Abstract
When cultivating the kelp Saccharina latissima, knowledge on the availability of seeding material for the production is essential. Applying a spore seeding approach requires spores from the reproductive organs of the fertile sporophytes (sori). As sori are generally not present during the time of seeding in late summer, the production of spores (sporogenesis) can be artificially induced by removing the meristematic part of the sporophyte and keeping the sporophyte under short day, temperate, and nutrient-replete conditions. Only limited information is available on the effect of light intensities including darkness on the sporogenesis of S. latissima. This study examined the natural pattern of sporogenesis in S. latissima from Middelfart, Denmark, and the effect of four different light regimes (0, 20, 60, or 120 μmol photons m−2 s−1) on the artificial induction of sporogenesis in S. latissima. Natural reproductivity and availability of spores in Denmark peaked in early winter, with 86% of the population being reproductive in November. Reproductive material was available from October until late spring, but with a variable spore release from 11 × 103 to 1.2 × 106 spores cm−2 sori. The artificial induction of sporogenesis was optimal in darkness with > 90% of sporophytes developing sori after 49 days, with an average spore release density of 1.15 ± 0.38 × 106 spores cm−2 sori. The results confirmed that S. latissima in Denmark follows the general pattern of reproduction of S. latissima in North Atlantic regions and demonstrated for the first time that sporogenesis in S. latissima can be efficiently induced in darkness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Europe, the cultivation of seaweed is currently being developed toward a more viable and economically sustainable industry, which can provide biomass for a wide range of applications (Zhang and Thomsen 2019). Sugar kelp, Saccharina latissima (Linnaeus) C.E.Lane, C.Mayes, Druehl & G.W.Saunders, is a commercially important kelp species, extensively cultivated in Europe. Regardless of cultivation strategies for S. latissima, there is a fundamental need to obtain spores for the seeding of cultivation substrates and two main approaches can be used to obtain the seed: Either spores can be obtained from spore-bearing tissue (sorus) of adult individuals (sporophytes) and applied directly to the substrates, or the spores can be suspended in cultures and propagated into “seed banks” of gametophytes, which can be induced to fertility, and following applied to cultivation substrates either as gametophytes or as juvenile sporophytes (Edwards and Watson 2011; Flavin et al. 2013; Kerrison et al. 2018). Both approaches have advantages and challenges: If using gametophyte cultures as seeding material, the producer can obtain spores for the buildup of gametophyte cultures at the time when sporophytes are naturally fertile in nature, and will be able to time when the culture is ready for seeding, independent of the seasonal availability of spores in nature (Edwards and Watson 2011). However, maintaining the gametophyte cultures is time consuming, and there is a risk of losing cultures if contaminated. When basing the production on using spores for seeding, the producer depends on obtaining sori/spores at the time of seeding, which typically takes place in late summer (Flavin et al. 2013). In some areas, S. latissima produces sori year round but in general sori are present during winter (Bartsch et al. 2008; Andersen et al. 2011; Andersen 2013), which does not match the period of seeding. Therefore, if using a spore seeding strategy, a sugar kelp producer needs to artificially mature sporophytes, to be sure to obtain spores at the time of seeding in summer. A solution for this is to artificially induce sporogenesis by removing the meristem of sporophytes and keeping sporophytes in a short day regime around 10 °C (Lüning 1988; Pang and Lüning 2004; Forbord et al. 2012). The removal of the meristem excludes sporulating inhibitors, originating from the meristematic region, in reaching the blade and the temperate, short day regime mimics winter conditions, thereby promoting the sori formation. There is evidence however that also tissue nutrient content and light quality control sorus formation (Mizuta et al. 1998). Nimura et al. (2002) proposed that fertile tissue of different Laminaria species must contain a critical concentration of nitrogen to reproduce, and Mizuta et al. (1999a) demonstrated that sorus formation is favored by high ambient nutrient concentrations. In addition, blue light is found to promote sorus formation in Saccharina japonica, whereas red light inhibits sorus formation (Mizuta et al. 2007). The quantitative effect of light on reproduction however is not well described. Maturation can proceed in a light regime with light intensities above 30 μmol photons m−2 s−1 (Lüning 1988; Pang and Lüning 2004; Forbord et al. 2012), but as high light intensities have been found to promote sorus formation in S. japonica (Mizuta et al. 1999a), optimal light intensities for sorus formation are not well described for S. latissima.
The aims and hypotheses of this study were:
-
1.
To document the seasonal pattern of reproduction of S. latissima in inner Danish waters, with the hypothesis that the seasonal pattern of sori formation in S. latissima in Danish waters is similar to the generally described pattern in North Atlantic waters, where S. latissima is reproductive during winter
-
2.
