Abstract
This paper reports the results of supercritical carbon dioxide (scCO2) extraction of β-carotene from Dunaliella salina as potential alternative to conventional organic solvent extraction. In pilot-scale scCO2 experiments, the pressure, temperature, and co-solvent concentration were varied. The supercritical extraction at 500 bar, 70 °C, and 10 wt% ethanol as co-solvent yielded in the highly efficient pigment recovery of over 90%. Techno-economic assessment demonstrated higher energy consumption for the scCO2 extraction that was compensated by lower solvent costs. Thus, comparable pigment production costs to the reference extraction with n-hexane were estimated for the scCO2 process. Due to the green solvent properties of scCO2 and ethanol, this approach is highly promising for extraction of algal biomass in industrial scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Products derived from natural resources enrich the market of the chemical and pharmaceutical industry. However, the extraction of natural products is challenging since their resources are composed of various components with different physical and chemical properties. One high-value natural product is the non-polar, red-orange pigment β-carotene. It is used as a colorant, antioxidant, and immunostimulator in the nutrition, cosmetic, and pharmaceutical industry (Dias Ribeiro et al. 2011; Kyriakopoulou et al. 2015). This precursor of vitamin A belongs to the chemical class of isoprenoids (terpenoids) and is produced by fungi, microalgae, or higher plants (Del Campo et al. 2007). One natural producer of β-carotene is the green microalga Dunaliella salina which is commercially cultivated in open ponds (Borowitzka 2013; Borovkov et al. 2020). The pigment is produced as a response of high solar irradiation, high salinity, and nitrogen deprivation of the microalga. Concentrations up to 14 wt% are accumulated together with TAGs in the lipid granules within the chloroplast (Borowitzka and Borowitzka 1990). With that, Dunaliella is the most important producer of natural β-carotene and subject of significant industrial and academic efforts. The industrial production of the microalga started 1986 in the USA, Israel, and Australia near the sea in natural formed shallow lakes or simple raceway ponds (Ben-Amotz and Avron 1989; Borowitzka and Borowitzka 1990).
The global market for all carotenoids was US$ 1.5 billion in 2017 and is expected to reach US$ 2.0 billion by year 2022. A growing demand for naturally derived products in the food, beverage, and dietary supplement industries is expected to be a significant driving force in the future global market.
Natural β-carotene by D. salina is composed of a mixture of 9-cis, all-trans, and 15-cis β-carotene. With this isomer composition, the algae-derived pigment is claimed to be healthier than the synthetically produced all-trans form (Ben-Amotz 1999; Tafreshi and Shariati 2009). The 9-cis β-carotene has been identified as an important biomolecule in the treatment of retinal dystrophy, chronic plaque psoriasis, and atherosclerosis (Xu and Harvey 2019). The relative difficulty in the synthesis of 9-cis β-carotene is reflected in the price ratio of all-trans to 9-cis β-carotene (1:8300) (Harvey and Ben-Amotz 2020).
The extraction of lipophilic compounds such as β-carotene from dried powder can be done by combinations of polar and non-polar petrochemical-based solvents (e.g., n-hexane, methanol, acetone, or various mixtures) (Harvey and Ben-Amotz 2020). The extraction of β-carotene and other carotenoids requires mild conditions regarding temperature, light exposure, and time to minimize auto-oxidation and isomerization (Arvayo-Enriquez et al. 2013; Herrero et al. 2006). Nowadays, n-hexane is one of the most commonly used solvents to extract non-polar carotenoids from natural materials (Yara-Varon et al. 2016). However, the risk of product contamination or loss of quality due to organic solvent residuals in the extracts provokes a rising need of innovative extraction methods. One potential approach in this direction is the supercritical fluid extraction which makes use of the unique solvent properties of supercritical fluids or gases which are heated and pressurized above their critical points Pc and Tc. The density of the supercritical fluid is comparable to liquids, whereas the viscosity is similar to that of gases. The supercritical physical property enhances the heat and mass transfer of the solute to the extract. Consequently, the diffusivity of the solvent is higher than that of conventional liquids resulting in an improved solvent power (Meullemiestre et al. 2015). Furthermore, the selectivity of a supercritical fluid can be influenced by altering the pressure and the temperature within the supercritical window, allowing a more precise extraction of specific products (Brunner 2005; Kitada et al. 2009; Macias-Sanchez et al. 2009a, 2009b; Ruen-ngam et al. 2012). The methodology has the advantage of a complete solvent recovery from the extracts without the need of distillation.
So far, a few studies have reported the supercritical fluid extraction of valuable lipophilic compounds, carotenoids (Obeid et al. 2018; Leone et al. 2019), or especially the β-carotene from algal biomass (Molino et al. 2019). However, most of the studies are carried out in the lab scale (Jaime et al. 2007; Macias-Sanchez et al. 2008; Macias-Sanchez et al. 2009a; Hosseini et al. 2017; Molino et al. 2019). On the industrial scale, the method is applied for astaxanthin recovery from Haematococcus pluvialis by Cyanotech in Hawaii. Recently, the environmental and economic perspectives of the extraction for β-carotene extraction from D. salina were assessed in a simulation study (Espada et al. 2020). The co-solvents have been reported as beneficial for extraction of carotenoids from various microalgae (Machmudah et al. 2006; Nobre et al. 2006). The addition of an entrainer can provide a significant boost; however, the function principles of the entrainers are still under debate (Shimizu and Abbott 2016). Ethanol was identified as the best co-solvent for extraction of β-carotene D. salina by the prediction based on the Hansen theory (Tirado and Calvo 2019). Ethanol as co-solvent was investigated experimentally in supercritical fluid extraction also by Molino et al. (2018) for astaxanthin and lutein extraction from H. pluvialis.
