Abstract
The biomass valorisation of the invasive brown alga Rugulopteryx okamurae (Dictyotales, Phaeophyceae) is key to curbing the expansion of this invasive macroalga which is generating tonnes of biomass on southern Spain beaches. As a feasible alternative for the biomass management, anaerobic co-digestion is proposed in this study. Although the anaerobic digestion of macroalgae barely produced 177 mL of CH4 g−1 VS, the co-digestion with a C-rich substrate, such as the olive mill solid waste (OMSW, the main waste derived from the two-phase olive oil manufacturing process), improved the anaerobic digestion process. The mixture improved not only the methane yield, but also its biodegradability. The highest biodegradability was found in the mixture 1 R. okamurae—1 OMSW, which improved the biodegradability of the macroalgae by 12.9% and 38.1% for the OMSW. The highest methane yield was observed for the mixture 1 R. okamurae—3 OMSW, improving the methane production of macroalgae alone by 157% and the OMSW methane production by 8.6%. Two mathematical models were used to fit the experimental data of methane production time with the aim of assessing the processes and obtaining the kinetic constants of the anaerobic co-digestion of different combination of R. okamurae and OMSW and both substrates independently. First-order kinetic and the transference function models allowed for appropriately fitting the experimental results of methane production with digestion time. The specific rate constant, k (first-order model) for the mixture 1 R. okamurae- 1.5 OMSW, was 5.1 and 1.3 times higher than that obtained for the mono-digestion of single OMSW and the macroalga, respectively. In the same way, the transference function model revealed that the maximum methane production rate (Rmax) was also found for the mixture 1 R. okamurae—1.5 OMSW (30.4 mL CH4 g−1 VS day−1), which was 1.6 and 2.2 times higher than the corresponding to the mono-digestions of the single OMSW and sole R. okamurae (18.9 and 13.6 mL CH4 g−1 VS day−1), respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The greatest biodiversity loss in the world is produced by the introduction of invasive alien species (IAS) (Convention on Biological Diversity 2017), with the seas the areas that suffer the most its consequences. A clear recent example of IAS in Mediterranean aquatic ecosystems is the dictotalean macroalga Rugulopteryx okamurae, a north-western Pacific native species (Huang 1994). Rugulopteryx okamurae specifically comes from temperate waters near China, Korea, the Philippines, and Japan. This macroalga has been recently included in the Spanish catalogue of invasive alien species according to the ministerial order TED/1126/2020 of the Ministry of Ecological Transition published in the state official bulletin (BOE, number 314 of 1 December 2020).
Rugulopteryx okamurae has proliferated on the Atlantic and Mediterranean coast in a short time displacing the local biota and generating an unprecedented ecological impact, causing a great concern for the sea-dependent local activities in the aforementioned geographical enclave (El Aamri et al. 2018; Navarro-Barranco et al 2019; García-Gómez et al. 2020; Sempere-Valverde et al. 2021). The high content of spatane type diterpenes, with herbivorous deterrent activity, of this species has allowed its high and rapid propagation and production. In fact, only in 1 year after being observed for first time in the Strait of Gibraltar area in the autumn of 2015 in Ceuta, the beach cleaning machines of this city removed more than 5000 t of upstream macroalga biomass of the IAS (Ocaña et al. 2016). It took only 4 years to occupy the entire Cadiz coastline and to expand along the coasts of Malaga and Granada, affecting very relevant spaces of great ecological value included in the Natura 2000 Network. This rapid expansion is unprecedented; the most similar case may be the spread of the seaweed Sargassum spp. in the Caribbean (van Tussenbroek et al. 2017).
Rugulopteryx okamurae not only does have negative effects on the biodiversity of the affected areas, but it also has a great impact on human activities and economic affairs (García-Gómez et al. 2018). Economic impact associated to fishing activities and beach management in Southern Spain has been estimated to be higher than one million Euros per year (Altamirano Jeschke et al. 2016).
Currently, tonnes of collected biomass of R. okamurae end up in landfills. However, this macroalga is known to contain polysaccharides (alginates and fucoidans) which are used in the food industry, nutraceutical and pharmaceutical industry, medical treatments, etc. (Hernández-Carmona et al. 2012). It is rich in secondary metabolites among which the terpene and polyphenolic compounds stand out presenting photoprotective properties, antioxidants, and immune-stimulants (De Paula et al. 2011). Interest in the study of these natural products has been growing in recent years due to their ecological role and their utility for human beings (Hernández-Carmona et al. 2012). Different groups, associations, and local administrations affected by this invasion are proposing for this macroalgae to not be treated as a waste but rather as a valued raw material for cosmetic, dermatological, or composting use, for example. It is about finding sustainable uses and exploitation of the large available biomass that will finance the removal of arrivals from the coast: the so-called elimination for its valorisation.
In addition to the uses or exploitations already mentioned, another alternative could be as a substrate in anaerobic digestion (AD). AD is an anoxic biological process with the participation of different microorganisms that transform organic matter until obtaining biogas as a final product (mainly methane, 60–70%) with a high calorific value, liable to be used energetically by combustion in engines, turbines, or boilers, either individually or mixed with other fuels (Abad et al. 2019). As a circular economy model, AD has been widely used for the degradation and stabilization of domestic and industrial wastes (Parra Huertas 2015). Recently, this biological process has been carried out in different species of red, green, and brown macroalgae obtaining methane yield coefficients of between 0.10 and 0.30 m3 CH4 kg−1 volatile solid (Morand and Briand 1996; Barbot et al. 2016; Saratale et al. 2018). Therefore, it could be a good alternative that would help eliminate and enhance the thousands of tonnes of biomass of R. okamurae deposited on the beaches, decreasing, in turn, the spread of this macroalga due to the removal of vegetative propagules.
