilation of phosphorus by Catenella nipae and Ulva lactuca can be used to indicate ambient phosphate availability. J Appl Phycol 16:181–194
Saunders GW, Kucera H (2010) An evaluation of rbcL, tufA, UPA, LSU and ITS as DNA barcode markers for the marine green macroalgae. Cryptogam Algol 31:487–528
Schuenhoff A, Shpigel M, Lupatsch I, Ashkenazi A, Msuya FE, Neori A (2003) A semi-recirculating, integrated system for the culture of fish and seaweed. Aquaculture 221:167–181
Shanmugam A, Palpandi C (2008) Biochemical composition and fatty acid profile of the green alga Ulva reticulata. Asian J Biochem 3:26–31
Shpigel M, Guttman L, Shauli L, Odintov V, Ben-Ezra D, Harpaz S (2017) Ulva lactuca from an integrated multi-trophic aquaculture (IMTA) biofilter system as a protein supplement in gilthead seabream (Sparus aurata) diet. Aquaculture 481:112–118
Shpigel M, Neori A, Popper DM, Gordin H (1993) A proposed model for “environmentally clean” land-based culture of fish, bivalves and seaweeds. Aquaculture 117:115–128
Shpigel M, Ragg NL, Lupatsch I, Neori A (1999) Protein content determines the nutritional value of the seaweed Ulva lactuca L. for the abalone Haliotis tuberculata L. and H. discus hannai Ino. J Shellfish Res 18:227–233
Silva DM, Valente LMP, Sousa-Pinto I, Pereira R (2015) Evaluation of IMTA-produced seaweeds (Gracilaria, Porphyra, and Ulva) as dietary ingredients in Nile tilapia, Oreochromis niloticus L., juveniles. Effects on growth performance and gut histology. J Appl Phycol 27:1671–1680
Tremblay-Gratton A, Boussin J-C, Tamigneaux É, Vandenberg GW, Le François NR (2018) Bioremediation efficiency of Palmaria palmata and Ulva lactuca for use in a fully recirculated cold-seawater naturalistic exhibit: effect of high NO3 and PO4 concentrations and temperature on growth and nutrient uptake. J Appl Phycol 30:1295–1304
Wiles PG, Gray IK, Kissling RC (2020) Routine analysis of proteins by Kjeldahl and Dumas methods: review and interlaboratory study using dairy products. J AOAC Int 81:620–632
WHO (2002) Protein and amino acid requirements in human nutrition: report of a joint FAO/WHO/UNU expert consultation. WHO Technical Report Series 935
Acknowledgements
The authors thank Willem Visser and Pim van Dalen for technical support.
Funding
The research was supported by the European Commission, H2020 Research Infrastructures (GENIALG; grant no. 727892), and national funding through the PROSEAWEED Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jansen, H.M., Bernard, M.S., Nederlof, M.A.J. et al. Seasonal variation in productivity, chemical composition and nutrient uptake of Ulva spp. (Chlorophyta) strains. J Appl Phycol 34, 1649–1660 (2022). https://doi.org/10.1007/s10811-022-02708-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02708-z