In the present study the potential of supercritical fluids as an alternative to conventional solvent extraction of D. salina biomass is evaluated. The first envisaged target of the study was the optimization of the extraction procedure by comparing seven solvents or solvent mixtures for the Dunaliella biomass. Secondly, the potential of supercritical fluid extraction was assessed by means of experimental investigation in a pilot scale. Finally, the energy consumption and the economics of the technique for the β-carotene recovery were compared to those of the state-of-art extraction with n-hexane.
Materials and methods
Biomass
Spray-dried Dunaliella salina powder (supplied by Denk Ingredients GmbH, Germany, Art. no: 967996) was used in all extraction experiments. The β-carotene content of the biomass varied slightly between the charges; however, maximally a content of 47.8 mg g−1 was determined in the charges by the analytical method of Lichtenthaler (1987). The biomass charges used in our study contained entirely all-trans isomer and negligible amount of 9-cis isomer as confirmed by HPLC measurement. The total neutral lipid amount of the biomass powder was 11.5 wt% (Pirwitz et al. 2016). According to Ben-Amotz and Avron (1989), spray drying, freeze-drying, and drum drying are appropriate for Dunaliella biomass, resulting in a minor variation of the quality of the biomass powder and high stability of the pigment. Therefore, we might assume a negligible effect of the used drying method on the exaction results.
Selection of appropriate organic solvents
For comparison, conventional solvent extraction experiments were conducted using THF (tetra hydro furan), acetone, n-hexane, ethanol (96 Vol% and 99 Vol%), ethyl acetate, and chloroform/methanol (2:2.5). The solvents were selected based on their experimental determined solubilities of β-carotene according to Craft and Soares (1992). Since chloroform/methanol mixture is frequently used in literature to extract the pigments (e.g., Lamers et al. 2010; Mezzomo and Ferreira 2016), a chloroform/methanol ratio of 2:2.5 was applied for β-carotene extraction instead of using the solvents separately. Ethanol was applied as 99 Vol% and 96 Vol% concentration in water since the presence of low amounts of water in ethanol is expectedly beneficial for the extraction.
Determination of β-carotene concentrations in the extracts
Standard calibration curves of β-carotene absorption were prepared in the above-mentioned solvents to determine the pigment concentration in the extracts according to the protocol of Craft and Soares (1992). In short, a stock solution containing 3 mg mL−1 of a β-carotene standard (Sigma-Aldrich, Germany) was prepared in THF supplemented with 0.1% (v/v) of the antioxidant butylated hydroxytoluene. The stock was diluted 1000-fold with the respective organic solvent (referred to as stock solution 2). In the next step, the wavelength λmax of the absorbance maximum was determined for each solvent stock solution 2 by analyzing the complete spectrum of β-carotene from 200 to 700 nm (see Table S1). Afterwards, the absorbance at λmax was measured spectrophotometrically in the respective solvent for various defined pigment concentrations. With the data, the observed linear regression between the absorbance and β-carotene concentration was used to calculate the β-carotene concentrations in the extraction samples.
Extraction of β-carotene by organic solvents
First, the influence of the extraction time and temperature as well as water content (in ethanol) on the extraction was investigated experimentally. The experiments were conducted in triplicates of sealed 100 mL shaking asks filled with 25 mL of the respective solvent and 100 mg of D. salina. The solvent to biomass ratio was defined according to the experimental determined solubility values of β-carotene (Craft and Soares 1992). The samples were extracted in the dark using a rotary shaking incubator with a frequency of 100 rpm. Extraction for 30, 60, 90, 120, and 180 min were applied at 20 °C to examine the influence of time on the extraction efficiency. Furthermore, the β-carotene extraction efficiency was analyzed at the temperatures of 20, 40, and 60 °C during a constant time of 30 min.
The efficiency of the extraction was quantified by absorbance. Therefore, duplicate suspension samples were taken from each shaking ask after extraction, transferred into non-transparent, brown tubes and centrifuged at 5000 rpm for 5 min to separate the algal sample and solvent. The supernatant was analyzed spectrophotometrically at the previously determined wavelengths λmax (Table S1). Pigment concentrations were quantified using the regression standards (Determination of β-carotene concentrations in the extracts Section ).
Extraction of β-carotene by scCO2
The extraction experiments with supercritical CO2 were carried out in pilot scale in the facilities of the Fraunhofer Center for Chemical-Biotechnological Processes (CBP, Leuna, Germany). Figure S2 schematically illustrates the design of the continuously operating extraction unit. For each experimental run, 100 g D. salina biomass was placed into a 2 L extractor vessel and mixed with approximately 1300 g stainless steel cylindrical filling rings (5 × 5 × 0.3 mm, Raschig GmbH, Germany). The Raschig rings in the extractor vessel mitigated the agglomeration of the biomass during the experiments. The extractor was sealed with sintered metal plates on both ends. The plates were covered by cellulose filter paper (2.5 μM, Cat. no.: 1005320, Whatman, UK) to avoid biomass intrusion through the plates into downstream pipelines and vessels. CO2 (operated at 62 bar) was delivered from a feed tank that was supplied by an external CO2 source. The solvent CO2 was pressurized up to the investigated extraction pressure by a compressor and tempered to the extraction temperature by a heat exchanger. The co-solvent ethanol (96 Vol%) was added to the CO2 stream from a separate feed tank connected close to the extractor vessel inlet. The influence of the co-solvent ethanol was analyzed by varying the ethanol mass fraction in the solvent stream at levels of 0, 4, and 10 wt%. The temperature and pressure of ethanol were adjusted similar to the CO2 stream by a heat exchanger and a compressor. The total mass ow of the extracting solvents CO2 and ethanol was measured by a flow meter, and it was approximately 4–5 kg h−1. The extraction time was 180 min in all supercritical CO2 extraction experiments.
