Abstract
Microorganisms play important roles in the growth and development of macroalgae. Still, the biodiversity of the epiphytic microbial community associated with the economically important red alga Pyropia haitanensis during the cultivation period remains uncharacterized, especially the effects of P. haitanensis cultivation on the microbial community of surrounding seawater. Here, we isolated epiphytic microbes from P. haitanensis during the thallus stage during oceanic cultivation and the conchocelis stage during industrial cultivation. The dynamic diversity patterns, as determined by 16S and 18S rRNA gene sequencing of the bacterial and fungal communities, respectively, associated with P. haitanensis and seawater in the presence and absence of algal cultivation were investigated. A notable distinction was observed between the microbial communities of seawater with and without P. haitanensis cultivation. Additionally, the alpha-diversity of seawater with P. haitanensis cultivation was significantly greater than without P. haitanensis cultivation. Cyanobacteria were the dominant species in the latter, while Rhodobacteraceae was enriched in the former. Furthermore, there were significant differences in the microbial community of P. haitanensis at the thallus and conchocelis stages. Seawater properties had significant direct effects on the microbial diversity of P. haitanensis and cultivation seawater, but not on non-cultivation seawater. The enriched microbial presence might promote thallus morphogenesis and be beneficial for the growth and development of both the thallus and conchocelis stages. These findings expand our knowledge of the bacteria and fungi that are beneficial for Pyropia nursery seeding and cultivation, as well as the effects of P. haitanensis cultivation on the seawater microbial community.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbes are widely distributed in seawater and selectively enrich the phycosphere (Bell and Mitchell 1972). These microbes can form communities with unique functions and structures, which may play important roles in marine food webs and biogeochemical cycles (Cole 1982; Armstrong et al. 2001; Farooq and Francesca 2007). Additionally, host microbe interactions play crucial roles in marine ecosystems (Egan et al. 2013; Dittami et al. 2019); van der Loos et al. 2019. The interactions of bacteria with algae have evolved as the result of the mutual exploitation of signals in the environment, such that the Roseovarius sp. MS2 and Maribacter sp. MS6 strains release morphogenetic compounds and ensure proper thallus morphogenesis of Ulva mutabilis (Kessler et al. 2018). Moreover, the surfaces of seaweeds also provide a habitat for microbial communities (Beleneva and Zhukova 2006; De Mesquita et al. 2019). The cultivation area of economically important seaweeds in China comprised about 145,000 ha in 2017 (China Fishery Bureau 2018). Thus, the microbes of seaweeds are an important component of marine ecosystems on the Chinese coastline, and interactions between cultivated seaweed and seawater may shape the marine microbial community.
The red alga Pyropia haitanensis, which naturally inhabits the coastal waters of southern China, has been extensively cultured in the Fujian, Zhejiang, and Guangdong provinces for more than 50 years. Its cultivation accounts for more than 70% of all Pyropia production in China (China Fishery Bureau 2018). The total annual harvest and cultivation area of P. haitanensis were about 116,000 t (dry weight) and 29,000 ha in the aforementioned three provinces in 2017. The annual life cycle of P. haitanensis consists of a thallus stage, which is subject to marine aquaculture in the cool season (from September to December), and a shell-living conchocelis stage, which is cultivated in industrial seedling nurseries in the hot season (from January to August) (Zeng 1985; Blouin et al. 2011). During the conchocelis stage, the filamentous conchocelis develops into sporangial branchlets from which conchospores are released. Subsequently, during conchospore germination, meiotic division gives rise to a four-celled thallus to initiate the thallus stage for oceanic cultivation. The thallus and conchocelis stages are entirely different in morphology, longevity, ploidy, and living environment between thallus stage and conchocelis stage. Therefore, microbes may play different roles during different cultivation stages of P. haitanensis.
Recently, attempts have been made to elucidate the relationship between Pyropia and the epiphytic microbial community. For example, an analysis of the epiphytic microbial community of P. haitanensis thalli using the denaturing gradient gel electrophoresis method revealed that the microbial community varied based on growth form, culture time, and environment (Shen et al. 2013). Microbial communities on Pyropia yezoensis thalli and in seawater were significantly different when infected with the oomycetic genus Pythium (Yan et al. 2019). In addition, industrial cultivation of the conchocelis stage is also an important component of the Pyropia industry. Gai and Zhang (2016) observed significant differences between the microbial community compositions of healthy and diseased shell-living conchocelis of P. yezoensis using next-generation sequencing. However, these studies focused on the impacts of pathogenic bacteria on the microbial community of Pyropia. Whether the epiphytic microbial community associated with P. haitanensis differs between different stages of its life cycle is unknown.
