Abstract
As the commercial use of seaweed for natural product extraction calls for abundant, uniform biomass, this study focused on the production and the variability of the harvested biomass of valuable compounds produced by a commercially relevant red algal species, Asparagopsis armata. Its tetrasporophyte stage was retrieved from two Irish localities and grown for over 2 years in indoor cultures at 13 °C and 17 °C, and bromoform, mycosporine-like amino acids and phycobiliprotein contents were monitored over time. Growth rates at different temperatures were specific to isolates, and one isolate failed to grow at 17 °C. All compounds of interest were detected by the end of the 2-year cultivation period, and most of them were produced at an exponential rate at 13 °C but not at the higher temperature. At 13 °C, bromoform reached concentrations of 10.00 ± 0.55 mg g−1, total mycosporine-like amino acids of 2.65 ± 0.10 mg g−1, phycoerythrin of 11.46 ± 0.35 mg g−1 and phycocyanin of 72.13 ± 1.74 mg g−1 in Irish isolates. The observed variability in compound content was statistically significant but not large enough to impede commercial utilization. Bromoform content in cultivated samples was almost 6-fold higher than in field-collected samples though natural bromoform variability remains to be elucidated. Our findings suggest that the tetrasporophytic phase of A. armata is a suitable candidate for indoor cultivation; abundant and homogeneous biomass composition can be obtained which can be further optimized by growth temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seaweeds (marine macroalgae) are sources of bioactive compounds with multiple commercial applications, ranging from food to pharmaceuticals as well as agriculture (Stengel and Connan 2015; Wang et al. 2019). Although over 30,000 marine compounds have already been characterized (Chuanyu et al. 2021), except in a few species, their commercial exploitation is currently restricted due to, among other things, limitations in the supply of biomass. Natural seaweed populations vary greatly in metabolite composition, and hence also often the associated bioactivity, both geographically and temporally (Stengel and Connan 2015). In addition, harvesting of wild populations should only be considered if detailed knowledge of the sustainability of such activity is available, as target or associated species may become diminished and non-reversible ecosystem decline may occur. However, broad geographical and seasonal variation in both content and activity of bioactive compounds actually presents a highly valuable opportunity for culturing opportunities, reflecting the richness and plasticity of wild populations, which can be exploited in indoor cultivation systems.
Strain domestication of marine biota in aquaculture is considered an important development towards the sustainable production of biomass (Hafting et al. 2015). However, domestication of seaweeds remains underdeveloped compared to that of terrestrial crops, and only two genera—Saccharina japonica (Areschoug) C. E. Lane, C. Mayes, Druehl and G. W. Saunders and several species of Eucheuma J. Agardh—provide the majority of the world’s seaweed mariculture production, concentrated in nine East and Southeast Asian countries (FAO 2020; Costa-Pierce and Chopin 2021). The expansion of seaweed farming depends on domestication of wild strains, which in turn requires a good understanding of the life cycle of the species to control reproduction and propagation. In this regard, optimal growth and stable chemical composition are essential attributes for commercial biomass production.
Invasive seaweed species are of special interest as biotechnological targets (Balboa et al. 2015; Epstein and Smale 2017; Máximo et al. 2018; Alburquerque et al. 2019); they are considered specialists at producing highly variable secondary metabolites to enable their success in host ecosystems, to avoid grazing and to increase competitiveness, and they display higher growth rates. The red seaweed Asparagopsis armata Harvey (Rhodophyta) is an invasive seaweed introduced into many temperate areas worldwide, arriving in Ireland in 1939 (de Valéra 1942). Since then, it has spread along the Irish coast (pers. obs). The species has proven to be a promising source of metabolites with antibacterial (Paul et al. 2006; Pinteus et al. 2015), antiviral (Haslin et al. 2001; Bouhlal et al. 2013), antioxidant and antitumor potential (Pinteus et al. 2018). Also, it has potential as a source of ultraviolet (UV) screening and antioxidant compounds, namely mycosporine-like amino acids (MAAs) (Dunlap and Yamamoto 1995; Bandaranayake 1998) and accessory pigments widely used as colour additives, such as phycocyanin (PC), phycoerythrin (PE) and allophycocyanin (APC) (Roda-Serrat et al. 2018). Lately, both species in the genus Asparagopsis have been in the media hotspot because of the reported in vitro (Kinley et al. 2016; Machado et al. 2016) and in vivo (Roque et al. 2019; Stefenoni et al. 2021) anti-methanogenic capacity of their halogenated compounds. Given that bromoform is also toxic for human consumption, bromoform accumulation by cattle and any potential negative impacts on animal tissues and milk production require further investigation. Biomass processing and preservation protocols need to be improved but, principally, sourcing proposed quantities to achieve an impact remains a challenge.
Although removing ‘naturally’ occurring biomass of invasive species would reduce the negative impact these have on native ecosystems, this activity might also be associated with ecological risks. There is evidence that dispersal of invasive species is enhanced when removed manually (Critchley et al. 1986) or when intentionally cultured in the open sea (Fletcher and Farrell 1998), including in Ireland (Kraan and Barrington 2005). Additionally, damage to the native ecosystem may be induced by the physical removal of the species, e.g. through changes in incident irradiance that might favour species altering their abundance and misidentification of native species. Therefore, on-land cultivation techniques provide ecologically safe options, with the benefit of high-level control of environmental parameters, which are species- and even life stage-specific, to optimize the yield of the targeted compound(s). Some disadvantages are related to high implementation costs and the difficulty of growing particular species or life stages indoors.