During the experiments, the temperatures of 50, 60, and 70 °C were applied for the extractor. The extraction pressures of 300, 400, and 500 bar were investigated. After passing the extractor, the solvent-extract mixture was conducted into a separation vessel operating at 50 °C and 62 bar. Here, CO2 evaporated and separated from the ethanol-extract mixture, and it was recycled back into the CO2 tank. For sampling of the liquid ethanol-extract, an outlet valve was opened allowing the complete draining of the separator vessel content into preweighed brown glass flasks. In experiments without co-solvent, the separator vessel was filled with 500 mL ethanol prior to an extraction experiment to facilitate the collection of extracted β-carotene.
Extract samples taken during the experiments were analyzed spectrophotometrically (spectrophotometer Type 7310, Jenway, UK) at the specific wavelength determined as described in Determination of β-carotene concentrations in the extracts Section. A standard curve of β-carotene in 96 Vol% ethanol was used to quantify the pigment concentration in the samples.
Determination of scCO2 extraction efficiency and β-carotene solubility
Due to the high extraction power of acetone (as reported in Organic solvent extraction of β-carotene from D. salina Section) for β-carotene, this solvent was used to compare the extraction efficiency under varied scCO2 extraction conditions. For this, after every scCO2 extraction experiment, the biomass samples were collected and stored until analysis in non-transparent, dark tubes at −20 °C. The remnant amount of β-carotene in the scCO2-extracted biomass was determined by a subsequent acetone extraction and compared to that of the fresh biomass. All samples were treated equally in order to quantitatively compare the residue amount of β-carotene in the extracted samples: The samples were dried in the dark at 40 °C for 1 h to remove residual traces of the co-solvent ethanol. Afterwards, the biomass samples were weighted into aluminum foil-covered 15 mL tubes (n = 3-4) in portions of 0.5–1.2 mg. One milliliter acetone per 0.1 mg biomass was added into the tubes. Samples were mixed and incubated in the dark at 500 rpm and 20 °C for 1 h. Subsequently, the tubes were centrifuged to separate the biomass from the solvent, and triplicates of supernatant acetone were measured at 455 nm. The β-carotene concentration was calculated using a standard curve (see Determination of β-carotene concentrations in the extracts Section). The extraction efficiency ηE was determined based on the following equation:
where ct0 and ctEND are the measured β-carotene concentrations in acetone using the fresh biomass or biomass after extraction in mg \( {g}_{dw}^{-1} \). The same re-extraction with acetone was applied for n-hexane-extracted biomass to quantify the extraction efficiency of n-hexane. The solubility Se of β-carotene in scCO2 in mg g−1 CO2 was calculated according to the method of Danielski et al. (2007) as the slope of the linear phase of the extraction curves (phase I in Figure S1) reached in the experiments described in Extraction of β-carotene by scCO2 Section .
Energy and operating cost estimation of scCO2 extraction
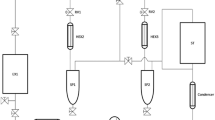
To quantify the energy demand and the operating costs of the scCO2 extraction, the process model described in Pirwitz et al. (2015) was modified by replacing the unit of n-hexane extraction with that of scCO2 extraction as schematically illustrated in Fig. 1. The energy demand for the product extraction comprises the energy required for solvent delivery as well as for compression, heating, and cooling.
For the techno-economic calculation, one extraction condition (500 bar, 70 °C, and a co-solvent addition of 10 wt% ethanol) was taken as reference as the best extraction yield was obtained under this condition in the pilot scale. The energy demand for the compression of CO2 and EtOH was estimated according to Eq. 2 (Attard et al. 2015). Therefore, the specific enthalpies hi of 320.97 kJ \( {\mathrm{kg}}_{\mathrm{CO}2}^{-1} \)and 854.79 kJ\( {\mathrm{kg}}_{\mathrm{EtOH}}^{-1} \) were used to quantify the energy demand of compression EEi in kJ (NIST 2016):
For the cost estimations, an extraction was assumed to be operated in a continuous mode using an isolated extractor with a working volume of 400 L and a biomass density of 0.5 kgdw L−1. The approach is similar to that applied for scCO2 extraction of ginger powder by Rosa and Meireles (2005). The estimated mass flows of the solvents were 2.12 kgCO2 L−1 h−1 and 0.24 kgEtOH L−1 h−1, and one sample was extracted 3 h. Accordingly, the solvent masses, msi, during the operation (tE= 3 h) was calculated to be 2550 kgCO2 and 288 kgEtOH. The heating up of the input flows to the extractor was estimated with an efficiency η of 80% (Delrue et al. 2013). The energy required for heating was calculated by Eq. 3:
where Qhi is the required heat energy, cpi is the heat capacity of the component i, ∆T is the temperature change, and mi is the mass of species i.
For the algal biomass, cpi = 1.25 kJ kg−1 K−1 was assumed (Orosz and Forney 2008). The cpi of EtOH and CO2 are 2.50 and 0.83 kJ kg−1 K−1, respectively.
After extraction, the mixture consisting of CO2, EtOH, and β-carotene was separated in a separation vessel at 62 bar and 50 °C. Therefore, the stream was cooled from 70 to 50 °C, and the energy for that was assumed to be provided by a cooling unit operated with cooling water. The required cooling energy Qci was estimated by Eq. 4 (Attard et al. 2015) using the coefficient of performance, COP = 1.875. At the given temperature, the heat capacities of EtOH and CO2 are 3.10 and 0.87 kJ kg−1 K−1, respectively.