In this study, we used high-throughput sequencing based on 16S rRNA gene and 18S rRNA gene to explore the dynamics of the microbial community on P. haitanensis during the entire cultivation period and the effects of P. haitanensis cultivation on the seawater microbial community. The samples were collected from all the important stages of the life cycle, including both the conchocelis stage (filamentous conchocelis, sporangial branchlets, and conchospores) and the thallus stage (young, adult, mature, and advanced mature thalli). Our main objectives were as follows: (i) to explore the dynamics of the microbial community on P. haitanensis during different life stages, (ii) to explore the effects of Pyropia cultivation on the microbial community in surrounding seawater, and (iii) to investigate the interactions between the seaweed and epiphytic microbes during the cultivation of P. haitanensis. Taken together, these findings will enhance our understanding of the interactions between seawater and P. haitanensis, and the dynamics diversity changes in the microbial community during the cultivation of P. haitanensis.
Materials and methods
Sample collection
Conchocelis-stage samples were collected from a nursery farm at Ningde City, Fujian Province, China. The filamentous conchocelis and sporangial branchlets growing inside calcium carbonate shells were scraped from the shell surface with a sterilized trowel. Each sample was collected from 30 to 50 shells. The shells with mature conchocelis were stimulated with flowing seawater by hanging from a boat overnight for about 12 h and then moved to the laboratory and placed upside-down on sterilized Petri dishes to encourage the release of conchospores. Thallus-stage samples were obtained from the coast at Quanzhou City, Fujian province, China. Young thalli (1–3 cm), adult thalli (20–30 cm), mature thalli bearing antheridia or carpogonia, and advanced mature thalli (decaying thalli after the formation and release of carpospores) were collected from culture ropes. Three biological replicates from each sample were used for subsequent experiments. All the samples were stored at − 20 °C until analysis.
Three cultivation seawater samples, three non-cultivation seawater samples, and three algal samples were collected for each of the afore-mentioned seven life cycle stages. These samples were collected concurrently. For the conchocelis stage, cultivation seawater was sampled from the culture ponds of the nursery farm, whereas non-cultivation seawater was collected from a reservoir that was used as the seawater source for the culture pond. For the thallus stage, cultivation seawater was collected from around the thalli, whereas non-cultivation seawater was collected ~ 100 m from the culture area. Two liters of each sample were passed through 0.22-μm microporous filter paper and stored at − 20 °C until analysis.
DNA extraction and PCR amplification
The filter papers used for the seawater samples were cut into small pieces and placed in a 50-mL centrifuge tube. Extraction buffer (4 g CTAB, 16.4 g NaCl, 20 mL of 1 M Tris-HCl, 8 mL of 0.5 M EDTA, and 200 mL distilled water) was added to the tube together with 500-mg glass beads. The tube was vortexed at maximum speed until the sample was thoroughly homogenized. Then, the sample tubes were incubated at 90 °C for 10 min to ensure that all unicellular organisms were lysed. Subsequently, the tubes were centrifuged at 4000×g for 10 min at 4 °C. The clear supernatant was transferred to a new tube, and an equal volume of phenol/chloroform/isoamyl alcohol (25:24:1) was added. The liquid phase was removed and extracted with an equal volume of chloroform/isoamyl alcohol (24:1). Next, 50 mL of 3 M sodium acetate was added together with a 60% volume of isopropanol to the solvent phase. The solution was then incubated at − 20 °C overnight. The DNA was pelleted (13,000×g, 15 min) and washed with 70% ethanol. An identical extraction procedure was used for the conchocelis, thallus, and seawater samples.
The hypervariable V4-V5 regions of the 16S and 18S rRNA genes were amplified using the primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) (Zhu et al. 2016) and the primers SSU0817F (5′-TTAGCATGGAATAATRRAATAGGA-3′) and 1196R (5′-TCTGGACCTGGTGAGTTTCC-3′) (Rousk et al. 2010), respectively. The PCR reactions were performed in a 50-μL volume containing 1× PCR buffer (20 mM Tris-HCl, 50 mM KCl, pH 8.4), 5.0 mM MgCl2, 0.5 μM of each primer, 200 μM of each dNTP, 4 μL DNA templates, and 2.5 U Taq polymerase. The PCR reactions for amplifying 16S and 18S rRNA genes were conducted using the following program: 3 min of denaturation at 95 °C, 27 cycles of 30 s at 95 °C, 30 s for annealing at 55 °C, and 45 s for elongation at 72 °C, followed by a final extension at 72 °C for 10 min.