The tetrasporophyte phase of A. armata (former Falkenbergia stage) has enormous potential as a source of bioactive compounds (Paul et al. 2006; Figueroa et al. 2008; Mata et al. 2011). Here, isolates from two localities on the Irish west coast were maintained for 2 years in indoor culture, with the aim of investigating the temporal production of commercially interesting compounds (MAAs: shinorine and palythine; phycobiliproteins: PE, PC and APC; and bromoform) at two temperatures. Genetic characterization of the isolates was performed to detect unique haplotypes at the locations. Given that compound production is a pivotal aspect of cultivation, we also estimated growth rates at each experimental temperature. To evaluate the tetrasporophytic stage of A. armata as a candidate for long-term, on-land cultivation, we studied the following characteristics: (1) importance of geographically distinct isolates regarding growth and metabolite content; (2) growth rates and compound production enhancement by culture conditions and through time, (3) consistency and stability of metabolite content in the harvested biomass as a prerequisite for industrial long-term (>2 years) cultivation where the physiological state of the species is not compromised.
Material and methods
Algal material and culture conditions
Isolates were obtained from two locations: Finavarra (Co. Clare, Ireland) (53° 09′ 09.8″ N 9° 07′ 11.6″ W) on 11/7/2017; and Lettermore (Co. Galway, Ireland) (53° 17′ 16.1″ N 9° 39′ 31.6″ W) on 23/07/2017. From each population, approx. five individual ‘pompoms’ ‘Falkenbergia’ stages were taken to the laboratory within 1 h, cleaned of epiphytes and treated against diatom fouling using germanium dioxide (GeO2) for 1 day in 10-mL Petri dishes. Subsequently, they were transferred to 2-L flasks with sterile seawater supplemented with 20 mL L−1 of Provasoli-enriched media (PES; Provasoli 1957). Asparagopsis armata, as all Florideophyceae, has a tri-phasic heteromorphic life cycle, where gametophytes alternate with carposporophytes (parasitic to gametophytes) and tetrasporophytes. Cultures were established using tetrasporophytes given that gametophytes failed to grow in laboratory conditions (pers. obs., data not shown). After 2 months, cultures were transferred to 20-L tanks (translucid, 29 cm diameter, 32.6 cm depth) with aeration provided by air pumps, connected to a tube placed in the middle of the bucket. Culture media were changed monthly. Two sets of cultures (n=1 per set) with an initial biomass density of 5 g FW L−1 were established under 15 μmol photons m−2 s−1 provided by Sylvania Activa 36W/172 (Feilo Sylvania, Germany) and a photoperiod of 12:12 (L:D) in walk-in culture chambers at 13 °C and 17 °C with the same aeration and monthly seawater enrichment (PES). The lower experimental temperature (13 °C) corresponded to the in situ water temperature at the time of sampling and the upper (17 °C) to the maximum photosynthetic efficiency (α) of tetrasporophytes from A. armata from the Mediterranean Sea (Zanolla et al. 2015), as well as maximum summer temperatures recorded locally in western Ireland (Guihéneuf et al. 2018). Each month, approximately a third of the biomass was removed using a strainer from the 20-L tanks to avoid overcrowding and self-shading and keep the ideal density for optimal growth for this life stage and species at 5 g FW L−1 (Mata et al. 2006). This fresh biomass was measured by removing water excess with lab paper, and then placed in a scale.
Molecular methods and analyses
DNA was extracted from three tetrasporophytes from each location and the mitochondrial cox2-3 spacer was sequenced following protocols detailed in Sherwood (2008). Newly generated and mined sequences of the cox2-3 spacer from A. armata from NCBI were treated as in Zanolla et al. (2018). Briefly, the alignment was assembled from forward and reverse reads using BioEdit (Hall 1999) with available sequences. Genealogical networks were computed using the median-joining algorithm in Network v4.5.1.6 (http://www.fluxus-technology.com; Bandelt et al. 1999) to identify unique haplotypes at locations.
Measurements of growth and chemical composition
Growth rate was assessed by determining the difference of total biomass weight over 5 consecutive months and then using the following formula: Growth (% day−1) = (Ln final FW-Ln initial FW)/Time (days) ×100 for each isolate at 13 °C and 17 °C. We studied individual, daily and monthly variations of shinorine, palythine, phycobiliproteins (phycoerythrin-PE, phycocyanin-PC and allophycocyanin-APC) and bromoform content. Monthly variation was monitored over a 2-year period. Every month, for both temperatures and geographical isolates, three replicates of approx. 3 g dry weight (DW) were taken for chemical analysis from the bulk culture vessel. Preliminary analysis of these data portrayed unexpected variability in spite of stable culture conditions. To investigate the underlying cause of this phenomenon, we assessed daily and individual variation in compound production. For the former, three replicates were sampled every 6 h for a 30-h period. For the latter, we picked four individual pompons, large enough to be split into three analytical replicates. All material for chemical analyses was frozen for 24 h, then freeze-dried (Labconco Freezone, USA) and ground, as recommended for bromoform, pigment and MAA detection (Hagerthey et al. 2006; Vucko et al. 2017; Guihéneuf et al. 2018; respectively). All material was stored at −20 °C until analysis. Phycobiliproteins and MAAs were measured within 2 months, and bromoform after 2 years.