To determine the quantitative effect of light on induction of sporogenesis in S. latissima sporophytes during summer, with the hypothesis that increased light intensities will lead to a faster sporogenesis and larger sori areas
Materials and methods
Natural fertility of Saccharina latissima in Danish waters
Collection of sporophytes
To assess the variation in fertility of natural populations of Saccharina latissima, fifty individuals of S. latissima were randomly collected by snorkeling at 1–2 m depth at the old harbor, Middelfart, Denmark (55° 30′ 25.8″ N, 9° 43′ 43.1″ E) (Fig. 1).
Map of the site where sporophytes were collected and the station where environmental information was gathered. The “dot” marks the site of sporophyte collection at Gammelhavn, Middelfart, Denmark, and the “star” marks the Danish National Monitoring and Assessment Programme (NOVANA) monitoring station from which temperature, dissolved inorganic nitrogen (DIN), and salinity were measured during the study period
The sampling was undertaken with monthly intervals during a full year from February 2018 to February 2019.
The intact sporophytes were stored in a plastic bag during transportation to the lab (1–2 h). In the lab, the number of fertile sporophytes was recorded and the ratio of fertile to non-fertile sporophytes was calculated. Hereafter, 10 randomly selected fertile sporophytes were submerged in saltwater at 10 °C and stored for 1–2 days until spore release was initiated. In June, August, and November, the 10 selected fertile sporophytes were stored in saltwater from 4 to 6 days.
Local seawater temperature, dissolved inorganic nitrogen (DIN) (ammonia, ammonium, nitrate, and nitrite), and salinity data were derived from the nearest sampling station monitored by the Danish National Monitoring and Assessment Programme (NOVANA) located 18 km north of the seaweed sampling site (Fig. 1).
Spore release density
Prior to spore release, a disc of 9.29 cm2 was cut out from sorus tissue of each of the 10 sporophytes. The discs were wiped clean with dry paper towel, layered with tissue paper and covered with a plastic bag and left to dry in a cooling room at 10 °C for 20 h. After drying for approximately 20 h, the discs were re-immersed for 45 min in separate, individual 15-mL Petri dish plates, each containing 10 mL artificial seawater (Instant Ocean, 30 ppt) to induce spore release. Straight after, the spore concentration in each Petri dish was counted for each sample using a Thoma hemocytometer Counting Chamber. The spore release density (spores cm−2 sori) for each month was calculated as AV ± SE, n = 5–10. Only motile spores were registered and counted as viable spores.
Induction of sporogenesis
Experimental setup
Induction of sporogenesis in non-fertile sporophytes was conducted from June 28 to August 16, 2018. On June 28, sixty intact S. latissima sporophytes of equal length (approximately 80 cm), with no signs of sori, were collected at the same site in Middelfart on 1–2 m depth. Twelve sporophytes served as start samples for analysis of DM, C, N, and P, and the remaining 48 sporophytes were cut 20 cm above the meristem to remove sporulation inhibitors (Pang and Lüning 2004) and distributed into the four experimental tanks (Fig. 2). In each experimental tank, twelve sporophytes of equal length (around 60 cm) were placed into separate compartments containing 2 sporophytes each. The separate compartments ensured that sporophytes were not floating on top of each other.
Setup of the four experimental tanks and the header tank. Twelve sporophytes were distributed in each experimental tank into separate compartments with two sporophytes per compartment. The numbers indicate the light intensity in each of the tanks (μmol photons m−2 s−1). In the bottom, one of the experimental tanks is viewed from the side. The light intensities were adjusted by adjusting the distance of the light sources to the surface of the water in the tanks
The experimental tanks (360 × 34 × 12 cm, each containing 100 L) were set up and connected in parallel to the same single header tank from which all water was distributed and collected. Consequently, each tank had its own water supply from the header tank providing a unidirectional flow in each tank of app. 500 L h−1. The setup ensured a total mixing of the water, providing all treatments with the same nutrient regime.
The system contained a total of 650 L of nutrient-enriched seawater. The whole system (experimental tanks and the header tank) was placed inside a refrigerated 40-foot container at 10 °C. The water in the system was changed after 8, 21, and 34 days with 10 °C (cooled) seawater from 2 m depth at Snaptun Harbor, Denmark, and added new nutrients. The nutrient concentration was adjusted to 100 μM NH4-N and 10.5 μM PO4-P at each water change using an organic fertilizer (500-μm filtered degassed manure from “Krogsminde BioEnergi,” Ølgod, Denmark). Nutrient concentrations were in range of what has previously been used for sporogenesis studies (Lüning 1988; Pang and Lüning 2004).