The evaporation process was simulated with an efficiency ηv of 70% to compensate heat transfer limitation and boiling point elevation due to impurities in the EtOH extract solution. The heat of evaporation Qv, ETOH for the co-solvent EtOH was calculated using an evaporation enthalpy hv, ETOH of 841 kJ kg−1 (boiling point Tb = 78.37 °C) (NIST 2016):
Regarding solvent losses during recycling, a recovery efficiency of 99% was used in the calculations. The energy consumption of the n-hexane extraction was estimated based on the parameters and assumptions given in Pirwitz et al. (2015). In the cost estimation, we assumed the extraction efficiency of ηE = 86.4%. This efficiency value for n-hexane was obtained experimentally in our study by the re-extraction of the n-hexane-extracted biomass with acetone (for details, see Extraction of β-carotene by organic solvents Section). A semi-quantitative techno-economic comparison of scCO2 and n-hexane extraction was carried out (Energy and cost assessment of the extraction strategies Section). The initial costs of CO2, EtOH, and n-hexane were included in the daily operation costs assuming 330 production days of the year. The numeric values of the energy consumption for the unit operations are given Table S2, and the model was implemented in MATLAB (MathWorks). Uncertainties of the data were obtained by Monte-Carlo simulations as described in Pirwitz et al. (2015).
Results
Organic solvent extraction of β-carotene from D. salina
The present study aims to investigate the influence of different process parameters on the extraction results of β-carotene from D. salina biomass. Initially, we aim to identify the optimal organic solvent extraction conditions, which were used as reference to reliably compare the costs of conventional and supercritical fluid extraction.
The stability of pigments is dependent on the temperature, light, and the selected solvent (Tang and Chen 2000). Therefore, we initially looked for the optimal extraction time and temperature for β-carotene extraction in the lab-scale batches. Figure 2a illustrates the effect of time on the yield of extracted β-carotene reached at 20 °C. As seen in Fig. 2a, the best solvents under these conditions are acetone, THF, ethyl acetate, or the chloroform/MeOH mixture. Ethanol and n-hexane demonstrate lower β-carotene extraction yields being roughly half of those with the other solvents. Apart from the time, also the temperature affects the extraction yield by influencing the solute-solvent interaction. Based on the results shown in Fig. 2a, a time of 30 min was used in the study of the temperature effect. Figure 2b demonstrates the strong influence of the extraction temperature on the β-carotene yield in the batch experiments using the organic solvents.
The results above demonstrated the impact of time and temperature on the extraction results. We used this information to fix the reference conditions for β-carotene extraction by conventional organic solvents. Unexpected, the extraction efficiency of the industrially commonly applied solvent n-hexane was low in our experiments. Considering all investigated experimental conditions, acetone achieved the best extraction yields, even at 20 °C and a short extraction time of 30 min. In line with our study, one should note that acetone is applied also in the analytical determination of β-carotene (Lichtenthaler 1987). In consequence, acetone was selected as a reference solvent to assess and quantify the results of the supercritical fluid extraction.
scCO2 extraction of β-carotene from D. salina
CO2 is non-polar and therefore a promising option for the extraction of the lipophilic pigment β-carotene (Casas Cardoso et al. 2012). The solubility of the pigment in scCO2 is further enhanced by the use of polar co-solvents in scCO2 extraction (Chemat and Abert Vian 2014; Tirado and Calvo 2019). To analyze the effect of co-solvent on the β-carotene yield in our study, EtOH was added to the CO2 feed at levels of 4 and 10 wt%. Table 1 lists the influence of ethanol concentration on the extraction efficiency in the scCO2 extraction experiments. Here, one biomass batch was extracted for 180 min at 50 °C and 300 bar, with an approximate flow of scCO2 70.4 g min−1 and varying EtOH mass flow, respectively. Without EtOH only 4.3% of the β-carotene was extracted. By adding 4 wt% EtOH to the CO2 stream, a nearly threefold extraction efficiency of 12.2% was achieved. Further increase of the EtOH fraction to 10 wt% led to an additional enhancement of the extraction efficiency to 25.0%.
Even with the inclusion of the co-solvent EtOH, only a partial recovery of β-carotene was achieved (Table 1). Therefore, the most important parameters of supercritical fluid extraction (pressure and temperature) were varied in our pilot-scale study. Figure 3a shows the extraction efficiency at temperatures 50, 60, and 70 °C under the constant pressure of 300 bar. At temperatures 50 to 60 °C, the amount of extracted β-carotene was closely equal, although the solubility of the pigment in scCO2 was estimated to be lower at 60 °C than at 50 °C.
Influence of (a) temperature T and (b) pressure p on the scCO2 extraction yields of β-carotene from D. salina. (c) Solubility Se of β-carotene in scCO2 as a function of pressure. Se accounts for the slope of the linear phase of the extraction curves. In all experiments, 10 wt% EtOH was used as co-solvent. The scCO2 flow rate of was about 70.5 ± 2.7 g min−1. Error bars indicate one standard deviation of the average measurement value (n = 6)
The effect of temperature is complex and is not directly predictable (Tirado and Calvo 2019). In some research studies, the negative effect of increased temperatures on carotenoid extraction is reported, especially at low operating pressures (Ruen-ngam et al. 2012), but this was not visible in our study (see Fig. 3a). Here, highest extraction temperature 70 °C led to highest final β-carotene extraction efficiency. The same behavior has been reported in lab scale by Macias-Sanchez et al., 2009a, b for the carotene extraction of D. salina by scCO2 with or without EtOH as co-solvent. The coincidence of the physical properties was also visible for scCO2 extraction of lutein from C. vulgaris for 60 and 70 °C at 300 bar (Kitada et al. 2009). In their study, comparable pigment yields were obtained at both temperatures. Comparable results are reported for the temperature increase of lutein extraction from Scenedesmus and Chlorella vulgaris as well as the astaxanthin extraction from H. pluvialis (Machmudah et al. 2006; Kitada et al. 2009; Yen et al. 2012).