Chemical analyses
Mycosporine-like amino acids were extracted and analysed according to Karsten et al. (2009) and adapted as in Guihéneuf et al. (2018). Identification of MAAs was obtained by co-chromatography with in-house (NUI Galway) purified standards of shinorine, palythine, asterina-330 and porphyra-334 as detailed in Guihéneuf et al. (2018). Phycobiliproteins were extracted from 30 mg of biomass using 2 mL of 0.1 M phosphate buffer (pH6.80) and left overnight at 4 °C with continuous stirring. After centrifugation at 8000 × g for 5 min (at 4 °C), the supernatant was analysed using a Cary UV50 Spectrophotometer and CaryWIN software (Varian Inc., USA). Absorbance was measured at wavelengths 455, 564, 592, 615, 618, 645 and 652 nm. PE and PC contents were calculated using Beer and Eshel formulas (Beer and Eshel 1985) and APC using Sudhakar et al. (2015). The bromoform content was determined by GC-MS analysis following a method previously developed and validated by Cawthron Institute, as described in detail by Romanazzi et al. (2021). Briefly, freeze-dried seaweed samples were extracted twice with methanol and the extracts were combined and further diluted before GC-MS analysis. Analysis was performed on an Agilent 7890B gas chromatograph equipped with a single quadrupole mass analyser (Agilent 5977A). The GC was fitted with an Agilent 19091N-133 HP-INNOWAX silica capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness) and the injector was maintained at 180 °C and operated in split-less mode with a split vent time of 0.5 min and an injection volume of 1 μL. The GC oven temperature was programmed to start at 40 °C, and then was ramped up to 250 °C at 16 °C min−1; hydrogen was used as the carrier gas, with a flow rate of 1.5 mL min−1. The ion source electrode was operated in positive mode and the mass analyser was set up in SIM mode with a dwell time of 50 ms for all signals. MassHunter software was used for data analysis. A bromoform calibration curve for calibration and quantitation of bromoform was generated by serial dilution of a bromoform reference standard obtained from Sigma-Aldrich (USA).
Photosynthetic measurements
We used photosynthesis-irradiance (P-E) curves to characterize the photosynthetic performance of Finavarra isolates at 13 °C at the end of the cultivation period, given it was the best growing isolate and growth rates are decisive aspects of cultivation. Four replicates, consisting of 0.04 g fresh weight (FW) each, and belonging to a single individual, were then transferred to the reaction chamber of a Clark-type oxygen electrode system (Hansatech Instruments, UK), in 2 mL sterile seawater at 13 °C to determine photosynthetic performance. Changes in dissolved oxygen concentration were used to estimate the dark respiration and photosynthetic rates under 10 levels of increasing irradiance, ranging from 0 to 475 μmol photons m−2 s−1. This maximum irradiance was chosen to avoid photoinhibition (Zanolla et al. 2015). Specific details of P-E curve measurements and photosynthetic parameter calculation followed Zanolla et al. (2015). The following parameters were estimated: maximum net photosynthetic rate (NPRmax), light compensation point (Ic), photosynthetic efficiency (α), light saturation point (Ik) and dark respiration. These parameters, taken together, are good indicators of the physiological state of the samples (Pmax, α, DR) and help to refine irradiance culture conditions (Ik, Ic).
Data analyses
Monthly MAA and phycobiliprotein contents were plotted using all replicates from each harvesting event (n=3) to determine the best fitting model (linear, exponential, none, indicated using trend lines in the figures) of their content over time. R values from these adjustments were used as indicators of significance. The variances (s2) of the replicates within 1 month were used to assess the homogeneity of the harvested biomass in the relevant compounds. To uncover optimal temperature (13 °C vs 17 °C) for MAAs content in Finavarra isolates, we performed a t student test. Daily and individual variations in MAAs and phycobiliproteins were analysed using one-way ANOVA, with ‘hour of sampling’ or ‘individual’ as fixed factor. Bromoform content was analysed every 2 months. Bromoform levels are presented as mean ± sd and are compared among locations (for the first year only, at 13 °C) and among temperatures (13 vs 17 °C, only for Finavarra isolates, 2 years) using two-way ANOVAs with ‘time and location’ and ‘time and temperature’, respectively, as fixed factors. Normality and homoscedasticity were checked prior to the ANOVAs, as implemented in the software used. No transformation of the data was needed. Significance was set at 0.05 for all tests, which were performed in Kaleidagraph (Synergy Software, Version 4.0).
Results
Molecular analyses
All replicates retrieved from Finavarra and Lettermore belonged to lineage 1 of A. armata (as in Dijoux et al. 2014, phylogenetic tree not shown) and more than one haplotype was found at Finavarra. All samples from Lettermore, on the other hand, belonged to same haplotype (in blue in Fig. 1). Most individuals, except one (in red in Fig. 1), were identical to those from the Mediterranean Sea (Fig. 1).
Growth rates
The isolate from Lettermore failed to grow at 17 °C, and growth at 13 °C was lower than for the Finavarra isolate (14.8 ± 1.7 % day−1 vs 18.4 ± 4.2 % day−1, respectively). Temperature had a drastic effect on the growth rate in Finavarra isolates; rates of 1.6 ± 0.5 % day−1 were measured at 17 °C, almost 10 times lower than at 13 °C. Cultures based on the Lettermore isolates were maintained for 1 year only, and those from Finavarra for more than 2 years.