Illumina MiSeq sequencing
The PCR products were extracted from 2% agarose gel and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, USA) in accordance with the manufacturer’s instructions and then quantified using a QuantiFluor ST fluorometer (Promega, USA). Sequencing was performed using the Illumina MiSeq platform at the Majorbio BioPharm Technology Co., Ltd., Shanghai, China.
Processing of sequencing data
Data from Illumina sequencing were quality-filtered by Trimmomatic and merged by FLASH with the following criteria: (i) the reads were truncated at any site receiving an average quality score < 20 over a 50 bp sliding window; (ii) sequences whose overlap was longer than 10 bp were merged according to their overlap with a mismatch of no more than 2 bp; (iii) sequences of each sample were separated according to barcodes (exactly matching) and primers (allowing 2 nucleotide mismatching), and reads containing ambiguous bases were removed. Operational taxonomic units (OTUs) were clustered with 97% similarity cutoff using UPARSE (version 7.1 http://drive5.com/uparse/) with a novel “greedy” algorithm that performs chimera filtering and OTU clustering simultaneously. The taxonomy of each 16S rRNA gene sequence was analyzed by the RDP Classifier algorithm (http://rdp.cme.msu.edu/) against the Silva (SSU123) 16S rRNA database using a confidence threshold of 70%, and each 18S rRNA gene sequence was analyzed against the silva128/18S_eukaryota database using a confidence threshold of 70% (Vilo and Dong 2012). The alpha-diversity level of microbial community was described for each sample using the metrics Chao1 and Shannon (Hartmann and Widmer 2006).
Structural equation models (SEMs) were used to evaluate direct and indirect effects of seawater properties, microbial diversity of Pyropia haitanensis and seawater without Pyropia cultivation on the microbial diversity of cultivation seawater (Eisenhauer et al. 2015). All the data used in the SEMs was standardized using Z scores. A covariance matrix was calculated by pairwise correlations using SPSS statistical software (version 20, SPSS Inc.,, USA) and was then imported into AMOS 21 (SPSS Inc.) for SEMs construction using maximum likelihood estimation. Goodness-of-fit index (N0.90), χ2 test (P N0.05), and root mean square errors of approximation (RMSEA b 0.05) were used to test the overall goodness of the model fit.
A non-metric multidimensional scaling (NMDS) analysis of Bray–Curtis distance (Kent 2011) and the alpha-diversity (diversity, richness and evenness) (Hartmann and Widmer 2006) were calculated using the “vegan” package in the R 3.4.0 software for investigating the dissimilarities among samples and visualized with Orginlab 2019. Adonis tests (Su et al. 2015) were used to determine significant differences among communities. An analysis of differences between samples was performed on the I-Sanger online platform (https://www.i-sanger.com). All statistical tests using one-way ANOVA test were conducted by SPSS Software V20.0 (IBM, USA).
Results
Characterization of prokaryotic and eukaryotic microbial communities of seawater with or without Pyropia haitanensis cultivation
A total of 4498 OTUs, with 3,085,384 high-quality prokaryotic sequences, were obtained, in which, 982,608 sequences of epiphytic microbes on P. haitanensis were distributed among 3824 OTUs, 1,925,622 cultivation seawater sequences were distributed among 3633 OTUs, and 2,278,090 non-cultivation seawater sequences were distributed among 1921 OTUs (Table 1). For the eukaryotic community, 1139 OTUs, with 3,590,560 high-quality sequences, were obtained, among which, 1,223,131 sequences of epiphytic microbes on P. haitanensis were distributed among 751 OTUs, 2,044,299 cultivation seawater sequences were distributed among 790 OTUs, and 2,398,942 non-cultivation seawater sequences were distributed among 617 OTUs (Table 1).
The alpha-diversity of diversity, evenness, and richness between the seawater with and without Pyropia cultivation samples showed that the biodiversity of cultivation seawater was significantly greater (P < 0.01) than that of non-cultivation seawater, but had no distinction from Pyropia samples base on the 16S rRNA gene analysis (Fig. 1). For the eukaryotic microbial diversity, the richness of cultivation seawater was also significantly higher (P < 0.01) than that of non-cultivation seawater (Fig. 1).