Although the temperature increase from 50 to 70 °C enhanced the final β-carotene yield of D. salina from 25 to 60%, further improvement can be expected by the variation of the pressure as an additional control parameter. The effect of pressure variation on β-carotene extraction using a constant temperature of 70 °C in our pilot-scale experiments is illustrated in Fig. 3b. Here, the highest extraction efficiency was obtained under the highest investigated pressure, 500 bar. Empirically, the solubility of the carotenoids increases with increased pressure, which is in line with Mendes et al. (1995). This observation was also confirmed by the results of the solubility estimation presented by Fig. 3c.
All in all, it was experimentally observed that the combined effect of high pressure and high temperature (500 bar, 70 °C) resulted in the most effective extraction of β-carotene from Dunaliella salina in the pilot-scale equipment as applied by us.
Discussion of the experimental results
The moderate extraction power of ethanol and n-hexane as shown in Fig. 2 is not in line with the study of Taungbodhitham et al. (1998), which reported the best carotenoid extraction results from different matrices for both solvents. Except for ethyl acetate, we could not identify a clear visible effect by the variation of the extraction time. Comparable conclusion was drawn for β-carotene extraction from tomato or carrot powder using different organic solvents (Ishida and Chapman 2009) as well as for lutein extraction of marigold biomass (Surendranath et al. 2016). In our present study, the expected kinetics of extraction as illustrated in Figure S1 was not visible, leading to the assumption that the linear phase of extraction was probably passed within the first 30 min. For ethyl acetate, the pigment yield even decreased over extraction time that might indicate instability of the pigment in the solvent. The temperature was found to influence on the extraction with organic solvents, see Fig. 2b. On one hand, the solubility and the diffusivity of the solute are influenced; on the other hand, the liquid viscosity is changed as a function of temperature (Palma et al. 2013). Furthermore, the temperature optimum for extraction was depending on the applied solvent.
For the extractions with THF, acetone, chloroform/MeOH, and ethyl acetate, a decrease of β-carotene yield was observed if the temperature raised. This phenomenon can be attributed to the thermolability of the pigment at higher temperatures, which is influenced by the surrounding solvent (Craft and Soares 1992). In contrast, the extraction yield of EtOH was improved at higher temperatures. With both investigated ethanol concentrations (96 and 99 Vol%), the yields increased in line with temperature, and the highest yields were obtained at 60 °C. For the extraction of β-carotene by n-hexane, we could not observe a clear visible effect of the temperature. ScCO2 is one of the frequently applied supercritical fluids in the industry as the gas is low in cost and toxicity aside with high purity and uncomplicated handling. It is already industrially applied for decaffeination, deoiling, fractionation, and refining of vegetable oil as well as the extraction of herbal flavorings and fragrances (Brunner 2005; Mukhopadhyay 2009). Furthermore, for the extraction of valuable lipophilic microalgal products such as pigments or polyunsaturated fatty acids, academic and industrial efforts to apply scCO2 is growing (e.g., for docosahexaenoic acid (He et al. 2017), carotenoids (Casas Cardoso et al. 2012), and lutein (Yen et al. 2012)). However, to the best of our knowledge, the industrial implementation for β-carotene extraction is still missing (Mäki-Arvela et al. 2014). The results presented in Organic solvent extraction of β-carotene from D. salina Section confirmed that neat EtOH is not appropriate to be used as a solvent without co-solvents for the extraction of β-carotene from D. salina. However, the work of Yen et al. (2012) pointed out that a solvent with weak performance in conventional solvent extraction can serve as an effective entrainer. In principle, there are two main effects causing an improved mass transfer of the solvent within the cells in multi-solvent systems. On the one hand, the use of polar entrainer increases cell permeability by solving polar ingredients of the cell membrane (Panesar et al. 2007). On the other hand, the co-solvent can lead to cell rupture or matrix swelling (Mäki-Arvela et al. 2014).
In the pilot experiments, we found a beneficial effect of the co-solvent, as shown in Table 1. This is in line with other experimental studies; preferential co-solvent-caused effects are previously reported for the extraction of different carotenoids from Nannochloropsis gaditana, Synechococcus sp., Scenedesmus sp., and H. pluvialis, respectively (Machmudah et al. 2006; Nobre et al. 2006; Casas Cardoso et al. 2012; Yen et al. 2012).
Theoretically, the beneficial effect of co-solvent for supercritical CO2 extraction has been successfully explained by the Hansen solubility theory (Tirado and Calvo 2019) or more recently by the important solute-entrainer interaction as predicted by the Kirkwood-Buff theory (Shimizu and Abbott 2016).
By applying the Hansen theory, Tirado and Calvo (2019) suggested ethanol to be a very good co-solvent for Dunaliella extraction which was confirmed in out pilot-scale experiments. However, too high EtOH concentrations in scCO2 might lead to a decrease of extraction power as reported in the extraction of carotenoids from different algal biomass matrices by Machmudah et al. (2006) and Yen et al. (2012). Furthermore, the use of high amounts of co-solvent additionally raises purification cost due to solvent separation. Consequently, the concentration of 10 wt% EtOH was taken as feasible co-solvent amount in the economical analysis, and higher concentrations were not investigated experimentally.
The comparable extraction efficiencies of approximately 60% were obtained for the extractions at 300 and 400 bar (shown in Fig. 3b). This seems to be relevant especially for the mass transfer controlled phases of extraction kinetics (phases II + III in Figure S1). At this point, most of the pigments are already solved from the solid-liquid interface (Macias-Sanchez et al. 2009b). The solubility values as presented Fig. 3c were estimated from the slope of the initial phase (phase I) where the diffusion limitation plays only a minor role. The mass transfer inhibition can be overcome by further increase of the pressure, where the temperature impact is less pronounced (Brunner 2005). Thus, in our pilot-scale study, the application of 500 bar at 70 °C led to the highest extraction yield as well as solubility.
Under these conditions, nearly 90% of the pigment could be extracted (in relation to the reference extraction with acetone) which motivated us to use these conditions in the cost assessment as discussed in Energy and cost assessment of the extraction strategies Section.