Chemical analyses
Mycosporine-like amino acids
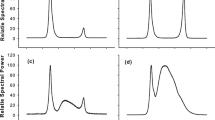
Our quantification method allowed us to detect palythine, shinorine and traces of asterine 330, though the latter could not be quantified. Total MAA content in Finavarra samples grown at 13 °C was 1.93 ± 0.82 mg g−1 DW and 2.13 ± 1.07 mg g−1 DW at 17 °C. Lettermore samples grown at 13 °C yielded average values of MAA of 2.64 ± 0.77 mg g−1 DW throughout the study. Shinorine content was always higher than palythine at all temperatures (Fig. 2). The samples retrieved directly from the field before cultivation were too small to analyse but, at 13 °C in Finavarra isolates, shinorine content decreased from the concentrations determined after 1 month and then increased exponentially (Fig. 2a, Supp. Table 2). Overall, higher content in shinorine was found at 13 °C (Supp. Table 1). At 17 °C, however, initial (month 1) values were surpassed after 6 months, but concentrations did not show any apparent trend (Fig. 2a, b, Supp. Table 2). With respect to biomass homogeneity, at 13 °C, Finavarra isolate variance was always below 0.01, and even decreased with time (Supp. Fig. 1a). At 17 °C, the variation in shinorine was almost three times greater than that at 13 °C, with higher values observed by the 18th month in culture (Supp. Fig. 1a).
Shinorine and palythine content of Finavarra isolates at 13 °C and 17 °C throughout the 2-year cultivation period. Circles highlight ‘initial’ content of each MAA determined after 1 month of cultivation when sufficient biomass for analysis was produced. Superimposed trend lines indicate the best fit, determined by adjusting all replicates (n=3 per month) to linear or exponential models (see text)
Palythine contents from Finavarra isolates also decreased from initial values (Fig. 2c, d), but were lower at 17 °C (Fig. 2d; Supp. Table 1). While there was a linear increase during the first 12 months of cultivation, at 13 °C, no clear trend was observed during year 2 (Fig. 2c). At 17 °C, palythine production was inconsistent (Fig. 2d). As for compound production uniformity, variance of the content of the MAAs increased by the end of the first year and then declined with time under 13 °C (Supp. Fig. 1b). Contrary to shinorine, palythine production under 17 °C was more uniform, remaining below 0.006 during the 2 years of cultivation (Supp. Fig. 1b).
To address the observed variability in MAA content at 13 °C in Finavarra isolates over time—which could suggest underlying variation between individuals or a dependency of the time of day the biomass was harvested—MAAs were also analysed in individual tetrasporophytes and in samples collected throughout a 24-h period. Variation between individuals (Supp. Fig. 2a) and among times of collection in shinorine, palythine or total MAA content was low and not significant (Supp. Fig. 2b, Supp. Table 1).
Shinorine in Lettermore isolates showed a similar trend to Finavarra isolates cultivated at the same temperature and for the same period (year 1) (Fig. 3a). However, 37 % more of that compound was detected in the Lettermore isolates (Supp. Table 1). Initial values dropped at the beginning of the trial, but then levels increased exponentially (Supp. Table 2). In terms of uniformity of biomass, variance was below 0.015 and decreased after 5 months in culture (Supp. Fig. 1g). Palythine content surpassed initial values by month 2, and while there was a maximum around month 6, no clear trend was observed (Fig. 3b). Overall values of palythine determined for Lettermore isolates were approx. 40 % higher than those of Finavarra (Supp. Table 1). Sample variance remained below 0.014, being almost 0 at the end of year 1 (Supp. Fig. 1h).
Shinorine (a) and palythine (b) production of Lettermore isolates at 13 °C throughout the 12-month cultivation period. Circles highlight ‘initial’ content of each MAA determined after 1 month of cultivation when sufficient biomass for analysis was produced. Superimposed trend lines indicate the best fit, determined by adjusting all replicates (n=3 per month) to linear or exponential models (see text)
Phycobiliproteins
Phycoerythrin was the most abundant pigment recovered in all samples (Figs. 4, 5). Contrary to what was observed for MAAs, levels of all phycobiliproteins increased over time and were higher than the ‘initial’ values determined after 1 month of cultivation (Fig. 4). Phycoerythrin in samples from Finavarra increased exponentially (Supp. Table 2) under both temperatures tested. Variation in phycoerythrin content was very small during the first months of the cultivation period but increased with time, and this trend was more obvious at 17 °C (Supp. Fig. 1c). Phycocyanin in Finavarra isolates also increased exponentially at 13 °C, while 35 % lower levels were detected after culture at 17 °C for 2 years. There was no obvious trend for phycocyanin levels in samples cultivated at 17 °C (Fig. 4, Supp. Table 2). Similar variation in the levels was detected at both temperatures. Allophycocyanin was the least abundant pigment (Fig. 4). Unlike the other two compounds, allophycocyanin levels did not show a marked trend at either temperature and remained remarkably constant during the culturing trial (Fig. 4).
Individual and daily variations of phycobiliproteins in Finavarra isolates grown at 13 °C were analysed (Supp. Fig. 3), and one-way ANOVA results demonstrated variation to be significant only among individuals, but not when daily variations were examined (Supp. Fig. 3b, Supp. Table 1).
Lettermore samples yielded lower levels of all pigments (Fig. 5), but they increased at an exponential rate over time (Supp. Table 2). Phycoerythrin content was similar to that presented by Finavarra samples at 13 °C, while phycocyanin and allophycocyanin were 50 % lower in Lettermore samples during the year. Initial values were exceeded by month 3 (Fig. 5). Variation, interestingly, increased with time only in the case of phycoerythrin (Supp. Fig. 1).