An NMDS analysis based on a Bray–Curtis dissimilarity matrix revealed significant (P < 0.001) differences between the seawater with and without Pyropia cultivation samples at prokaryotic and eukaryotic microbial level (Fig. 2). Additionally, the thallus and conchocelis stages among cultivation seawater samples were also significantly (P < 0.001) distinctive and clustered with the thallus and conchocelis stages of Pyropia samples, respectively (Fig. 2).
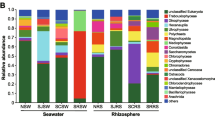
The differences in the bacterial and eukaryotic community compositions between the seawater with and without Pyropia cultivation were further demonstrated in detail at phylum and family levels. For the bacterial community composition at the phylum level, Proteobacteria and Cyanobacteria were the dominant species among seawater samples. Cyanobacteria were present at a greater level in the non-cultivation seawater compared with in the cultivation seawater during the thallus stage, while Bacteroidetes, Actinobacteria, and Proteobacteria were present at greater levels in the cultivation seawater compared with in the non-cultivation seawater (Fig. 3). For the eukaryotic community composition at the phylum level, Ciliophora, SAI-3C06, and Cryptophyta were present higher levels in Pyropia cultivation seawater than in the seawater without thallus cultivation, while Ascomycota were higher in seawater without conchocelis cultivation than in cultivation seawater (Fig. 3).
The compositions of bacterial and eukaryotic communities in the seawater with and without Pyropia cultivation during the thallus and conchocelis stages at family level (with > 1% relative abundance) are shown in Fig. 4. For the bacterial community, during the conchocelis stage, Rhodobacteraceae, Flavobacteriaceae, Oceanospirillaceae, Litoricolaceae, Saprospiraceae, Rhodospirillaceae, Hyphomonadaceae, and Alteromonadaceae were significantly higher (P < 0.05) in cultivation seawater than in non-cultivation seawater (Fig. 4). During thallus stage, Rhodobacteraceae, Flavobacteriaceae, Rhodospirillaceae, Oceanospirillaceae, Methylophilaceae, and Cryomorphaceae were significantly higher (P < 0.05) in cultivation seawater than in non-cultivation seawater (Fig. 4). For the eukaryotic community, during conchocelis stage, Stephanoecidae and Trichocomaceae were significantly higher (P < 0.05) in non-cultivation seawater than in cultivation seawater (Fig. 4).
The effects of Pyropia haitanensis cultivation on the microbial community in surrounding seawater
One important capability of SEM is to partition direct and indirect effects that one variable may have on another. Our models explained 18 and 60% of the variances found in patterns of bacterial diversity on P. haitanensis and in seawater without Pyropia cultivation, respectively (Fig. 5a). However, for the bacterial diversity in the cultivation seawater, 22% of the variance was explained by our model. Seawater properties had a direct positive effect on P. haitanensis bacterial diversity (λ = 0.42*, P < 0.05), which strongly positively affected the cultivation seawater bacterial diversity (λ = 0.46*, P < 0.05; Fig. 5a). In addition, seawater properties had a direct negative effect on bacterial diversity in non-cultivation seawater (λ = − 0.78***, P < 0.05), but this had no significantly direct effect on cultivation seawater bacterial diversity (Fig. 5a). For the eukaryotic community, our models explained 43 and 7% of the variances found in patterns of eukaryotic diversity on P. haitanensis and in the seawater without Pyropia cultivation, respectively (Fig. 5b). However, for the eukaryotic diversity of cultivation seawater, 81% of the variance was explained by our model. Seawater properties had a direct positive effect on P. haitanensis eukaryotic diversity (λ = 0.66**, P < 0.01), which strongly positively affected cultivation seawater eukaryotic diversity (λ = 0.75***, P < 0.001; Fig. 5b). However, seawater properties had no significantly direct effects on the eukaryotic diversity of the seawater with and without Pyropia cultivation. The eukaryotic diversity in non-cultivation seawater also had no significantly direct effect on the eukaryotic diversity in cultivation seawater (Fig. 5b).