Energy and cost assessment of the extraction strategies
The obtained parameters and the results of the experimental extraction studies were integrated into the process model of D. salina for β-carotene production introduced in Pirwitz et al. (2015) to compare conventional solvent extraction and scCO2 extraction regarding their costs and energy demand.
In principle, conventional n-hexane extraction comprises two main energy-consuming steps: (1) the heating of the solvent up to the extraction temperature and (2) the recovery of the solvent by evaporation. In contrast, scCO2 extraction requires electrical energy for compression to achieve the critical process pressure as well as a cooling step for the separation of scCO2 and the solute. Furthermore, heat energy for evaporation needs to be provided if a co-solvent is used. Thus, higher energy consumption can be expected for the supercritical solvent extraction.
Figure 4 illustrates the estimated energy demand of β-carotene extraction from D. salina based on a production plant with a production area of 10 ha and 200 kg biomass yield per day. Obviously, with 63% of the overall energy demand, the compression of scCO2 and EtOH requires the highest energy amount in the extraction process. This seems to be characteristic for scCO2 extraction (Shariaty-Niassar et al. 2009). Another considerable energy input is needed for the evaporation of the co-solvent EtOH. According to the extraction energy and cost estimation results listed in Table 2, conventional n-hexane extraction shows a lower energy demand compared to that of scCO2 extraction. With 57.42 kWh kg−1 β-carotene, the extraction by scCO2 consumes twice as much energy as the n-hexane extraction. However, the required solvent-to-biomass ratio is significantly lower for scCO2 extraction. Consequently, the solvents costs are even lower in this case. Considering all operation costs for the solvents including the first fill and make-up as well as the expended energy, n-hexane extraction was estimated to be more expensive than scCO2. Here, extraction costs of 10.38 ± 0.99 US$ kg−1 β-carotene were calculated, whereas the scCO2 extraction requires 7.7 ± 0.83 US$ kg−1 β-carotene. Regarding the yield of the pigment, no considerable differences are visible in the simulation results. Both technologies are expected to achieve an annual production of approximately 2.7 t β-carotene. With 1.6 M$, also the calculated annual selling value is comparable. Nevertheless, scCO2 extraction has the advantage to save approximately 5 $ kg−1 β-carotene which is visible in the cumulative production cost. However, the annual production costs which include the costs of all production steps differ only slightly for both scenarios. Here, values of 409 ± 31 k$ a−1 and 402 ± 31 k$ a−1 were calculated for the n-hexane or scCO2 extraction, respectively.
Nonetheless, it is crucial to consider the environmental aspect of product extraction. Espada et al. concluded lower energy consumption and greenhouse gas emissions for the supercritical extraction without co-solvent in an environmental evaluation study for Dunaliella (Espada et al. 2020). However, the lower extraction yield as assumed in their simulation for supercritical process contributed severely on the cultivation and nutrient demand and so to the overall cost. They concluded twofold higher price for β-carotene in supercritical extraction, which is not in line with our findings, but is explained by different assumptions in the simulations. In recent years, the awareness for more sustainable products and goods has been risen constantly. Natural products such as β-carotene should be extracted also with green solvents, especially if they are used in cosmetic, pharmaceutical, or food industry. The toxicity of solvent residues in the final product after n-hexane extraction is always a critical issue and controlled by quality constraints (European Parliament 2009). Due to the favorable properties of scCO2 as well as its co-solvent EtOH, this solvent system is more appropriate for environmental-friendly and sustainable pigment extraction than n-hexane. Furthermore, unpressurized CO2 volatilizes under ambient conditions, leading to simple and complete solvent removal from the extract that is important for the quality of the product (Chemat and Abert Vian 2014).
Conclusions
Supercritical fluid extraction is an extensively investigated methodology for a wide range of applications. The present work compared conventional and supercritical fluid extraction by using the example of β-carotene extraction from D. salina biomass. In pilot-scale experiments, 500 bar, 70 °C, and the addition of 10% EtOH were identified as optimal parameter set-up to extract 90% of the pigment from D. salina biomass by scCO2. Based on our detailed energy demand and operating cost calculations, we conclude that scCO2 extraction consumes twofold the energy of conventional n-hexane extraction. This is mainly attributed to the high compression work needed to achieve the critical pressure. However, the lower solvent to biomass ratio during scCO2 extraction lowers the extraction costs by 20% compared to n-hexane. Therefore, we can indicate here the possibility of economic viable scCO2 extraction of microalgal β-carotene for the first time.
References
Arvayo-Enriquez H, Mondaca-Fernandez I, Gortarez-Moroyoqui P, Lopez-Cervantes J, Rodriguez-Ramirez R (2013) Carotenoids extraction and quantification: a review. Anal Meth 5:2916–2924
Attard TM, McElroy CR, Hunt AJ (2015) Economic assessment of supercritical CO2 extraction of waxes as part of a maize stover biorefinery. Int J Mol Sci 16:17546–17564
Ben-Amotz A (1999) Dunaliella β-carotene. From science to commerce. (Seckbach N (ed) Enigmatic Microorganisms and Life in Extreme Environments. Kluwer, Dordrecht pp 399-410.