The four experimental tanks were exposed to each their specific light intensity of either 0, 20, 60, or 120 μmol photons m−2 s−1 as measured at the water surface. The no-light treatment was achieved by covering one of the experimental tanks with black plastic. The light source was two light fixtures (120 cm) each with two fluorescent lamps (Philips, MASTER, TL-D 90 De Luxe, 58 W/950) per tank (excluding the no-light treatment). All light treatments followed a short daylight regime of 8 h:16 h (light:dark) to mimic winter conditions (Lüning 1988). During the experiment, the power to the light sources was unintendedly interrupted for a period of time between July 15 and 19.
Measurements of sporogenesis and spore release density
At each water change, the number of fertile sporophytes in each treatment was registered. The number of fertile sporophytes (defined as sori presence) in each treatment, and the total area of sori on each sporophyte were registered measuring the length and width of the fertile area. At the end of the experiment, a tissue disc of 9.29 cm2 was stamped from the sorus tissue of 10 individuals, and the spores were released and counted as described above, with the modification that 25 mL seawater was used instead of 10 mL.
Tissue samples
At the experiment start, one tissue disc sample of 9.29 cm2 was randomly taken from each of 12 sporophytes collected at the same site, time, and depth as the sporophytes used in the experiment. The 12 discs were pooled into three samples each containing four discs. These discs constituted the “start” samples representing the tissue composition at the beginning of the experiment. At the end of the experiment, one disc was taken at the same place, from the middle part, of each of the 48 involved sporophytes, regardless if the tissue had sori or not. For each of the four treatments, the 12 discs were pooled into three replicates per treatment, with each pooled treatment replicate containing 4 discs. As some of the sporophytes were fertile, the number of fertile discs per pooled replicate sample was registered. All the pooled tissue samples (not the individual discs) were analyzed for the content of dry matter (DM), carbon (C), nitrogen (N), and phosphorous (P).
Analysis of tissue composition: DM, C, N, P
The DM content of the tissue discs was determined by drying the discs at 105 °C until the weight was stabilized, and hereafter calculated as percentage of fresh biomass: dry weight (DW)/fresh weight (FW) × 100. Hereafter, the dry disc was finely milled and homogenized before further analysis.
For determination of ash content, a known amount of DM was combusted at 550 °C for 2 h, and the ash fraction was calculated as percentage of DM. Tissue concentrations of C and N were determined by Pregl-Dumas ignition in pure oxygen atmosphere followed by chromatographic separation of C and N with detection of the individual elements by thermal conductivity. Total P content was analyzed spectrophotometrically following standard methods (Grasshoff et al. 1983).
Statistics
A Gaussian general linear model was fit to the datasets, using the identity function in Rstudio (version 1.3.1073). The fit was then tested by Shapiro-Wilk test for normality and Bartlett’s test for homogeneity of variance. If the tests were non-significant (p value > 0.05), the model was further validated against a null model using an ANOVA F-test to confirm the effect of the treatments. Further differences between treatments were tested using Tukey HSD post hoc test. If the criteria of normal distribution of the data were not met, a Kruskal-Wallis test was used. If this test was significant (p < 0.05), a Wilcoxon rank sum post hoc test was used to test the differences between treatments.
Results
Natural fertility of S. latissima in Danish waters
Reproductive tissue was present in the Middelfart population from October throughout June, and peaked in December with 86% of the total natural population having visible sori (Fig. 3). In the 3-month period from July to September, only one sporophyte with sori was observed. The seawater temperature varied from 0 to 22 °C during the period of observations, with the lowest temperature in March 2018 and the highest temperature in July 2018 (Fig. 3). The DIN concentration varied from 0.32 to 8.41 μM N, with the lowest concentration of 0.32 to 0.76 μM N from April to November.
The fraction of S. latissima sporophytes with sori of the total natural population (% of total population, bars), the local seawater temperature (°C) and dissolved inorganic nitrogen (DIN - ammonia, ammonium, nitrite, and nitrate-N) from February 2018 to February 2019 (dashed line). Temperature and DIN data were derived from the nearest sampling site monitored by Danish National Monitoring Programme (NOVANA) located 18 km north of the sampling site, measured at a depth of 1 m
The fraction of the fertile sporophytes capable of releasing spores varied between 70 and 100% from November to May, but in June and August none of the collected fertile sporophytes released viable spores, and in October it was estimated that only 10% of the total population released viable spores, despite the fact that more than 40% of the total population were bearing sori.
The spore release density was highest in November with an average spore release of 1.2 ± 0.41 × 106 spores cm−2 sori (Table 1). From November to February, the number of viable spores released decreased, coinciding with reduced natural fertility in the population (Fig. 3). In March and May however, the fertile sporophytes were also releasing viable spores in large quantities (0.51 ± 0.27 and 0.74 ± 0.36 × 106 spores cm−2 sori, respectively). The months with the lowest spore release density were April and October but no statistically significant differences were found between months due to large variations in spore release.