Bromoform content
As field-derived materials used to start the cultures had insufficient biomass to test bromoform concentrations, new materials obtained from Finavarra were analysed in October 2018, and revealed concentrations of 0.21 ± 0.01 mg g−1 DW. Concentrations in Finavarra cultures varied from 3.83 ± 0.45 to 10.01 ± 0.55 mg g−1 DW at 13 °C and from 0.19 ± 0.01 to 13.27 ± 0.25 mg g−1 DW at 17 °C. Lettermore samples yielded a range of 2.76 ± 0.45 to 7.51 ± 0.55 mg g−1 DW (Fig. 6). Both isolates responded differently to 13 °C during the first year of cultivation; effects of time or isolate origin alone were not significant, but their interaction was (Supp. Table 1). A reduction in bromoform content occurred in Lettermore samples by month 8, and then levels remained constant (5.85 ± 0.99 mg g−1 DW). The only significant decrease in concentrations in Finavarra isolates occurred between months 5 and 8, and levels remained similar for the rest of the year (Supp. Table 1). By the end of the 12th month of cultivation, bromoform content was higher in Lettermore samples compared to Finavarra (Supp. Table 1). As with the other metabolites investigated, bromoform levels differed between Finavarra isolates grown at different temperatures, but also depended on the cultivation time (Supp. Table 1). Overall values indicated that cultivation at 13 °C enhanced concentrations, except for a peak recorded at 17 °C by month 5, when 13.27 ± 0.25 mg g−1 DW was recorded, which represented the highest level of bromoform observed across all samples. A peak occurred by the end of the culturing period at 13 °C, which was lower than that recorded at 17 °C. Regarding uniformity, variance among replicates was smaller in Finavarra samples cultivated at 13 °C (below 0.8 throughout the culturing period). Lettermore isolates displayed even less variance, remaining under 0.6 throughout the year (Supp. Fig. 1).
Photosynthetic measurements
In order to portray the physiological status of the best performing isolate (Finavarra at 13 °C) at month 24, the photosynthetic parameters of maximum net photosynthetic rate, photosynthetic efficiency, light compensation and saturation point and dark respiration were determined, with results presented in Table 1.
Discussion
Genetic analysis revealed that Irish isolates of A. armata used in this study were derived from Mediterranean populations, due to the shared haplotype composition of the analysed samples. As was the case with A. taxiformis lineage 2 (Zanolla et al. 2018) and also the invasive Codium fragile (Suringar) Hariot (Provan et al. 2005), the use of genetic haplotypes allowed the reconstruction of a likely route of the European invasion of A. armata: from the Australian/New Zealand region moving eastwards through the Mediterranean Sea until it reached Ireland.
Our study indicates that the tetrasporophytic phase of A. armata represents a suitable candidate for long-term, land-based cultivation systems. A. armata tetrasporophytes displayed consistent, continuous vegetative propagation, and no tetrasporangia were detected in either isolate at either temperature. This was advantageous since cultures were very stable; a shift in life stages in A. armata typically is a response to narrow changes in the combination of several environmental factors (Guiry and Dawes 1992). Additionally, the physiological state of the algae was healthy after 24 months under the given culture conditions, indicating that even longer cultivation periods are possible. Comparing the physiological performance A. armata tetrasporophytes from Finavarra to that of isolates from the Mediterranean Sea (Zanolla et al. 2015) at a similar temperature, Irish isolates had a higher NPRmax, Ic, Ik and photosynthetic efficiency. This, related to growth conditions, means that less incident light is required to maintain cultures, thus reducing cultivation costs that might be significant given the amount of energy needed to maintain a stable controlled temperature in the culturing rooms. According to the growth rate presented by the Finavarra isolates at 13 °C, using an initial biomass of 5 g L−1, 27.5 g L−1 per month of fresh biomass could be harvested monthly, which indicates good potential for upscaling. Additionally, in haploid-diploid seaweeds, the manipulation of clonal propagation can facilitate genetic selection (Valero et al. 2017). Our results indicate that long-term maintenance under constant conditions had no effect on growth and reproductive responses of the species, which are important considerations for commercial cultivation.
Highly relevant when facing distributional logistics from biomass producers to end users is the bromoform content of biomass following processing, storage and transport. As a volatile compound, bromoform measured in samples varies greatly depending on biomass storage conditions and, additionally, the chosen method of detection. Thus, a direct comparison of bromoform levels reported from isolates gathered in Portugal (0.00126 mg g−1 DW, Muizelaar et al. 2021), Australia (16.7 ± 1.0 mg g−1 DW, Magnusson et al. 2020), Tasmania (1.32 mg g−1 DW, Roque et al. 2019) and Italy (23 mg g−1 DW approx. Mata et al. 2011) is difficult. Here, we provide evidence that bromoform levels depend on growth temperature and also on the origin of the isolates, even on small spatial scale (< 100 km)—this suggests that worldwide differences among populations are likely even greater. In fact, similar results have been found for A. taxiformis tetrasporophytes, A. armata sibling species (Mata et al. 2017). Differences between pre- and post-harvest treatment also seem to be crucial; previous research has shown that production can be optimized by the addition of H2O2 3 h prior to biomass harvesting (Mata et al. 2011), and increased bromoform content also depended on growth temperature, as in our study.
Regarding storage, Stefenoni et al. (2021) could not detect bromoform in freeze-dried and ground biomass after 4 months when stored at 4 °C, which contrasts with results of this study, where the compound was detectable even after storage for almost 4 years (initial samples were collected in August 2017 and were analysed in May 2021). These differences may, at least partially, be explained by the preservation and storage processes and final storage temperature (4 °C vs −18 °C), the different freezing temperatures prior to freeze-drying (−40, −25 or −20 °C vs −18 °C), the stored life stage (gametophytes vs tetrasporophytes) or different analytical methods used for bromoform determination. Biomass produced during the first year of cultivation and stored for 4 years rendered similar bromoform levels to samples collected during the following year, indicating that Asparagopsis can be stored over several years, with bromoform retained in the biomass under appropriate storage conditions.