Ben-Amotz A, Avron M (1989) The biotechnology of mass culturing Dunaliella for products of commercial interest. In: Cresswell R, Rees T, Shah N (eds) Algal and Cyanobacterial Biotechnology. Longman Scientific and Technical, New York, pp 91–114
Borovkov AB, Gudvilovich IN, Avsiyan AL (2020) Scale-up of Dunaliella salina cultivation: from strain selection to open ponds. J Appl Phycol 32:1545–1558
Borowitzka MA (2013) Dunaliella: biology, production, and markets. In: Richmond A, Hu Q (eds) Handbook of Microalgal Culture. Wiley, Chichester, pp 359–368
Borowitzka LJ, Borowitzka MA (1990) Commercial production of β-carotene by Dunaliella salina in open ponds. Bull Mar Sci 47:244–252
Brunner G (2005) Supercritical fluids: technology and application to food processing. J Food Eng 67:21–33
Casas Cardoso L, Mantell Serrano C, Rodriguez Rodriguez M, Martinez de la Ossa EJ, Lubian LM (2012) Extraction of carotenoids and fatty acids from microalgae using supercritical technology. Am J Anal Chem 3:877–883
Chemat F, Abert Vian M (eds) (2014) Alternative solvents for natural products extraction. Green Chemistry and Sustainable Technology. Springer, Heidelberg.
Craft NE, Soares JH (1992) Relative solubility, stability, and absorptivity of lutein and beta-carotene in organic solvents. J Agric Food Chem 40:431–434
Danielski L, Campos LMAS, Bresciani LFV, Hense H, Yunes RA, Ferreira SRS (2007) Marigold (Calendula officinalis L.) oleoresin: solubility in scCO2 and composition profile. Chem Eng Process 46:99–106
Del Campo JA, Garcia-Gonzalez M, Guerrero M (2007) Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl Microbiol Biotechnol 74:1163–1174
Delrue F, Li-Beisson Y, Setier PA, Sahut C, Roubaud A, Froment AK, Peltier G (2013) Comparison of various microalgae liquid biofuel production pathways based on energetic, economic and environmental criteria. Bioresour Technol 136:205–212
Dias Ribeiro B, Weingart Barreto D, Zarur Coelho MA (2011) Technological aspects of β-carotene production. Food Bioprocess Technol 4:693–701
Espada JJ, Perez-Antolin D, Vicente G, Bautista LF, Morales V, Rodriguez R (2020) Environmental and techno-economic evaluation of beta-carotene production from Dunaliella salina. Biofuels Bioprod Biorefin 14:43–54
European Parliament (2009) Directive 2009/32/EC of the European parliament and of the council of 23 April 2009 on the approximation of the laws of the member states on extraction solvents used in the production of foodstuffs and food ingredients. Official J Eur Union 141:3–11
Harvey PJ, Ben-Amotz A (2020) Towards a sustainable Dunaliella salina microalgal biorefinery for 9-cis beta-carotene production. Algal Res 50:102002
He B, Wang Y, Dou X, Chen Y-F (2017) Supercritical CO2 extraction of docosahexaenoic acid from Schizochytrium limacinum using vegetable oils as entrainer. Algal Res 21:58–63
Herrero M, Jaime L, Martin-Alvarez PJ, Cifuentes A, Ibanez E (2006) Optimization of the extraction of antioxidants from Dunaliella salina microalga by pressurized liquids. J Agric Food Chem 54:5597–5603
Hosseini SRP, Tavakoli O, Sarrafzadeh MH (2017) Experimental optimization of SC-CO2 extraction of carotenoids from Dunaliella salina. J Supercrit Fluids 121:89–95
Ishida BK, Chapman MH (2009) Carotenoid extraction from plants using a novel, environmentally friendly solvent. J Agric Food Chem 57:1051–1059
Jaime L, Mendiola JA, Ibanez E, Martin-Alvarez PJ, Cifuentes A, Reglero G, Senorans FJ (2007) Beta-carotene isomer composition of sub- and supercritical carbon dioxide extracts. Antioxidant activity measurement. JAgric Food Chem 55:10585–10590
Kitada K, Machmudah S, Sasaki M, Goto M, Nakashima Y, Kumamoto S, Hasegawa T (2009) Supercritical CO2 extraction of pigment components with pharmaceutical importance from Chlorella vulgaris. J Chem Technol Biotechnol 84:657–661
Kyriakopoulou K, Papadaki S, Krokida M (2015) Life cycle analysis of β-carotene extraction techniques. J Food Eng 167:51–58
Lamers PP, van de Laak CCW, Kaasenbrood PS, Lorier J, Janssen M, De Vos RCH, Bino RJ, Wijffels RH (2010) Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol Bioeng 106:638–648
Leone GP, Balducchi R, Mehariya S, Martino M, Larocca V, Di Sanzo G, Iovine A, Casella P, Marino T, Karatza D, Chianese S, Musmarra D, Molino A (2019) Selective extraction of omega-3 fatty acids from Nannochloropsis sp. using supercritical CO2 extraction. Molecules 24:2406
Lichtenthaler H (1987) Chlorophylls and carotenoids - pigments of photosynthetic biomembranes. Meth Enzymol 148:350–382
Machmudah S, Shotipruk A, Goto M, Sasaki M, Hirose T (2006) Extraction of astaxanthin from Haematococcus pluvialis using supercritical CO2 and ethanol as entrainer. Ind Eng Chem Res 45:3652–3657
Macias-Sanchez MD, Mantell Serrano C, Rodriguez Rodriguez M, de la Ossa EM, Lubian LM, Montero O (2008) Extraction of carotenoids and chlorophyll from microalgae with supercritical carbon dioxide and ethanol as cosolvent. J Sep Sci 31:1352–1362
Macias-Sanchez MD, Mantell C, Rodriguez M, Martinez de la Ossa E, Lubian LM, Montero O (2009a) Comparison of supercritical fluid and ultrasound-assisted extraction of carotenoids and chlorophyll a from Dunaliella salina. Talanta 77:948–952
Macias-Sanchez MD, Serrano CM, Rodriguez MR, Martinez de la Ossa E (2009b) Kinetics of the supercritical fluid extraction of carotenoids from microalgae with CO2 and ethanol as cosolvent. Chem Eng J 150:104–113