The position of the sori on the sporophytes changed through the season: During fall and winter, the fertile material was predominantly located on the distal part of the frond, with a large degree of biofouling by especially epiphytic algae. In May and June on the contrary, the fertile material was located on the basal part of the frond, which was not yet overgrown by epiphytes. The sori releasing the largest number of viable spores, in general, had the darkest appearance, but some individuals with lighter sori areas also released a high amount of spores (Table 2).
General seasonality in reproduction of S. latissima
The data on natural fertility in S. latissima in the Middelfart population was compared to data from other North Atlantic regions ranged by latitude (Table 3). Generally, the main reproductive period of S. latissima was observed in winter from November to February, but in some areas, sori could be found year round. No clear pattern relating to latitude could be extracted from the data.
Induction of sporogenesis in S. latissima
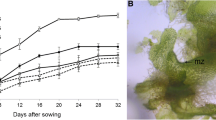
The first signs of visible sori were evident already after 8 days in the induction of sporogenesis experiment (Fig. 4). Hereafter, a gradual increase in the number of fertile sporophytes was observed during the experiment. The maturation happened faster and was more pronounced in the treatment where the sporophytes were not exposed to light, and this treatment resulted in 75% of all sporophytes being fertile after 34 days. After 49 days, 92% of the sporophytes were fertile in the no-light treatment, compared to 42% in both the 20 and 60 μmol photons m−2 s−1 treatment and only 25% in the 120 μmol photons m−2 s−1 light treatment.
The average sorus area of the fertile sporophytes was gradually increasing during the 49 days of the maturation experiment, reaching an average of around 120 cm2 of sori per fertile sporophyte after 34 days. Light had no significant effect on the final average sori area (Fig. 5).
Average sorus area per sporophyte (cm2) of the reproductive sporophytes from light treatments (0, 20, 60, and 120 μmol photons m−2 s−1) after 49 days of the maturation experiment. Data are shown as a box-whisker diagram with a vertical line representing the median, an “x” representing the mean and includes outliers. The box plot is divided into four quantiles, with the box containing the second and third quantiles and whiskers marking the lower and upper adjacent values. The quantiles are calculated using the “inclusive mean” method, n = 3–11
In all light treatments, a number of sporophytes were able to release viable spores; however, there was a non-significant tendency for the sporophytes from the no-light treatment to release a higher number of spores than the sporophytes matured with 20, 60, and 120 μmol photons m−2 s−1 (Table 4).
The DM and C contents of the sporophytes did not change significantly during the induction of sporogenesis experiment, but there was a tendency observed for the DM content to decrease in the no-light treatment: from 25.8 ± 1.7% of FW (start) to 22.2 ± 0.68% of FW (day 49) (Table 5). The tissue N content increased in all samples, but only significantly in the sporophytes from the 0, 20, and 60 μmol m−2 s−1 treatments, from 1.57 ± 0.015% of DM (start) to respectively 2.67 ± 0.066, 2.20 ± 0.19, and 2.38 ± 0.21% of DM. The P content only increased significantly in sporophytes from the no-light treatment, up to 0.547 ± 0.028% of DM from 0.393 ± 0.012% of DM (start).
At the final sampling after 49 days, some of the pooled disc samples contained no fertile discs, so a comparison of tissue N and P contents was made between the pooled disc samples containing fertile discs and those not containing fertile discs. The pooled samples containing fertile discs had significantly higher tissue concentration of both N and P, compared to the pooled samples with no fertile discs. This was observed across all light treatments (Table 5).