The importance of isolate selection and culture conditions in Asparagopsis armata
An important outcome of our experiments was that isolate origin appeared to be critical. Lettermore isolates responded differently to temperature conditions: they grew more slowly and contained lower levels of MAAs and accessory pigments than Finavarra isolates under equivalent conditions. Also, MAA concentrations in Irish isolates following our extraction and detection protocol were higher than in isolates from southern Portugal (Figueroa et al. 2008). Their phycoerythrin content, when cultured at 13 °C, was similar to those of other commercial species, Pyropia ssp. (ex Porphyra) and Spirulina sp. (Osório et al. 2020), but 40 % higher when grown at 17 °C. Regarding phycocyanin, strikingly high levels were found in Finavarra isolates grown at 13 °C (13 times higher than Pyropia ssp. and 3 times higher than Spirulina sp.; Osório et al. 2020), and somewhat higher levels, in samples grown at 17 °C. Chemical diversity, on a geographical scale, may be due to both genetic differentiation of locally adapted strains, or phenotypically acclimated individuals exposed to combinations of environmental variables (Stengel et al. 2011; Hafting et al. 2015). Several detailed profiling studies have documented the natural chemical diversity within and between populations, further demonstrating the richness of wild raw material derived from different natural populations. For example, genetically different strains of Porphyra yezoensis Ueda from different locations may exhibit distinct adaptive features to local environments with regard to phycobiliprotein and chlorophyll contents (Zhang et al. 2012). For the Irish Asparagopsis strains, genetic differences were detected among isolates; while Lettermore-derived cultures seemed to be represented by only one haplotype, samples from Finavarra contained at least two, in spite of the amount of original material being the same. Additionally, Asparagopsis shows consistently strong phenotypic variation among clones in terms of growth rates (Monro and Poore 2004) and thermal photosynthetic performance among lineages and species (Zanolla et al. 2015). In this present study, we were able to detect such phenotypic variation in both growth and metabolite production, which might be the result of genetic differences among localities. This indicates that there is potential for compound composition to be optimized by modifying culture conditions using pure or mixed isolates. While less variation in shinorine, palythine, bromoform, allophycocyanin and phycocyanin was observed for Lettermore isolates in pure cultures (one haplotype detected), higher overall production values could benefit from the contribution of several genetic variants. Contrary to the case of Gracilaria chilensis C.J. Bird, McLachlan and E.C. Oliveira, where artificial and natural population levels are mostly maintained by thallus fragmentation, and clonal lineages display notable differences in morphology and growth rates when cultured under the same conditions (Santelices and Varela 1993), our cultures showed little, though some statistically significant, variation between harvests.
Long-term compound production in Asparagopsis armata: repercussions for industry applications
While the higher temperature treatment enhanced levels of some compounds for our A. armata isolates, the drastic reduction in growth rate coupled with the higher variability of the bulk biomass indicates that culture of these isolates is more reliable at 13 °C. This study demonstrates that bromoform, pigments and mycosporine-like amino acids are retained at high levels in cultivation for over 2 years using PES-enriched media at 20 mL L−1, white light at 15 μmol photons m−2 s−1 and a photoperiod of 12:12 (L:D). However, even though experimental conditions were consistent over time, we found significant changes in quantity and variance of the compounds. No consistent effect of harvest time of day, or individual variation-based culture heterogeneity (given that possibly there is more than one haplotype), could be detected, though >24-h sampling periods might be necessary to uncover cycles of production in MAA that went unnoticed in this study. While the lack of diurnal variation enables more flexible harvesting times, future work is needed to determine how pigment, MAA or bromoform production may be associated with short-term fluctuations in daily growth rates which increase shading and, in turn, pigment production.
Conclusions
-
1.
Irish isolates of A. armata represent a potentially valuable source for phycobiliproteins (especially phycocyanin) and MAA and exhibit high bromoform content in long-term indoor cultivation.
-
2.
Industry needs stable product composition and high biomass yields to incorporate macroalgae as a source of bioactive compounds (with multiple high-value applications, other than animal feed); this can be achieved for A. armata tetrasporophytes through indoor cultivation.
-
3.