The co-digestion of different residues for improvement in the yields obtained from individual anaerobic digestion processes is a frequent practice (Saratale et al. 2018). In this way, the combination of different organic substrates generating a homogeneous mixture increases the performance and methane yield of the process. Moreover, anaerobic digestion of single macroalgae has been studied, although they are substrates with a low C/N ratio, which largely limits the proper functioning of the anaerobic process, generating low methane yields (Tabassum et al. 2016; 2017). Given these results, some authors have recently proposed co-digestion processes of macroalgae with nitrogen-poor but carbon-rich substrates to improve and compensate for the C/N ratio of anaerobic processes (Grosser 2017; Saratale et al. 2018). In this sense, R. okamurae, as nitrogen-rich biomass (C/N around 10:1–15:1, Geider and La Roche 2002), could potentially be used in AD as a co-substrate for C-rich by-products. One of these by-products could be the olive mill solid waste (OMSW), the main solid by-product generated in the process of producing olive oil by centrifugation in two phases, which is a mixture of olive pulp, skin, and pit (Fernández-Rodríguez et al. 2020). This by-product has a high organic content and its treatment and management represents an important environmental problem. Spain, as the world’s leading producer of olive oil, generates 2–4 million tonnes of OMSW per year (Moreno-Maroto et al. 2019). The C/N ratio of the OMSW is above the value indicated by Paul and Dutta (2018) as optimal for the process (C/N of OMSW 32, C/N optimal 25–30). Furthermore, the lignocellulosic content of the OMSW, along with the presence of certain AD inhibitor compounds, such as polyphenols, limits the AD process of this compound alone (Varnero et al. 2014). The use of macroalgae as a nitrogen-rich co-substrate would help balance the C/N ratio towards the values indicated as optimal and would help to dilute the concentration of toxic substances that the OMSW possesses (Ferreira et al. 2018; Li et al. 2018), thus, obtaining an improvement in the kinetics of the process, higher methane performance coefficients, and an increase in the biodegradability of the substrates (Fernández-Rodríguez et al. 2019a, b).
Therefore, the aim of this study was to evaluate the methane potential and the stability of the anaerobic co-digestion of OMSW and the invasive macroalga R. okamurae in order to propose a possible alternative for waste management that contributes to avoid the spread and new colonization of this invasive alien species as well as to assess the viability of a higher methane production from this alga. The influence of the C/N ratio on the kinetics of the anaerobic processes and ultimate methane yields of both substrates individually and different mixtures of them was also evaluated in biochemical methane potential (BMP) tests.
Materials and methods
Influents
The experimental olive oil mill factory located in the Instituto de la Grasa was selected for the collection of the two-phase OMSW. The OMSW was collected and frozen to − 4 °C until used. Then, OMSW was sieved (2 mm) in order to remove any piece of olive pit trapped in the pulp prior use. The invasive macroalgae R. okamurae was provided by the Laboratory of Marine Biology of the University of Seville.
The inoculum used during the anaerobic study was collected from an industrial up-flow anaerobic sludge blanket reactor treating brewery wastewater located at Heineken factory (Seville, southern Spain). The inoculum was chosen due to its high methanogenic activity, which was determined in previous anaerobic assays (Fernández-Rodríguez et al. 2021b).
Both inoculum and substrates were characterized and the results are summarized in Table 1.
Experimental procedure
The mesophilic batch experiments (BMP tests) were performed with an inoculum to substrate ratio fixed at 2 (as volatile solid (VS)) as described elsewhere (Fernández-Rodríguez et al. 2019a). A 10% micronutrient solution (Fernández-Rodríguez et al. 2019b) and nitrogen gas was added at the beginning of the experiment in order to keep anaerobic conditions. The anaerobic reactors of 250 mL total volume were placed in a thermostatic water bath at a controlled temperature of 35 ± 2 °C. Three replicates for each setting were carried out. Data are presented as means ± standard deviations of the means (n = 3). The agitation rate was constant by magnetic bars at 380 rpm.
For the purpose of the experiment, the produced biogas was passed through a 3 N NaOH solution in order to trap the CO2, while the remaining gas was assumed to be methane. The anaerobic digestion experiments were stopped when the gas production remained essentially unchanged; this period was c.a. 28 days across the board.
Five different VS ratios of the substrates R. okamurae-OMSW were evaluated in triplicate. The different co-digestion mixtures studied were as follows: 1 R. okamurae-0 OMSW; 1 R. okamurae-3 OMSW; 1 R. okamurae-1.5 OMSW; 1 R. okamurae-1 OMSW; and 0 R. okamurae-1 OMSW. As controls, a triplicate with only inoculum and trace elements solution but without the addition of substrate was performed, in order to evaluate the endogenous methane production.
Analytical methods
The substrates and inoculum were analyzed before the beginning of the experiments. The digestates or effluents of each set of experiments were also analyzed at the end of each experiment. The performed analyses were as follows: total solids (TS) and volatile solids (VS) were performed according to the standard method 2540E (APHA 2012), soluble chemical oxygen demand (SCOD) was also determined following the standard method 5220D (APHA 2012), using the closed digestion and the colorimetric method. Total chemical oxygen demand (COD) was analyzed as described by Raposo et al. (2008). pH and total alkalinity (TA) were determined in fresh samples using a pH meter model Crison 20 Basic. TA was measured by pH titration to 4.3 (APHA 2012). Total ammonia nitrogen (TAN) was measured by distillation and titration according to the standard method 4500-NH3 (APHA 2012). C and N were determined through an LECO CHNS-932 Elemental Analyzer (Leco Corporation, USA). Soluble parameters were determined after sample centrifugation (Eppendorf, 9000 xg, 10 min) and filtration (47 mm glass fiber filter).
Kinetic models
First-order kinetic model
In order to study the process kinetics and estimate the process performance in the anaerobic digestion and co-digestion of the two substrates studied, the following first-order kinetic model was used:
where G is the cumulative specific methane production (mL CH4 g−1 VSadded), Gm is the ultimate methane production (mL CH4 g−1 VSadded), k is the specific rate constant (day−1), and t is the digestion time (day). This kinetic model is generally applied to evaluate the kinetics of the batch anaerobic digestion processes for different types of biodegradable substrates (Li et al. 2012; Scarcelli et al. 2020). This model is based on the premise that methane generation is proportional to the amount of substrate and not limited by microbial cell mass (Wang et al. 2017).
Transference function model
The transference function (TF) model was also used to fit the experimental data of methane production during biochemical methane production (BMP) tests (Eq. (2)). The transference function (Reaction curve-type model) (RC), utilized mainly for control purposes, contemplates that any process might be analyzed as a system receiving inputs and producing outputs (Donoso-Bravo et al. 2010). The TF model has been successfully applied by several authors for the biomethanization of different organic wastes (Donoso-Bravo et al. 2010; Li et al. 2012; Pinto-Ibieta et al. 2016). The TF model is given by the following expression:
where B (mL CH4 g−1 VSadded) is the cumulative specific methane production, Bmax (mL CH4 g−1 VSadded) is the ultimate methane production, Rmax is the maximum methane production rate (mL CH4 (g−1 VSadded·day−1)), t (day) is the digestion time, and γ (day) is the lag time.