Discussion
Natural fertility of S. latissima in Danish waters
The reproductive pattern of S. latissima in Middelfart, Denmark, confirmed results from other North Atlantic regions, with a main reproductive period during winter, and almost no reproductive sporophytes present during summer. The main reproductive period in Middelfart was concentrated in early winter (November/December), but persisted into late spring, with sori present until June. In November/December, > 70% of the total population was reproductive, whereas < 50% of the total population was reproductive in the rest of the winter/spring. The main period of reproduction in November/December also coincided with a high spore release density from sori. Presence of sori is generally related to periods of slow growth (Van Patten and Yarish 1993; Bartsch et al. 2008), and the main reproductive period in November/December, found in this study, is also the period with known slow growth of S. latissima in Denmark (Nielsen et al. 2014). Fertile tissue however was also observed in May, where the highest growth rates are normally observed, supporting that environmental factors, such as temperature and nutrient availability, also affect the period of reproduction (Bartsch et al. 2008; Liu et al. 2017). The results from this study clearly support that the combination of high water temperatures and low nutrient availability impairs the production of sori in S. latissima. However, the results also show that S. latissima is able to produce sori in periods of low nutrient availability (spring and fall), when temperatures are below 15 °C, most likely relying on internal nitrogen stores for production of sori. The spore release density of fertile sporophytes observed in this study varied from 11 × 103 to 1.2 × 106 spores cm−2 sori with a high variability for each month. Van Patten and Yarish (1993) calculated that 1.70–1.87 × 107 spores cm−2 sori could be obtained assuming 32 meiospores per sporangia and 5.30–5.84 × 105 sporangia cm−2 sori. The spores obtained in this study therefore represented only 0.1–6.7% of the spores theoretically available in the sorus tissue, but were in line with previous studies for S. latissima and other kelp species, documenting spore release densities of 10 × 104–80 × 104 spores cm−2 sori (Lee and Brinkhuis 1986; Olischläger and Wiencke 2013). Generally, 70–90% of the fertile sporophytes released viable spores, but in June and October, none and only 10% of the fertile sporophytes released spores, respectively. Lee and Brinkhuis (1986) described the same pattern from Long Island Sound, NY, as fertile sporophytes were not releasing viable spores in August, possibly due to high seawater temperatures. As the spores need time to develop within the sori (Andersen 2013), the low spore release could have been a consequence of the spores not yet being fully mature and ready for release, or the fact that the same experimental temperature was used for the release experiments in all months. As pointed out by Flavin et al. (2013), the temperature sometimes has to be increased up to 16 °C before the sori release spores. Other studies have included a fertility index or maturation class of sorus maturation state (Olischläger and Wiencke 2013; Bartsch et al. 2013), which can help in forecasting the ripeness of the sori. However, simply judging the fertility of sori by tissue color/appearance is not enough to obtain precise estimates of the expected spore release (Joska and Bolton 2007), as also found in this study. The general large variation in spore release from sori collected in the same month could be approached in future studies to find methods to better predict/control spore release from sori.
The finding that the basal and distal parts of the sporophytes were reproductive in different periods of the season was also described from Norway (Andersen 2013). In contrast to this study however, the basal tissue was primarily bearing sori in December–February, whereas distal tissue primarily had sori in February–March. Another important consideration in areas with stratified water columns is the abiotic differences along a depth gradient affecting growth conditions of S. latissima. Both temperature, light, and nutrient variability potentially vary with depth at the same site, potentially differentiating the patterns of reproduction at various depths (Bartsch et al. 2008); and therefore, the pattern of reproduction seen in this study is not necessarily the same for populations in other areas, or in the same area at other depths.
Induction of sporogenesis in S. latissima
The artificial induction of sori produced fertile sporophytes in all treatments, demonstrating that sporogenesis in S. latissima can be successfully induced during summer, when no fertile sporophytes are present in nature, also confirming previous studies (Pang and Lüning 2004; Forbord et al. 2012). The sporogenesis and formation of sori however were most pronounced in darkness, considering both the number of sporophytes successfully induced to fertility and the spore release density from the fertile tissue. This was in contrast to our hypothesis, as previous studies have shown that short day lengths and high light intensities are optimal for the formation of sori in S. latissima and S. japonica (Mizuta et al. 1999a; Pang and Lüning 2004). In this study, there was no positive effect of increasing light intensities and no benefit of exposing the sporophytes to a short day photoperiod as compared to darkness. Even though the light sources were unintendedly interrupted for up to 5 days during this experiment, this interruption did not break the continuity of the dark treatment, and the result of obtaining spores from sporophytes matured in darkness is therefore not questionable. The release of viable spores from fertile sporophytes artificially induced in darkness was comparable to and in the high end of what was observed from fertile sporophytes collected in nature from the same population, suggesting that this method can successfully be used to obtain viable spores for hatchery operations.