Considering its invasive characteristics, land-based cultivation of A. armata is a recommended option in locations where the species is not native, due to the potential negative ecological impacts on native ecosystems.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alburquerque N, Faize L, Faize M, Nortes MD, Bernardeau J, Fernandez JMR, Burgos L (2019) Towards the valorization of the invasive seaweeds Caulerpa cylindracea and Asparagopsis taxiformis in the Mediterranean Sea: applications for in vitro plant regeneration and crop protection. J Appl Phycol 31:1403–1413
Balboa EM, Moure A, Domínguez H (2015) Valorization of Sargassum muticum biomass according to the biorefinery concept. Mar Drugs 13:3745–3760
Bandaranayake WM (1998) Mycosporines: are they nature’s sunscreens? Nat Prod Rep 15:159–172
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48
Beer S, Eshel A (1985) Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Aust J Mar Freshw Res 36:785–792
Bouhlal R, Riadi H, Bourgougnon N (2013) Antibacterial activity of the extracts of Rhodophyceae from the Atlantic and the Mediterranean coasts of Morocco. J Microbiol Biotech Food Sci 2:2431–2439
Chuanyu L, Tong C, Bo Q, Ningfeng L, Heyu W, Liangren Z, Zhenming L (2021) CMNPD: a comprehensive marine natural products database towards facilitating drug discovery from the ocean. Nucleic Acids Res 49:D509–D515
Costa-Pierce BA, Chopin T (2021) The hype, fantasies and realities of aquaculture development globally and its new geographies. World Aquac 52:23–35
Critchley AT, Farnham WF, Morrell SL (1986) An account of the attempted control of an introduced marine alga, Sargassum muticum, in southern England. Biol Cons 35:313–332
de Valéra M (1942) A red alga new to Ireland: Asparagopsis armata Harv. on the west coast. Irish Nat J 8:30–33
Dijoux L, Viard F, Payri C (2014) The more we search, the more we find: discovery of a new lineage and a new species complex in the genus Asparagopsis. PloS One 9:e103826
Dunlap WC, Yamamoto Y (1995) Small-molecule antioxidants in marine organisms: antioxidant activity of mycosporine-glycine. Comp Biochem Physiol B 112:105–114
Epstein G, Smale DA (2017) Undaria pinnatifida: a case study to highlight challenges in marine invasion ecology and management. Ecol Evol 7:8624–8642
FAO (2020) The State of World Fisheries and Aquaculture: Sustainability in Action. FAO, Rome. p. 206. https://doi.org/10.4060/ca9229en
Fletcher RL, Farrell P (1998) Introduced brown algae in the North East Atlantic, with particular respect to Undaria pinnatifida (Harvey) Suringar. Helgol Meeresunters 52:259–275
Figueroa FL, Bueno A, Korbee N, Santos R, Mata L, Schuenhoff A (2008) Accumulation of mycosporine-like amino acids in Asparagopsis armata grown in tanks with fishpond effluents of gilthead sea bream, Sparus aurata. J World Aquacult Soc 39:692–699
Guihéneuf F, Gietl A, Stengel DB (2018) Temporal and spatial variability of mycosporine-like amino acids and pigments in three edible red seaweeds from western Ireland. J Appl Phycol 30:2573–2586
Guiry MD, Dawes CJ (1992) Daylength, temperature and nutrient control of tetrasporogenesis in Asparagopsis armata (Rhodophyta). J Exp Mar Biol Ecol 158:197–217
Hafting JT, Craigie JS, Stengel DB, Loureiro RR, Buschmann AH, Yarish C, Critchley AT (2015) Prospects and challenges for industrial production of seaweed bioactives. J Phycol 51:821–837
Hagerthey SE, Louda JW, Mongkronsri P (2006) Evaluation of pigment extraction methods and recommended protocol for periphyton chlorophyll a determination and chemotaxonomic assessment. J Phycol 42:1125–1136
Hall TA (1999) BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Haslin C, Lahaye M, Pellegrini M, Chermann JC (2001) In vitro anti-HIV activity of sulfated cell-wall polysaccharides from gametic, carposporic and tetrasporic stages of the Mediterranean red alga Asparagopsis armata. Planta Med 67:301–305
Karsten U, Escoubeyrou K, Charles F (2009) The effect of re-dissolution solvents and HPLC columns on the analysis of mycosporine-like amino acids in the eulittoral macroalgae Prasiola crispa and Porphyra umbilicalis. Helgol Mar Res 63:231–238
Kinley RD, de Nys R, Vucko MJ, Machado L, Tomkins NW (2016) The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces methane production during in vitro fermentation with rumen fluid. Anim Prod Sci 56:282–289
Kraan S, Barrington KA (2005) Commercial farming of Asparagopsis armata (Bonnemaisoniceae, Rhodophyta) in Ireland, maintenance of an introduced species? J Appl Phycol 17:103–110
Machado L, Magnusson M, Paul NA, Kinley RD, de Nys R, Tomkins N (2016) Dose-response effects of Asparagopsis taxiformis and Oedogonium sp. on in vitro fermentation and methane production. J Appl Phycol 28:1443-1452
Magnusson M, Vucko MJ, Neoh TL, de Nys R (2020) Using oil immersion to deliver a naturally-derived, stable bromoform product from the red seaweed Asparagopsis taxiformis. Algal Res 51:102065
Mata L, Silva J, Schuenhoff A, Santos R (2006) The effects of light and temperature on the photosynthesis of the Asparagopsis armata tetrasporophyte (Falkenbergia rufolanosa), cultivated in tanks. Aquaculture 252:12–19
Mata L, Gaspar H, Justino F, Santos R (2011) Effects of hydrogen peroxide on the content of major volatile halogenated compounds in the red alga Asparagopsis taxiformis (Bonnemaisoniaceae). J Appl Phycol 23:827–832
Mata L, Lawton JR, Magnusson M, Andreakis N, Nys R, Andreakis N, Paul NA (2017) Within-species and temperature-related variation in the growth and natural products of the red alga Asparagopsis taxiformis. J Appl Phycol 29:1437–1447
Máximo P, Ferreira LM, Branco P, Lima P, Lourenço A (2018) Secondary metabolites and biological activity of invasive macroalgae of Southern Europe. Mar Drugs 16:265–293