Determination coefficients (R2) and standard errors of estimates (SEE) were calculated to evaluate the goodness-of-fit and the accuracy of the results for both models. The kinetic parameters for each experiment and mathematical adjustment were determined numerically from the experimental data obtained by non-linear regression using the software Sigma-Plot (version 11).
Results
Geographical distribution and main characteristics of the substrates
The invasive alien macroalga R. okamurae was detected in the south of Spain for the first time in the Strait of Gibraltar area in 2015 (Altamirano Jeschke et al. 2016; García-Gómez et al. 2020), and in just a few years, it has spread rapidly over a wide area of the southern coast of Spain. Rugulopteryx okamurae is becoming the dominant macroalga species in the area of the Strait of Gibraltar (Sempere-Valverde et al. 2021). On the west coast of southern Spain, the presence of this invasive alien species has been detected up to Chipiona, and in the east, its presence has been described up to the coast of Benalmadena (García-Gómez et al. 2020). The presence of this macroalga is accumulating tons of biomass on the Andalusian coasts of southern Spain. The management and valorisation of the macroalga biomass is vital to reduce the spread of R. okamurae by vegetative propagules.
The TS content of R. okamurae biomass was 197.6 ± 7.3 g kg−1 and the VS/TS ratio was 0.52. The lower VS content reduces its potential as a raw material for the AD process (Lee et al. 2015). The C content of the invasive macroalgae biomass was 30.29 ± 0.50% and the N content of 1.99 ± 0.03%, reaching a C/N ratio of 15.2 ± 0.4 (Table 1).
The OMSW presents a high VS/TS ratio content (0.81). The C content of the OMSW was 49.70 ± 0.38%, meanwhile its N content was 1.58 ± 0.06%, keeping a C/N ratio of 30.4. The main characteristics of the inoculum and both substrates (TS, VS, C/N, COD, pH) are given in Table 1.
Co-digestion methane yield
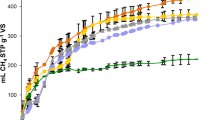
Accumulated volumes of methane for the different substrates and combinations of substrates tested are presented in Fig. 1. The methane yield versus time curves of the evaluated experiments were characterized by having a typically exponential form up to 23 days and then keeping the methane production about constant until day 28 (Fig. 1). The methane yield for all the substrates and mixtures tested reached an asymptotic value until the experiment concluded (Fig. 1).
The highest methane production in the study was found in the 1 R. okamurae-3 OMSW co-digestion mixture, for which 454 ± 69 mL CH4 g−1 VS was obtained. The co-digestion mixture 1 R. okamurae- 3 OMSW improved the methane yield of 0 R. okumurae-1 OMSW and 1 R. okamurae-0 OMSW by 8.6% and 157%, respectively (0 R. okamurae-1 OMSW = 418 ± 56 mL CH4 g−1 VS and 1 R. okamurae-0 OMSW = 177 ± 15 mL CH4 g−1 VS). The co-digestion mixture 1 R. okamurae- 1.5 OMSW achieved a methane yield of 368 ± 42 mL CH4 g−1 VS. Similar value was found in the co-digestion mixture 1 R. okamurae- 1 OMSW (380 ± 15 mL CH4 g−1 VS), not observing significant differences between these co-digestion mixtures. The lowest methane yield values were obtained for the AD of the invasive alien macroalgae alone (R. okamurae, 177 ± 15 mL CH4 g−1 VS).
Benefits of anaerobic co-digestion
The inoculum is a microbial consortium that carries out the anaerobic digestion process. One of the major drawbacks of AD can be found in the susceptibility of the microbial community to changes in operational parameters and the presence of inhibitory substances, mainly in the final step of the process, methanogenesis. The accumulation of inhibitory substances during AD process is often a result from feeding the reactor with unbalanced C/N ratio substrates. There are numerous anaerobic co-digestion studies where it has been seen that a feeding substrate C/N ratio of between 25 and 30 is the optimal one to avoid the formation of inhibitory substances in the process and, therefore, to facilitate the anaerobic process of methane production (Allen et al. 2013). Rugulopteryx okamurae biomass used in this study had a C/N ratio of 15.2 (Table 1), being below the optimum for AD process. Co-digestion with a C-rich substrate, such as OMSW (C/N ratio 31.4), helped to balance the ratio, obtaining values of 27.4, 24.9, and 23.3 for co-digestions 1 R. okamurae- 3 OMSW; 1 R. okamurae- 1.5 OMSW; and 1 R. okamurae- 1 OMSW, respectively (Table 2).
Table 2 shows the biodegradability values (percentage of organic matter eliminated after AD process) which were obtained for each co-digestion mixture and for the sole OMSW and macroalgae. The highest biodegradability was obtained with the 1 R. okamurae- 1 OMSW mixture (87%). Very similar values were obtained with the co-digestions of 1 R. okamurae-3 OMSW (85%) and 1 R. okamurae- 1.5 OMSW (81%). On the other hand, with the mono-digestions of R. okamurae and OMSW, biodegradability values of 77% and 63%, respectively, were reached. The AD of the sole R. okamurae presented a C/N ratio of 15.2 at the beginning of the experiment, being the final concentration of ammonium in its digestate 1645 ± 22 mg L−1. At the end of the study, in OMSW reactors the final ammonium concentration was 970 ± 33 mg L−1, indicating the lowest nitrogen concentration among the different substrates tested. The co-digestion mixture 1 R. okamurae- 3 OMSW, with a C/N ratio of 27.4, presented a final ammonium concentration of 1125 ± 12 mg L−1. The 1 R. okamurae-1.5 OMSW mixture reached a C/N ratio of 24.9, obtaining a final ammonium concentration in the anaerobic reactors of 1352 ± 25 mg L−1. And finally, the last mixture tested (1 R. okamurae- 1 OMSW) presented a C/N ratio at the beginning of the experiment of 23.3 and the final concentration of ammonium in the anaerobic reactor was 1338 ± 14 mg L−1.
Study of possible synergic effects
The experimental methane yields observed for each co-digestion mixture (Fig. 1) were compared to the calculated or theoretical methane yields based on the OMSW and R. okamurae methane yields separately, according to Eq. (3):
where 418 and 177 are the experimental methane yields (mL CH4 g−1 VSadded) obtained for OMSW and R. okamurae, respectively. X OMSW and Y R. okamurae are the proportions of OMSW and R. okamurae, respectively, in each co-digestion mixture.