Regardless of light intensity, all sporophytes from the induction of sporogenesis experiment demonstrated increased tissue concentrations of N and P during the experiment, and significantly more in fertile than non-fertile thalli. Previous studies of S. japonica have shown that the formation of sori is intensified in nutrient-rich environments (Mizuta et al. 1999a), and that tissue N and P concentrations are increased in sori tissue (Nimura et al. 2002), which was also the case in this study. Species of Laminariaceae are known to have internal translocation of nutrients (Schmitz and Lobban 1976; Lobban 1978; Davison and Stewart 1984; Mizuta et al. 1999b; Boderskov et al. 2016), which is presumably used to supply N and P for cell division in sorus tissue during formation of sori (Mizuta et al. 1999b). The sporophytes matured in darkness increased their tissue concentrations of N and P significantly more than the sporophytes from the treatments receiving light, which could have been a factor driving the fast and pronounced production of sori in this treatment. Nutrient uptake rates of macroalgae follow a diurnal periodicity, with higher nutrient uptake rates in light than in darkness (Roleda and Hurd 2019), and in periods of prolonged darkness, the energy for nutrient uptake relies on stored carbohydrates (Huppe and Turpin 1994). However, Henley and Dunton (1997) showed that the growth of Laminaria solidungula stopped in both newly formed basal tissue and older distal tissue when grown in darkness with nutrients added, consequently increasing the concentration of N in both the basal and distal blade parts. In this study, the meristem was removed, and the growth potential diminished, but growth in S. latissima is known to take place in other parts of the blade than the meristem, although in much lower rates (Parke 1948). Therefore, the faster tissue increase of N and P in the dark treatment could have been due to growth in blades receiving light. However, since growth was not measured in this experiment, we can only speculate this. Another important notion for the difference observed in N and P concentrations between the dark and light treatments is that, as almost all sporophytes were fertile in the dark treatment and significantly less in the light treatments, and all samples were taken at the same place of the blade to counteract possible differences in concentrations along the blade (Boderskov et al. 2016), some samples from the light treatments were not from fertile tissue. As shown in this and previous studies, the concentrations of N and P are increased in fertile tissue; and therefore differences in N and P concentrations seen between the light treatments could have been driven by the difference in fertility of the tissue. In this study, the discs were pooled before analysis, so individual differences in N and P concentrations could not be verified.
When collecting S. latissima sporophytes in early summer, as was the case in this study, the sporophytes contain a high tissue concentration of C (Manns et al. 2017) but relatively low tissue concentrations of N and P (Marinho et al. 2015). As S. latissima is able to survive with no light for prolonged periods (Borum et al. 2002), the tissue C stores could have supported the formation of sori without any light to sustain photosynthesis, when supplied sufficient nutrients. Further studies would need to include tissue sampled in other seasons, and/or at other locations/depths, to ensure that the induction of fertility in darkness also successfully produces spores when the sporophytes collected have lower C tissue concentrations. The maturation of spores includes the formation of lipid granules (Motomura 1993) that serve as storage material for the germination of spores before fully developed to use a photoautotroph energy strategy (Brzezinski et al. 1993; Steinhoff et al. 2011). The maturation of spores in darkness could potentially negatively affect the lipid content and thereby energy reserves of the spores, which could reduce their swimming ability and/or following germination capacity, which is known to vary greatly in different seasons (Lee and Brinkhuis 1988). Future studies could look into this aspect when using this method. Furthermore, this study used a novel nutrient source (degassed organic manure), and the dependency of the process on the actual fertilizer used should also be investigated.
Implications for production of S. latissima
As the natural reproductive season did not overlap with the period of need for fertile material for seeding, a producer of S. latissima in Denmark would need to rely on either gametophyte cultures for seeding, or artificially induced maturation. Still, if using fertile sporophytes collected in the sea for a production, a producer could optimally plan to collect fertile sporophytes during November/December in inner Danish waters. During winter however, the sporophytes were partly covered with epiphytes during this period, so an extensive cleaning was needed before the sporophytes could be used for a spore release to obtain clean cultures or seeded lines (Edwards and Watson 2011). Therefore, it could be more feasible to collect the sporophytes in May, when a significant part of the population was bearing sori, viable spores were released, and the sori were emerging from the basal part of the thalli, which had no fouling. This study is only based on 1 year of observations, and interannual variations could be expected (Parke 1948; Andersen et al. 2011).
In contrast to our hypothesis, the sporophytes induced in darkness developed sori faster and more consistently than when induced in light. This technique has the potential to reduce costs for seaweed producers, as the sorus induction is less resource demanding when no light sources need to be installed and run for the fertility induction process to succeed. As the whole maturation study was based on using degassed organic manure as a nutrient source, the results can also be applied for organic production, complying with the present EU legislation (European Commission 2017).
Conclusion
The present findings demonstrate that the natural abundance of fertile sporophytes of S. latissima peaked in November–December in Denmark and supported the general pattern of reproduction described for other North Atlantic populations of S. latissima. Therefore, an artificial induction of fertility is needed for sugar kelp production relying on direct seeding of spores, as the period of seeding did not overlap with the season of reproduction in S. latissima. The study also demonstrated a simpler and resource-efficient technique of inducing sporogenesis in S. latissima in darkness, which eliminates the need for exposing the sporophytes to light during artificial induction of sporogenesis.
Data availability
All data and material can be supplied upon request.