Mäki-Arvela P, Hachemi I, Murzin DY (2014) Comparative study of the extraction methods for recovery of carotenoids from algae: extraction kinetics and effect of different extraction parameters. J Chem Technol Biotechnol 89:1607–1626
Mendes RL, Fernandes HL, Coelho JP, Reis EC, Cabral JMS, Novais JM, Palavra AF (1995) Supercritical CO2 extraction of carotenoids and other lipids from Chlorella vulgaris. Food Chem 53:99–103
Meullemiestre A, Breil C, Maryline A-V, Chemat F (2015) Modern techniques and solvents for the extraction of microbial oils. Springer-Briefs in Molecular Science, Springer, Switzerland
Mezzomo N, Ferreira SRS (2016) Carotenoids functionality, sources, and processing by supercritical technology: a review. J Chem 2016:3164312
Molino A, Mehariya S, Iovine A, Larocca V, Di Sanzo G, Martino M, Casella P, Chianese S, Musmarra D (2018) Extraction of astaxanthin and lutein from microalga Haematococcus pluvialis in the red phase using CO2 supercritical fluid extraction technology with ethanol as co-solvent. Mar Drugs 16:432
Molino A, Larocca V, Di Sanzo G, Martino M, Casella P, Marino T, Karatza D, Musmarra D (2019) Extraction of bioactive compounds using supercritical carbon dioxide. Molecules 24:No 782
Mukhopadhyay M (2009) Extraction and processing with supercritical fluids. J Chem Technol Biotechnol 84:6–12
NIST (2016) NIST Webbook, Thermophysical properties of fluid systems. https://webbook.nist.gov/chemistry/fluid/. Accessed 07 Jul 2016
Nobre B, Marcelo F, Passos R, Beirao L, Palavra A, Gouveia L, Mendes R (2006) Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur Food Res Technol 223:787–790
Obeid S, Beaufils N, Camy S, Takache H, Ismail A, Pontalier P-Y (2018) Supercritical carbon dioxide extraction and fractionation of lipids from freeze-dried microalgae Nannochloropsis oculata and Chlorella vulgaris. Algal Res 34:49–56
Orosz MS, Forney D (2008) A comparison of algae to biofuel conversion pathways for energy storage off-grid. Massachusetts Institute of Technology, Report 2:62
Palma M, Barbero G, Pineiro Z, Liazid A, Barroso C, Rostagno M, Prado J, Meireles M (2013) Extraction of natural products: principles and fundamental aspects. In: Rostagno MA (ed) Natural product extraction principles and applications. Royal Society of Chemistry, Cambridge, pp 56–58
Panesar PS, Panesar R, Singh RS, Bera MB (2007) Permeabilization of yeast cells with organic solvents for β-galactosidase activity. Res J Microbiol 2:34–41
Pirwitz K, Flassig RJ, Rihko-Struckmann LK, Sundmacher K (2015) Energy and operating cost assessment of competing harvesting methods of D. salina in a β-carotene production process. Algal Res 12:161–169
Pirwitz K, Rihko-Struckmann L, Sundmacher K (2016) Valorization of the aqueous phase obtained from hydrothermally treated Dunaliella salina remnant biomass. Bioresour Technol 219:64–71
Rosa PTV, Meireles MAA (2005) Rapid estimation of the manufacturing cost of extracts obtained by supercritical fluid extraction. J Food Eng 67:235–240
Ruen-ngam D, Shotipruk A, Pavasant P, Machmudah S, Goto M (2012) Selective extraction of lutein from alcohol treated Chlorella vulgaris by supercritical CO2. Chem Eng Technol 35:255–260
Shariaty-Niassar M, Aminzadeh B, Azadi P, Soltanali S (2009) Economic evaluation of herb extraction using supercritical fluid. Chem Ind Chem Eng Q 15:143–148
Shimizu S, Abbott S (2016) How entrainers enhance solubility in supercritical carbon dioxide. J Phys Chem B 120:3713–3723
Surendranath R, Ganga M, Jawaharlal M, Anitha K (2016) Extraction and quantification of marigold lutein using different solvent systems. Int J Pharm Sci Rev Res 37:187–191
Tafreshi HA, Shariati M (2009) Dunaliella biotechnology: methods and applications. J Appl Microbiol 107:14–35
Tang YC, Chen BH (2000) Pigment change of freeze-dried carotenoid powder during storage. Food Chem 69:11–17
Taungbodhitham AK, Jones GP, Wahlqvist ML, Briggs DR (1998) Evaluation of extraction method for the analysis of carotenoids in fruits and vegetables. Food Chem 63:577–584
Tirado DF, Calvo L (2019) The Hansen theory to choose the best cosolvent for supercritical CO2 extraction of beta-carotene from Dunaliella salina. J Supercrit Fluids 145:211–218
Xu Y, Harvey PJ (2019) Red Light control of β-carotene isomerisation to 9-cis β-carotene and carotenoid accumulation in Dunaliella salina. Antioxidants 8: No 148
Yara-Varon E, Fabiano-Tixier AS, Balcells M, Canela-Garayoa R, Bily A, Chemat F (2016) Is it possible to substitute hexane with green solvents for extraction of carotenoids? A theoretical versus experimental solubility study. RSC Advances 6:27750–27759
Yen HW, Chiang W-C, Sun C-H (2012) Supercritical fluid extraction of lutein from Scenedesmus cultured in an autotrophical photobioreactor. J Taiwan Inst Chem Eng 43:53–57
Acknowledgements
Open Access funding enabled and organized by Projekt DEAL.
Funding
This work was partly supported by the Ministry of Science and Economy of Saxony Anhalt (Germany) within the EMIBEX project ZS\2017\01\83925.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 29 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ludwig, K., Rihko-Struckmann, L., Brinitzer, G. et al. β-Carotene extraction from Dunaliella salina by supercritical CO2. J Appl Phycol 33, 1435–1445 (2021). https://doi.org/10.1007/s10811-021-02399-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02399-y