In all cases tested, experimental methane yield or BMP values were higher than the calculated methane yields for the three co-digestion mixtures tested (Table 3). 26.8% for the co-digestion mixture 3 OMSW-1 R. okamurae, 13.9% for the co-digestion mixture 1.5 OMSW-1 R. okamurae, and 27.5% for the co-digestion mixture 1 OMSW-1 R. okamurae. Therefore, according to the increase in the BMP values, the biodegradability of the above mentioned co-digestion mixtures was also much higher than the biodegradability of the sole macroalga.
Kinetic studies
First-order kinetic model
Table 4 shows the kinetic parameters obtained from the application of the first-order model to the experimental data of methane production-time corresponding to the different batch anaerobic digestion assays of all the mixtures and single substrates carried out. Values after ± represent the standard deviations of each parameter. Figure 2 shows as an example the experimental points of methane production versus time in the same plot that the theoretical curve predicted by the first-order model for some of the different substrates (100% R. okamurae) and mixtures (1 R. okamurae—1.5 OMSW) tested. The good fit of the data indicated that the model adequately predicted the experimental data.
Variation in the experimental methane production with time (points) as well as the theoretical curve (solid lines) predicted by the first-order kinetic model for some of the cases tested (1 Rugulopteryx okamurae (R.o.)- 0 olive mill solid waste (OMSW) and 1 R.o.- 1.5 OMSW). Values represent mean ± standard error
Among the different co-digestion mixtures tested, the highest specific rate constants, k, was found for the mixtures 1 R. okamurae—1.5 OMSW and 1 R. okamurae- 3 OMSW with values of 0.066 and 0.042 days−1, respectively. These values were 5.1 and 3.2 times higher than that obtained for the single or mono anaerobic digestion of OMSW. On the other hand, the kinetic constant for the mixture 1 R. okamurae- 1.5 OMSW was 29% higher than that obtained for the anaerobic digestion of the sole macroalga.
Transference function model
Table 5 summarizes the parameters obtained from the application of the transference function model to the experimental data of cumulative methane production time (Fig. 1). The R2 values were higher than 0.988 in all cases. In addition, the low values of the standard deviations of the kinetic parameters and standard errors of estimates also pointed out a good fit of the experimental data to this suggested model for all cases assayed (Table 5). Figure 3 shows the experimental points of methane production versus digestion time as well as the theoretical curve predicted by the transference function model for some of the different mixtures (1 R. okamurae—3 OMSW and 1 R. okamurae—1.5 OMSW) tested as an example. The good adjustment of the data indicated that the model appropriately predicted the experimental data.
Variation in the experimental methane production with time (points) as well as the theoretical curve (solid lines) predicted by the transference function model for some of the cases tested (1 Rugulopteryx okamurae (R.o.)-3 olive mill solid waste (OMSW) and 1 R.o.- 1.5 OMSW). Values represent mean ± standard error
Discussion
The low VS content of the macroalgae is a major drawback for optimizing the anaerobic digestion process. Similar VS/TS ratio values of those obtaining in the experiment were previously described by Milledge and Harvey (2016), obtaining low methane yield values. The low C/N ratio value indicates the high nitrogen content of the biomass, placing the value of the C/N ratio below of the optimum value established for the biomethanization process (Zheng et al. 2021). In this work, co-digestion is proposed for optimizing the anaerobic process as a solution to valorise the R. okamurae biomass to prevent it from accumulating on Andalusian beaches and to be able to stop its expansion and for the need to adjust the C/N ratio to optimize the anaerobic digestion process. Due to its proximity and because it is one of the more polluting and main by-products in Andalusia (south Spain), the OMSW (main semi-solid by-product of the olive oil production process) is proposed in this study as C-rich co-substrate for anaerobic co-digestion together with the invasive alien macroalgae biomass. The high amount of VS present in OMSW makes it a highly potential substrate for AD process (Lee et al. 2015). This high C/N ratio value indicated the lignocellulosic nature and composition of the OMSW. Therefore, co-digestion was used in this study because the optimal C/N ratio for AD process is known to be between 25 and 30. All the C/N ratio values obtained with the co-digestion mixtures assayed were within the values established as optimal for the AD process (Allen et al. 2013).
In previous biomethanization studies, the high nitrogen content of the macroalgae was indicated as one of the main factors that inhibit methane production (Costa et al. 2012). Co-digestion would not only help to balance the C/N ratio, but it also helps to dilute the presence of toxins and inhibitory substances for the anaerobic digestion process, such as salt, in the case of macroalgae and phenols and lignocellulosic material for the OMSW (Fernández-Rodríguez et al. 2020).
The highest methane production in the study was found in the 1 R. okamurae-3 OMSW co-digestion mixture. The co-digestion mixture 1 R. okamurae- 3 OMSW improved the methane yield of 0 R. okumurae-1 OMSW and 1 R. okamurae-0 MSW by 8.6% and 157%, respectively. Sharply lower values were found by Elalami et al. (2020), who recorded a methane production value of 188 ± 19 mL CH4 g−1 VS for the mixture 50% olive pomace (by-product of the traditional olive oil extraction mill) and 50% macroalgal residues of Gelidium sesquipedale. The lowest methane yield values were obtained for the AD of the invasive alien macroalgae alone. Previous studies carried out in batch mode using as substrate the macroalgae Saccharina latissima (brown alga) and Palmaria palmata (red alga) reported a methane yield of 209 ± 15 mL CH4 g−1 VS and 257 ± 22 mL CH4 g−1 VS, respectively (Jard et al. 2012). These values are somewhat higher than those obtained for R. okamurae (177 ± 15 mL CH4 g−1 VS). Maiguizo-Diagne et al. (2019) used green macroalgae as AD substrate (Ulva lactuca and Codium tomentosusm) in BMP test, and achieved a final methane yield of 216 mL CH4 g−1 VS.