Monro K, Poore AG (2004) Selection in modular organisms: is intraclonal variation in macroalgae evolutionarily important? Am Nat 163:564–578
Muizelaar W, Groot M, van Duinkerken G, Peters R, Dijkstra J (2021) Safety and transfer study: Transfer of bromoform present in Asparagopsis taxiformis to milk and urine of lactating dairy cows. Foods 10:584–600
Osório C, Machado S, Peixoto J, Bessada S, Pimentel FB, Alves CR, Oliveira MBPP (2020) Pigments content (chlorophylls, fucoxanthin and phycobiliproteins) of different commercial dried algae. Separations 7:33
Paul NA, de Nys R, Steinberg PD (2006) Chemical defence against bacteria in the red alga Asparagopsis armata: linking structure with function. Mar Ecol Progr Ser 306:87–101
Pinteus S, Alves C, Monteiro H, Araújo E, Horta A, Pedrosa R (2015) Asparagopsis armata and Sphaerococcus coronopifolius as a natural source of antimicrobial compounds. World J Microbiol Biotechnol 31:445–451
Pinteus S, Lemos MF, Alves C, Neugebauer A, Silva J, Thomas OP, Pedrosa R (2018) Marine invasive macroalgae: Turning a real threat into a major opportunity-the biotechnological potential of Sargassum muticum and Asparagopsis armata. Algal Res 34:217–234
Provan J, Murphy S, Maggs CA (2005) Tracking the invasive history of the green alga Codium fragile ssp. tomentosoides. Mol Ecol 14:189–94
Provasoli L, McLaughlin JJA, Droop MR (1957) The development of artificial media for marine algae. Arch Microbiol 25:392–428
Roda-Serrat MC, Christensen KV, El-Houri RB, Fretté, Christensen LP (2018) Fast cleavage of phycocyanobilin from phycocyanin for use in food colouring. Food Chem 240:655–661
Romanazzi D, Sanchez-Garcia C, Svenson J, Mata L, Pes K, Hayman CM, Wheeler TT, Magnusson M (2021) Rapid analytical method for the quantifcation of bromoform in the red seaweeds Asparagopsis armata and Asparagopsis taxiformis using gas chromatography–mass spectrometry. ACS Ag Sci Technol 1:436–44
Roque BM, Salwen JK, Kinley R, Kebreab E (2019) Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J Clean Prod 234:132–138
Santelices B, Varela D (1993) Intra-clonal variation in the red seaweed Gracilaria chilensis. Mar Biol 116:543–552
Sherwood AR (2008) Phylogeography of Asparagopsis taxiformis (Bonnemaisoniales, Rhodophyta) in the Hawaiian Islands: two mtDNA markers support three separate introductions. Phycologia 47:79–88
Stefenoni HA, Räisänen SE, Cueva SF, Wasson DE, Lage et al (2021) Effects of the macroalga Asparagopsis taxiformis and oregano leaves on methane emission, rumen fermentation, and lactational performance of dairy cows. J Dairy Sci 104:4157–4173
Stengel DB, Connan S (2015) Marine algae: a source of biomass for biotechnological applications. In: Stengel DB, Connan S (eds) Natural Products From Marine Algae. Humana Press, NY, pp 1–37
Stengel DB, Connan S, Popper ZA (2011) Algal chemodiversity and bioactivity: sources of natural variability and implications for commercial application. Biotechnol Adv 29:483–501
Sudhakar MP, Jagatheesan A, Perumal K, Arunkumar K (2015) Methods of phycobiliprotein extraction from Gracilaria crassa and its applications in food colourants. Algal Res 8:115–120
Valero M, Guillemin ML, Destombe C, Jacquemin B, Gachon CM, Badis Y, Faugeron S (2017) Perspectives on domestication research for sustainable seaweed aquaculture. PiP 4:33–46
Vucko MJ, Magnusson M, Kinley RD, Villart C, de Nys R (2017) The effects of processing on the in vitro antimethanogenic capacity and concentration of secondary metabolites of Asparagopsis taxiformis. J Appl Phycol 29:1577–1586
Wang X, Sun G, Feng T, Zhang J, Huang X, Wang T, Geng M (2019) Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res 29:787–803
Zanolla M, Altamirano M, Carmona R, De La Rosa J, Sherwood A, Andreakis N (2015) Photosynthetic plasticity of the genus Asparagopsis (Bonnemaisoniales, Rhodophyta) in response to temperature: implications for invasiveness. Biol Invas 17:1341–1353
Zanolla M, Altamirano M, Carmona R, De la Rosa J, Souza-Egipsy V, Sherwood A, Andreakis N (2018) Assessing global range expansion in a cryptic species complex: insights from the red seaweed genus Asparagopsis (Florideophyceae). J Phycol 54:12–24
Zhang T, Shen Z, Xu P, Zhu J, Lu Q, Shen Y, Wang Y, Yao C, Li J, Wang Y, Jiang H (2012) Analysis of photosynthetic pigments and chlorophyll fluorescence characteristics of different strains of Porphyra yezoensis. J App Phycol 24:881–886
Acknowledgements
The authors appreciate Claudia Cara Ortega’s assistance with culture maintenance. Funding from the Marine Institute Ireland (‘A National Marine Biodiscovery Laboratory’, PBA/MB/16/01) and the AXA Research Fund (2019-AXA-THEME1-021, ‘Valorisation potential of invasive seaweed species in Ireland’) is gratefully acknowledged.
Funding
Open Access funding provided by the IReL Consortium. This work was supported by Marine Institute Ireland (‘A National Marine Biodiscovery Laboratory’, PBA/MB/16/01) and the AXA Research Fund (2019-AXA-THEME1-021, ‘Valorisation potential of invasive seaweed species in Ireland’).
Author information
Authors and Affiliations
Contributions
Marianela Zanolla—experimental design, sample and data analysis and manuscript elaboration
Donato Romanazzi—bromoform content analysis, manuscript elaboration
Johan Svenson—funding, bromoform content analysis, manuscript elaboration
Alison Sherwood—genetic delineation of the cultures, manuscript elaboration
Dagmar B. Stengel—funding, provision of analytical and culture facilities, experimental and manuscript design and elaboration
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zanolla, M., Romanazzi, D., Svenson, J. et al. Bromoform, mycosporine-like amino acids and phycobiliprotein content and stability in Asparagopsis armata during long-term indoor cultivation. J Appl Phycol 34, 1635–1647 (2022). https://doi.org/10.1007/s10811-022-02706-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02706-1