References
Andersen GS (2013) Patterns of Saccharina latissima recruitment. PLoS One 8 (12):e0081092
Andersen GS, Steen H, Christie H, Fredriksen S, Moy FE (2011) Seasonal patterns of sporophyte growth, fertility, fouling, and mortality of Saccharina latissima in Skagerrak, Norway: implications for forest recovery. J Mar Biol 2011:1–8
Bartsch I, Vogt J, Pehlke C, Hanelt D, Buschmann A (2013) Prevailing sea surface temperatures inhibit summer reproduction of the kelp at Helgoland (North Sea). J Phycol 49(6):1061–1073
Bartsch I, Wiencke C, Bischof K, Buchholz CM, Buch BH, Eggert A, Feuerpfeil P, Hanelt D, Jacobsen S, Karez R, Karsten U, Molis M, Roleda MY, Schubert H, Schumann R, Valentin K, Weinberger F, Wiese J (2008) The genus Laminaria sensu lato: recent insights and developments. Eur J Phycol 43:1–86
Boderskov T, Schmedes PS, Bruhn A, Rasmussen MB, Nielsen MM, Pedersen MF (2016) The effect of light and nutrient availability on growth, nitrogen, and pigment contents of Saccharina latissima (Phaeophyceae) grown in outdoor tanks, under natural variation of sunlight and temperature, during autumn and early winter in Denmark. J Appl Phycol 28:1153–1165
Borum J, Pedersen MF, Krause-Jensen D, Christensen PB, Nielsen K (2002) Biomass, photosynthesis and growth of Laminaria saccharina in a high-arctic fjord, NE Greenland. Mar Biol 141:11–19
Brzezinski MA, Reed DC, Amsler CD (1993) Neutral lipids as major storage products in zoospores of the giant kelp Macrocystis pyrifera (Phaeophyceae). J Phycol 29:16–23
Davison IR, Stewart WDP (1984) Studies on nitrate reductase activity in Laminaria digitata (Huds.) Lamour. I. Longitudinal and transverse profiles of nitrate reductase activity within the thallus. J Exp Mar Biol Ecol 74:201–210
Edwards M, Watson L (2011) Aquaculture explained. Aquaculture 26:1–71
European Commission (2017) Commission Regulation (EC) No 889/2008. Off J Eur Union 51:1173–1182
Flavin K, Flavin N, Flahive B (2013) Kelp farming manual. A guide to the processes, techniques and equipment for farming kelp in New England waters. Ocean Approved. 123p. https://doi.org/10.1007/s10811-015-0547-z
Forbord S, Skjermo J, Arff J, Handå A, Reitan KI, Bjerregaard R, Lüning K (2012) Development of Saccharina latissima (Phaeophyceae) kelp hatcheries with year-round production of zoospores and juvenile sporophytes on culture ropes for kelp aquaculture. J Appl Phycol 24:393–399
Grasshoff K, Ehrhardt M, Kramling K (1983) Methods of sea-water analysis. Verlag Chemie, Weinheim
Henley WJ, Dunton KH (1997) Effects of nitrogen supply and continuous darkness on growth and photosynthesis of the arctic kelp Laminaria solidungula. Limnol Oceanogr 42:209–216
Huppe HC, Turpin DH (1994) Integration of carbon and nitrogen metabolism in plant and algal cells. Annu Rev Plant Physiol Plant Mol Biol 45:577–607
Joska MAP, Bolton JJ (2007) In situ measurement of zoospore release and seasonality of reproduction in Ecklonia maxima (Alariaceae, Laminariales). Br Phycol J 22 (2):209–214
Kerrison PD, Stanley MS, Hughes AD (2018) Textile substrate seeding of Saccharina latissima sporophytes using a binder: an effective method for the aquaculture of kelp. Algal Res 33:352–357
Lee J, Brinkhuis BH (1988) Seasonal light and temperature interaction effects on development of Laminaria Saccharina (Phaeophyta) gametophytes and juvenile sporophytes. J Phycol 24:181–191
Lee JA, Brinkhuis BH (1986) Reproductive phenology of Laminaria Saccharina (L.) Lamour. (Phaeophyta) At the southern limit of its distribution in the Nothwestern Atlantic Ocean. J Phycol 22:276–285
Liu X, Bogaert K, Engelen AH, Leliaert F, Roleda MY, De Clerck O (2017) Seaweed reproductive biology: environmental and genetic controls. Bot Mar 60:89–108
Lobban CS (1978) Translocation of 14C in Macrocystis pyrifera (Giant Kelp). Plant Physiol 61:585–589
Lüning K (1988) Photoperiodic control of sorus formation in the brown alga Laminaria saccharina. Mar Ecol Prog Ser 45:137–144
Manns D, Nielsen MM, Bruhn A, Saake N, Meyer AS (2017) Compositional variations of brown seaweeds Laminaria digitata and Saccharina latissima in Danish waters. J Appl Phycol 29:1493–1506