Recently, Thompson et al. (2021) studied different VS co-digestions mixtures of pelagic Sargassum (PS) and food waste (FW) in BMP tests. The mixture 75 PS:25 FW obtained a final methane yield of 97.46 ± 1.05 mL CH4 g−1 VS. For the 50 PS:50 FW co-digestion mixture, the biomethane obtained was 182.33 ± 2.61 mL CH4 g−1 VS. Meanwhile, the highest methane production was obtained in the mixture with the lowest PS concentration, 25 PS:75 FW; 201.67 ± 6.36 mL CH4 g−1 VS. A similar pattern has been found in the present study, where the highest biomethane production was found in the mixture with the lowest content of the macroalgae R. okamurae (1 R. okamurae-3 OMSW; 454 ± 69 mL CH4 g−1 VS).
The low biodegradability values obtained (Table 2) showed that the substrates used had a high complexity content. This result agrees with Sun et al. (2019). They reported a 75% of biodegradability of Laminaria digitata during anaerobic assays. Fernández-Rodríguez et al. (2019b) also found an OMSW biodegradability of 56%, very similar to that obtained in this experiment. In addition, the mentioned study also found an improvement in the biodegradability of different microalgae-OMSW mixtures compared with those obtained for the substrates separately. The biodegradability depends on the composition of the substrate used, being highly affected by the presence of organic compounds which are difficult to biodegrade, such as lignin (Sun et al. 2019). During AD process of the single invasive macroalgae (1 R. okamurae- 0 OMSW), a methane production of 177 ± 15 mL CH4 g−1 VS was obtained, and at the end of the experiment, the biodegradability of the macroalgae was 77%. Previous studies found that the methane production during the anaerobic digestion of brown algae is limited by the inaccessibility of bacteria to complex sugars (Thompson et al. 2020). In addition, it should be considered that marine macroalgae tend to have a high content of salt, which can accumulate in the reactor, being able to achieve the inhibition concentration for the AD process (Ometto et al. 2018; Sun et al. 2019).
According to Varnero Moreno (2011), for substrates with low C/N ratios, bacterial activity can be inhibited, due to the excessive amount of ammonia that can accumulate in the anaerobic reactor. It has been reported in the literature that ammonium values higher than 1500 mg L−1 are toxic for the anaerobic digestion process (Yenigün and Demirel 2013). However, during the AD of sole OMSW, the C/N ratio is above the upper limit established in the bibliography as optimal for the anaerobic digestion process (C/N = 31.4). OMSW is a substrate with high carbon content which would lead to a slower degradation due to the low nitrogen content reducing the multiplication and development rate of bacteria (Varnero Moreno 2011). In the different co-digestion mixtures tested, the C/N ratio was balanced, and therefore, values below the limit established as ammonium toxic in the anaerobic reactors were obtained.
In all cases tested, experimental methane yield or BMP values were higher than the calculated methane yields for the three co-digestion mixtures tested (Table 3). The synergy effects of the OMSW and R. okamurae co-digestions with the above percentages were clearly shown with these results, being the co-digestion mixture 1 OMSW-1 R. okamurae the combination that achieved the highest synergic effect. In addition, the methane yields of the three mixtures tested were always higher than the methane yield of the sole R. okamurae (177 mL CH4 g−1 VS), which is within the range of the methane yields reported for anaerobic digestion of other marine macroalgae such as Sargassum, Gracilaria, Laminaria, Ascophyllum, and Ulva, for which methane yields of between 140 and 280 mL CH4 g−1 VS were achieved (Saratale et al. 2018). High contents in salts, phenolic compounds, heavy metals (in some cases), sulfides, and volatile acids stand out, along with low nitrogen content which has been reported as the characteristics that hinder and inhibit the anaerobic digestion process of macroalgae, and determine their low biodegradability (Saratale et al. 2018). Particularly, regarding R. okamurae, recent information (https://www.elestrechodigital.com/tag/rugulopterix-okamurae/ accessed 13 June 2021) indicates that the increase in sea water temperature, the lengthening of the summer months, and the changes in the wind regimes are promoting the proliferation of this macroalga, in places such as the Strait of Gibraltar, a phenomenon common in the hottest months of the year. This is causing the population of the area and tourists to have to live every summer with these seaweeds that are gaining more and more ground (García-Gómez et al. 2020). Added to the serious damage that the algae is causing to sectors such as fishing or tourism is the costs that the withdrawal of tonnes of this algal biomass means for municipalities such as Tarifa and Algeciras, which only in 2020 have already invested more than 100,000 euros each to carry out these tasks, given the flooding suffered by the beaches every time the Levante wind drags tons of these macroalgae towards the coast (https://www.elestrechodigital.com/tag/rugulopterix-okamurae/ accessed 13 June 2021).
The first order kinetic model (Table 4) shown the high R2 values as well as the low values of standard deviations of the kinetic constants and standard errors of estimates indicated that the experimental data correctly fit the proposed model. Beltran et al. (2016) showed that the co-digestion of waste activated sludge (WAS) and the microalga Chlorella sorokiniana (Ch.s.) with percentages of 75%WAS-25% Ch.s. and 50% WAS-50% Ch.s. were 12% and 42% higher than that obtained for the single microalga. The high kinetic constant values for the abovementioned mixtures (1 R. okamurae—1.5 OMSW and 1 R. okamurae- 3 OMSW) can be attributed to the appropriate C/N ratios of the both mixtures (24.9 and 27.4, respectively) compared with that observed for the single substrates (31.4 and 15.2 for 1 OMSW and 1 R. okamurae, respectively). Recent studies have demonstrated efficient anaerobic processes when the C/N ratio is within the range of 25 and 30 (Yan et al. 2015). Carbon helps to supply energy to the anaerobic microorganisms whereas nitrogen is crucial for the growth of the bacterial population.
In addition, very recent studies (Fernández-Rodríguez et al. 2021a) have shown that the anaerobic co-digestion of 95% OMSW-5% Dunaliella salina achieved specific rate constant values of 0.19 days−1, a value 2.8 times higher than the maximum value reached in the present study for the mixture 1.5 OMSW-1 R. okamurae. The mentioned kinetic constant value for the mixture 95% OMSW-5% D. salina was also 12% higher than that obtained for the single OMSW, similar trend than that observed in the present research work. Dunaliella salina is a halophilic flagellate alga able to tolerate and acclimate to a wide range of salinities (Fernández-Rodríguez et al. 2021a).
On the other hand, the anaerobic co-digestion of the macroalga Sargassum sp. with glycerol (Gly) (with 0.5% TS Sargassum sp. and 3.0 g L−1 of Gly) and with waste fried oil (WFO) (1.31% TS Sargassum sp. and 0.88 g L−1 of WFO) increased 38% and 19%, respectively, the specific rate constant when compared to the value obtained in the mono-digestion of the single macroalga (Oliveira et al. 2015). In addition, similar first order kinetic constant values to that obtained for 1 R. okamurae (0.051 day−1) were reported for anaerobic mono-digestion of fresh Ulva lactuca (0.06 day−1), commonly known as sea lettuce. However, the kinetic constant increased up to values of 0.12 and 0.15 day−1 when fresh Ulva was co-digested with dairy slurry at ratios of 75% fresh Ulva-25% slurry and 50% fresh Ulva-50% slurry, respectively (Allen et al. 2013).
The good adjustment of the data indicated that the transference model appropriately predicted the experimental data. As can be seen, the highest value of the maximum methane production rate (Rmax) was found for the mixture 1 R. okamurae—1.5 OMSW (30.4 mL CH4 g−1 VS day−1), which was 1.6 and 2.2 times higher than the corresponding to the mono-digestions of the single OMSW (18.9 mL CH4 g−1 VS day−1) and sole R. okamurae (13.6 mL CH4 g−1 VS day−1), respectively. In the same way, it was recently reported that the maximum methane production rate, Rmax, for the anaerobic co-digestion of the mixture 95% OMSW- 5% D. salina increased by 34.7% compared with the value obtained for the single OMSW (Fernández-Rodríguez et al. 2021a).
The lowest values of Rmax and ultimate methane production (Bmax) were found for the single mono-digestion of R. okamurae. Most macroalgae usually have high contents in salts, which can lead to an accumulation of them in anaerobic digesters, causing an inhibition of the microorganisms responsible for the process of biomethanization (Tabassum et al. 2017). Others macroalgae, such as U. latuca and Ascophyllum nodosum, may contain high levels of sulfide and polyphenols, respectively, compounds that from certain concentrations are inhibitors of the anaerobic digestion process (Tabassum et al. 2016). In general, this type of organisms also tends to have a high protein content that often limits the energy produced in anaerobic digestion processes due to their inhibition by the high concentration of ammoniacal nitrogen released (Tabassum et al. 2017), which have an inhibitory effect on anaerobic microorganisms, especially methanogenic archaea.
On the other hand, the maximum methane production rate (Rmax) values found in anaerobic co-digestion of mixtures of defatted spent coffee grounds (DSCG) and the macroalga Cladaphora glomerata (C.g.) at ratios of 75% DSCG-25% C.g. and 50% DSCG-50% C.g. (7.8 and 9.1 mL CH4 g−1 VS day-1), respectively, were much lower than those obtained in the present research (29.6–30.4 mL CH4 g−1 VS day-1) for similar ratios of OMSW- R. okamurae. The lower Rmax values achieved for the mixtures of DSCG-C.g. can be attributed to the higher lignin contents of C. glomerata (44.5% w/w), value much higher than that contained in the other substrates (Atelge et al. 2021). By contrast, R. okamurae as most brown macroalgae are characterized by its little or no contain in lignin, being laminarin, mannitol, and alginate their major carbohydrates (Tagaki et al. 2018).
Conclusions
The valorisation of the invasive alien macroalga biomass of R. okamurae is the key for stopping its quick expansion. This work shows the feasibility of the anaerobic digestion and biogas recovery from this macroalga. Methane yield and biodegradability values of up to 177 mL CH4 g−1 VSadded and of 77%, respectively, were achieved for the AD of the macroalga alone. However, this work also demonstrated an improvement in biomass valorisation by anaerobic co-digestion of the macroalga with a C-rich substrate such as the olive mill solid waste (OMSW). The co-digestion improved the methane yield of the macroalga alone by 157% as well as the biodegradability of the single R. okamurae, achieving an increase in its degradability of up to 11%. The first-order and the transference function models showed a good fit to the experimental results in all cases studied and, thus, could describe the kinetics of the co-digestion of different mixtures of R. okamurae and OMSW as well as the mono-digestions of the sole substrates. The highest values for the kinetic constant (k) and the maximum methane production rates (Rmax) were achieved for the co-digestion mixture 1 R. okamurae.—1.5 OMSW, which revealed the robustness of the co-digestion of this mixture compared with the single digestions of OMSW and single R. okamurae.
Data availability
All data generated or analyzed during this study are included in the article.
References
Abad V, Avila R, Vicent T, Font X (2019) Promoting circular economy in the surroundings of an organic fraction of municipal solid waste anaerobic digestion treatment plant: biogas production impact and economic factors. Bioresour Technol 283:10–17
Allen E, Browne J, Hynes S, Murphy JD (2013) The potential of algae blooms to produce renewable gaseous fuel. Waste Manag 33:2425–2433
Altamirano Jeschke M, De la Rosa Álamos J, Martínez Medina J (2016) Arribazones de la especie exótica Rugulopteryx okamurae (E.Y. Dawson) en el estrecho de Gibraltar. RIUMA, http://hdl.handle.net/10630/12433
APHA, Awwa, WEF (2012) Standard Methods for examination of water and wastewater, 22nd edn. American Public Health Association, Washington
Atelge AR, Atabani AE, Abut S, Caya M, Eskicioglu C, Semaan C, Lee Ch, Yildiz YS, Unalan S, Mohanasundaram S, Duman F, Kumar G (2021) Anaerobic co-digestion of oil-extracted spent coffee grounds with various wastes: experimental and kinetic modeling studies. Bioresour Technol 322:124470
Barbot IN, Hashem AG, Roland B (2016) A review on the valorization of macroalgal wastes for biomethane production. Mar Drugs 14:120