Marinho GS, Holdt SL, Birkeland MJ, Angelidaki I (2015) Commercial cultivation and bioremediation potential of sugar kelp, Saccharina latissima, in Danish waters. J Appl Phycol 27:1963–1973
Mizuta H, Hayasaki J, Yamamoto H (1998) Relationship between nitrogen content and sorus formation in the brown alga Laminaria japonica cultivated in southern Hokkaido, Japan. Fish Sci 64:909–913
Mizuta H, Nimura K, Yamamoto H (1999a) Inducible conditions for sorus formation of the sporophyte discs of Laminaria japonica Areschoug (Phaeophyceae). Fish Sci 65:104–108
Mizuta H, Nimura K, Yamamoto H (1999b) Sorus development on median and marginal parts of the sporophyte of Laminaria japonica Areschoug (Phaeophyceae). J Appl Phycol 11:585–591
Mizuta H, Kai T, Tabuchi K, Yasui H (2007) Effects of light quality on the reproduction and morphology of sporophytes of Laminaria japonica (Phaeophyceae). Aquac Res 38:1323–1329
Motomura T (1993) Ultrastructural and immunofluorescence studies of zoosporogenesis in Laminaria angustata. Hokkaido Univ Collect Sch Acad Pap 3:1–32
Nielsen MM, Krause-Jensen D, Olesen B, Thinggaard R, Christensen PB, Bruhn A (2014) Growth dynamics of Saccharina latissima (Laminariales, Phaeophyceae) in Aarhus Bay, Denmark, and along the species’ distribution range. Mar Biol 161:2011–2022
Nimura K, Mizuta H, Yamamoto H (2002) Critical contents of nitrogen and phosphorus for sorus formation in four Laminaria species. Bot Mar 45:184–188
Olischläger M, Wiencke C (2013) Seasonal fertility and combined effects of temperature and UV-radiation on Alaria esculenta and Laminaria digitata (Phaeophyceae) from Spitsbergen. Polar Biol 36:1019–1029
Pang SJ, Lüning K (2004) Breaking seasonal limitation: year-round sporogenesis in the brown alga Laminaria saccharina by blocking the transport of putative sporulation inhibitors. Aquaculture 240:531–541
Parke M (1948) Studies on British Laminariaceae. J Mar Biol Assoc UK 27:651–709
Roleda MY, Hurd CL (2019) Seaweed nutrient physiology: application of concepts to aquaculture and bioremediation. Phycologia 58:552–562
Schmitz K, Lobban CS (1976) A survey of translocation in Laminariales (Phaeophyceae). Mar Biol 36:207–216
Steinhoff FS, Graeve M, Wiencke C, Wulff A, Bischof K (2011) Lipid content and fatty acid consumption in zoospores/developing gametophytes of Saccharina latissima (Laminariales, Phaeophyceae) as potential precursors for secondary metabolites as phlorotannins. Polar Biol 34:1011–1018
Van Patten MS, Yarish C (1993) Allocation of blade surface to reproduction in Laminaria longicruris of Long Island Sound (USA). Hydrobiologia 260:173–181
Zhang X, Thomsen M (2019) Biomolecular composition and revenue explained by interactions between extrinsic factors and endogenous rhythms of Saccharina latissima. Mar Drugs 17:1–39
Acknowledgments
We would like to thank Kitte Linding Gerlich for analyzing disc samples for C, N and P contents and Tinna Christensen for help with setting up figures and tables.
Funding
The work was supported by the Innovation Fund Denmark, grant no 7038-00133B, and Hjarnø Havbrug A/S through the commercial PhD project “Økologisk Sukkertang – industriel produktion af en ny dansk bioressource”.
Author information
Authors and Affiliations
Contributions
Teis Boderskov: Conceptualization, methodology, investigation, data curation, writing - original draft, writing - review and editing Annette Bruhn: Conceptualization, methodology, data curation, writing - review and editing Michael Bo Rasmussen: Conceptualization, methodology
Corresponding author
Ethics declarations
Conflict of interest
Although Hjarnø Havbrug A/S is a private company engaged in sugar kelp production, no conflict of interests could be found influencing this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boderskov, T., Rasmussen, M.B. & Bruhn, A. Obtaining spores for the production of Saccharina latissima: seasonal limitations in nature, and induction of sporogenesis in darkness. J Appl Phycol 33, 1035–1046 (2021). https://doi.org/10.1007/s10811-020-02357-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02357-0