Beltran J, Jeison D, Fermoso FG, Borja R (2016) Batch anaerobic co-digestion of waste activated sludge and microalgae (Chlorella sorokiniana) at mesophilic temperature. J Environ Sci Health A 51:847–850
Costa JC, Gonçalves PR, Nobre A, Alves MM (2012) Biomethanation potential of macroalgae Ulva spp. and Gracilaria spp. and in co-digestion with waste activated sludge. Bioresour Technol 114:320–326
Convention on biological Diversity (2017) Decision Strategic Plan for Biodiversity 2011–2020. Available at: https://www.cbd.int/decision/cop/?id=12268. Accessed Sept 2016
De Paula JCD, Vallim MA, Teixeira VL (2011) What are and where are the bioactive terpenoids metabolites from Dictyotaceae (Phaeophyceae). Rev Bras Farmacog 21:216–228
Donoso-Bravo A, Perez-Elvira SI, Fernández-Polanco F (2010) Application of simplified models for anaerobic biodegradability tests. Evaluation of pre-treatment processes. Chem Eng J 160:607–614
El Aamri F, Idhalla M, Tamsouri MN (2018) Occurrence of the invasive brown seaweed Rugulopteryx okamurae (E.Y. Dawson) I.K. Hwang, W.J. Lee & H.S. Kim (Dictyotales, Phaeophyta) in Morocco (Mediterranean Sea). MedFAR 1:92–96
Elalami D, Monlau F, Carrere H, Abdelouahdi K, Oukarroum A, Zeroual Y, Barakat A (2020) Effect of coupling alkaline pretreatment and sewage sludge co-digestion on methane production and fertilizer potential of digestate. Sci Total Environ 743:140670
Fernández-Rodríguez MJ, De la Lama D, Jiménez AM, Borja R, Rincón B (2019a) Influence of the cell wall of Chlamydomonas reinhardtii on anaerobic digestion yield and its anaerobic co-digestion with a carbon-rich substrate. Process Saf Environ Prot 128:167–175
Fernández-Rodríguez MJ, De la Lama D, Jiménez AM, Borja R, Rincón B (2019b) Anaerobic co-digestion of olive mil solid waste and microalgae Scenedesmus quadricauda: effect of different carbon to nitrogen ratios on process performance and kinetics. J Appl Phycol 31:3583–3591
Fernández-Rodríguez MJ, de la Lama-Calvente D, Jiménez-Rodríguez A, Pino-Mejías R, Borja R, Rincón B (2020) Impact of soft hydrothermal pre-treatments on the olive mill solid waste characteristics and its subsequent anaerobic digestion. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-00759-1
Fernández-Rodríguez MJ, de la Lama D, Jiménez-Rodríguez A, Borja R, Rincón B (2021a) Evolution of control parameters in biochemical methane potential tests of olive mill solid waste (OMSW), thermal pre-treated OMSW, and its co-digestion with Dunaliella salina. J Appl Phycol 33:419–429
Fernández-Rodríguez MJ, Puntano NF, Mancilla-Leytón JM, Borja R (2021b) Batch mesophilic anaerobic co-digestion of spent goat straw bedding and goat cheese whey: comparison with the mono-digestion of the two sole substrates. J Environ Manage 280:111733
Ferreira JS, Volschan I Jr, Cammarota MC (2018) Enhanced biogas production in pilot digesters treating a mixture of sewage sludge, glycerol, and food waste. Energy Fuels 32:6839–6846
García-Gómez JC, Sempere-Valverde J, Ostalé-Valriberas E, Martínez M, Olaya-Ponzone L, González AR, Parada JA (2018) Rugulopteryx okamurae (EY Dawson) IK Hwang, WJ Lee & HS Kim (Dictyotales, Ochrophyta), alga exótica ‘explosiva’en el Estrecho de Gibraltar. Observaciones preliminares de su distribución e impacto. Almoraima 48:97–113
García-Gómez JC, Sempere-Velarde J, Roi-Gónzalez A, Martínez-Chacón M, Olaya-Ponzone L, Sánchez-Moyano E, Ostalé-Valriberas E, Megina C (2020) From exotic to invasive in record time: the extreme impact of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in the strait of Gibraltar. Sci Total Environ 704:135408
Geider R, La Roche J (2002) Redfield revisited: variability of C:N: P in marine microalgae and its biochemical basis. Eur J Phycol 37:1–17
Grosser A (2017) The influence of decreased hydraulic retention time on the performance and stability of co-digestion of sewage sludge with grease trap sludge and organic fraction of municipal waste. J Environ Manag 203:1143–1157
Hernandez-Carmona G, Rodríguez YE, Arvizu DL, Reyes R, Murillo DI, Muñoz M (2012) Avances tecnológicos en la producción de alginatos en Mexico. Ingeniería, Investigación y Tecnología 13:2–11
Huang ZG (1994) Marine species and their distributions in China’s Seas. China Ocean Press, Beijing
Hwang IK y otros (2009) Taxonomic reappraisal of Dilophus okamurae (Dictyotales, Phaeophyta) from the western Pacific Ocean. Phycologia 48:1–12
Jard G, Jackowiak D, Carrère H, Delgenes JP, Torrijos M, Steyer JP, Dumas C (2012) Batch and semi-continuous anaerobic digestion of Palmaria palmata: comparison with Saccharina latissima and inhibition studies. Chem Eng J 209:513–519
Jha B, Isha A, Trivedi A, Chandra R, Subbarao PMV (2021) Anaerobic co-digestion of rice straw and de-oiled rice bran for biomethane production. Energy Rep 7:704–710
Lee D-J, Lee S-Y, Bae J-S, Kang J-G, Kim K-H, Rhee S-S, Park J-H, Cho J-S, Chung J, Seo D-C (2015) Effect of volatile fatty acid concentration on anaerobic degradation rate from field anaerobic digestion facilities treating food waste leachate in South Korea. J Chem 2015:640717
Li L, Kong X, Yang F, Li D, Yuan Z, Sun Y (2012) Biogas production potential and kinetics of microwave and conventional thermal pretreatment of grass. Appl Biochem Biotechnol 166:1183–1191
Li L, Li Y, Sun Y, Yuan Z, Lv P, Kang X, Zhang Y, Yang G (2018) Influence of the feedstock ratio and organic loading rate on the co-digestion performance of Pennisetum hybrid and cow manure. Energy Fuels 32:5171–5180
Maiguizo-Diagne H, Ndiaye NA, Ndour-Badiane Y, Masse D, Torrijos M, Sousbie P, Lamine Gaye M, Ndoye I, Hamelin J, Fall S (2019) The use of green macroalgae (Ulva lactuca and Codium tomentosum) that have a high methane potential, as a source of biogas in Senegal. J Appl Biosci 132:13404
Milledge JJ, Harvey PJ (2016) Ensilage and anaerobic digestion of Sargassum muticum. J Appl Phycol 28:3021–3030
Morand P, Briand X (1996) Excessive growth of macroalgae: a symptom of environmental disturbance. Bot Mar 39:491–516
Moreno-Maroto JM, Uceda-Rodríguez M, Cobo-Ceacero CJ, de Hoces MC, MartínLara MÁ, Cotes-Palomino T, Martinez-Garcia C (2019) Recycling of ‘alperujo’ (olive pomace) as a key component in the sintering of lightweight aggregates. J Clean Prod 239:118041
Navarro-Barranco C, Muñoz-Gómez B, Saiz D, Ros M, Guerra-García JM, Altamirano M, Moreira J (2019) Can invasive habitat-forming species play the same role as native ones? The case of the exotic marine macroalga Rugulopteryx okamurae in the Strait of Gibraltar. Biol Invasions 21:3319–3334
Ocaña O, Alfonso-Carrillo JM, Ballesteros E (2016) Massive proliferation of a dictyotalean species (Phaeophyceae, Ochrophyta) through the strait of Gibraltar. Rev Acad Canaria Cienc 28:165–169
Oliveira JV, Alves MM, Costa JC (2015) Optimization of biogas production from Sargassum sp. using a design of experiments to assess the co-digestion with glycerol and waste frying oil. Bioresour Technol 175:480–485
Ometto F, Berg A, Bjorn A, Safaric L, Svensson H, Karlsson A (2018) Inclusion of Saccharina latissima in conventional anaerobic digestion systems. Environ Technol 39:628–639
Parra Huertas RA (2015) Digestión anaeróbica: mecanismos biotecnológicos en el tratamiento de aguas residuales y su aplicación en la industria alimentaria. Producción Limpia 10:142–159
Paul S, Dutta A (2018) Challenges and opportunities of lignocellulosic biomass for anaerobic digestion. Resour Conserv Recycl 130:164–174
Pinto-Ibieta F, Serrano A, Jeison D, Borja R, Fermoso FG (2016) Effect of cobalt supplementation and fractionation on the biological response in the biomethanization of olive mill solid waste. Bioresour Technol 211:58–64
Raposo F, de la Rubia MA, Borja R, Alaiz M (2008) Assessment of a modified and optimised method for determining chemical oxygen demand of solid substrates and solutions with high suspended solid content. Talanta 76:448–453
Saratale RG, Kumarb G, Banuc R, Xias A, Periyasamye G, Satarale GD (2018) A critical review on anaerobic digestion of microalgae and macroalgae and co-digestion of biomass for enhanced methane generation. Bioresour Technol 262:319–332
Scarcelli PG, Serejo ML, Paulo PL, Boncz MÁ (2020) Evaluation of biomethanization during co-digestion of thermally pretreated microalgae and waste activated sludge, and estimation of its kinetic parameters. Sci Total Environ 706:135745
Sempere-Valverde J, Ostalé-Valriberas E, Maestre M, Aranda RG, Bazairi H, Espinosa F (2021) Impacts of the non-indigenous seaweed Rugulopteryx okamurae on a Mediterranean coralligenous community (Strait of Gibraltar): The role of long-term monitoring. Ecol Indic 121:107135
Sun H, Kovalovszki A, Tsapekos P, Alvarado-morales M (2019) Co-digestion of Laminaria digitata with cattle manure: a unimodel simulation study of both batch and continuous experiments. Bioresour Technol 276:361–368
Tabassum MR, Wall DM, Murphy JD (2016) Biogas production generated through continuous digestion of natural and cultivated seaweeds with dairy slurry. Bioresour Technol 219:228–238
Tabassum MR, Xia A, Murphy JD (2017) Potential of seaweed as a feedstock for renewable gaseous fuel production in Ireland. Renew Sustain Energy Rev 68:136–146
Tagaki K, Kuroda K, Ueda M (2018) Platform construction of molecular breeding for utilization of brown macroalgae. J Biosci Bioeng 125:1–7
Thompson TM, Young BR, Baroutian S (2020) Efficiency of hydrothermal pretreatment on the anaerobic digestion of pelagic sargassum for biogas and fertiliser recovery. Fuel 279:118527
Thompson TM, Young B, Baroutian S (2021) Enhancing biogas production from caribbean pelagic Sargassum utilising hydrothermal pretreatment and anaerobic co-digestion with food waste. Chemosphere 275:130035
van Tussenbroek BI, Arana HAH, Rodríguez-Martínez RE, Espinoza-Avalos J, Canizales-Flores HM, González-Godoy CE, Collado-Vides L (2017) Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Mar Pollut Bull 122:272–281
Varnero Moreno MT (2011) Manual de biogás. Proyecto CHI/00/G32 Chile: Remoción de Barreras Para La Electrificación Rural Con Energías Renovables
Varnero MT, Galleguillos K, Guerrero D, Suárez J (2014) Producción de Biogás y Enmiendas Orgánicas a Partir del Residuo Olivícola (Alperujo). Información Tecnológica 25:73–78
Wang M, Lee E, Dilbeck MP, Liebelt M, Zhang Q, Ergas SJ (2017) Thermal pre-treatment of microalgae for biomethane production: experimental studies, kinetics and energy analysis. J Chem Technol Biotechnol 92:399–407
Yan Z, Song Z, Li D, Yuan Y, Liu X, Zheng T (2015) The effects of initial substrate concentration, C/N ratio, and temperature on solid-state anaerobic digestion from composting rice straw. Bioresour Technol 177:266–273
Yenigün O, Demirel B (2013) Ammonia inhibition in anaerobic digestion: a review. Process Biochem 48:901–911
Zheng Z, Cai Y, Zhang Y, Zhao Y, Gao Y, Cui Z, Hu Y, Wang X (2021) The effects of C/N (10–25) on the relationship of substrates, metabolites, and microorganisms in “inhibited steady-state” of anaerobic digestion. Water Res 188
Acknowledgements
J. Llanos wishes to thank the academic mobility and exchange program (PIMA). The PIMA program is a mobility initiative for undergraduate students promoted by the Ibero-American States Organization. The authors also wish specially to thank J.C. García-Gómez and the Laboratory of Marine Biology of the University of Seville for providing the Invasive alien macroalga Rugulopteryx okamurae.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was financed by the Spanish Ministry of Science and Innovation through Project PID2020-114975RB-100.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de la Lama-Calvente, D., Fernández-Rodríguez, M.J., Llanos, J. et al. Enhancing methane production from the invasive macroalga Rugulopteryx okamurae through anaerobic co-digestion with olive mill solid waste: process performance and kinetic analysis. J Appl Phycol 33, 4113–4124 (2021). https://doi.org/10.1007/s10811-021-02548-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02548-3