Structural equation models show the direct and indirect effects of seawater properties, bacterial (a) and eukaryotic (b) diversity of Pyropia haitanensis and seawater without Pyropia cultivation on the microbial bacterial (a) and eukaryotic (b) diversity of seawater with Pyropia cultivation. Solid and dashed arrows indicate significant and non-significant relationships, respectively. Numbers adjacent to the arrows are path coefficients, and the width of the arrows is proportional to the strength of the path coefficients. R2 denotes the proportion of variance explained. Significance levels are indicated: *P < 0.05, **P < 0.01 and ***P < 0.001. Standardized effects (total, direct, and indirect effects) are derived from the structural equation models. The hypothetical models fit our data well: χ2 = 0.658, P = 0.417, CFI = 1, GFI = 0.984, and RMSEA < 0.001 (a); χ2 = 1.381, P = 0.240, CFI = 0.991, GFI = 0.968, and RMSEA < 0.001 (b)
Characterization of prokaryotic and eukaryotic microbial communities of Pyropia haitanensis during different cultivation stages
To evaluate the biodiversity at the same sequencing depth, 34,200 bacterial sequences and 45,602 eukaryotic sequences per sample were randomly selected to generate rarefaction curves. The alpha-diversity (Fig. 6) showed a distinct level of epiphytic microbes on P. haitanensis among different cultivation stages based on diversity, richness and evenness. For the bacterial community, adult thallus showed the highest diversity and richness levels compared with other cultivation stages. For the eukaryotic community, cochospores exhibited the highest diversity and evenness levels compared with other cultivation stages (Fig. 6).
Variations in the bacterial community composition, visualized by the NMDS analysis based on a Bray–Curtis dissimilarity matrix, revealed a significant difference (P < 0.001) between the thallus and conchocelis stages of epiphytic bacterial and eukaryotic community on P. haitanensis (Fig. 7). Additionally, different developmental periods among the thallus or conchocelis stages of epiphytic bacterial on P. haitanensis were also separated, as were those of the eukaryotic community (Fig. 7).
Shifts in the bacterial and eukaryotic community composition were demonstrated in detail by examining community compositions at phylum and family levels. At the phylum level, Proteobacteria, Cyanobacteria, and Bacteroidetes were dominant among the epiphytic bacterial community on P. haitanensis during the entire cultivation period. Compared with the conchocelis stage, Proteobacteria decreased during the thallus stage, while Actinobacteria and Cyanobacteria increased (Fig. 8). Ciliophora and Cryptophyta were the dominant eukaryotic microbes of P. haitanensis during the entire cultivation stages at phylum level. Compared with the conchocelis stage, Ciliophora decreased during the thallus stage, while Cryptophyta increased (Fig. 8). The composition of the epiphytic bacterial and eukaryotic community on P. haitanensis between the thallus and conchocelis stages at the family level (with > 1% relative abundance) is shown in Fig. 8. Oceanospirillaceae was present at a significantly higher level (P < 0.05) during the conchocelis stage than the thallus stage, while Acidimicrobiaceae, SAR116_clade and SAR86_clade were present at significantly higher levels (P < 0.05) during the thallus stage than the conchocelis stage. Among the fungal community members on P. haitanensis, the level of Choreotrichia was significantly higher (P < 0.05) during the thallus stage than the conchocelis stage (Fig. 8). Conversely, Choanoflagellida, Cryptophyceae, Capsasporidae, and Trichocomaceae were present at significantly higher levels (P < 0.05) during the conchocelis stage than the thallus stage (Fig. 8).
Discussion
Macroalgae are living hosts, performing essential and defining roles in coastal ecosystems (Burke et al. 2011). Microbial biofilm communities in the marine environment positively affect the health, growth, and development of their hosts (Marshall et al. 2006; Burke et al. 2011). However, studies concerning the effects of the mariculture of economic red algae Pyropia on microbial communities are scarce. Additionally, the Pyropia life cycle contains two clearly different stages: the conchocelis and thallus stages (Blouin et al. 2011). However, the degree of microbial variation between the two stages is unknown. In the present study, therefore, deep sequencing was applied to investigate the microorganisms associated with P. haitanensis during the entire cultivation period.
The effects of P. haitanensis cultivation on the microbial community of the surrounding seawater
The present data showed that P. haitanensis cultivation had obvious impact on the microbial community of the surrounding seawater (Figs. 1, 2, 3, 4, and 5), such that P. haitanensis bacterial diversity strongly positively affected cultivation seawater bacterial diversity and eukaryotic diversity (Fig. 5). Many bacteria were enriched remarkably in P. haitanensis cultivation seawater. This result is consistent with the previous study that Gracilaria lemaneiformis cultivation altered the microbial community composition and structure in cultivation seawater (Xie et al. 2017). The surfaces of marine macroalgae support diverse microbial biofilms (Lemay et al. 2018). The seaweed can also secrete various organic substances that can be utilized by bacteria (Singh and Reddy 2014). The algal polysaccharides (e.g., glucose, mannose and galactose) are potential sources of carbon and energy for numerous marine bacteria (Goecke et al. 2010; Hehemann et al. 2012). For example, Proteobacteria are known to digest galactan sulfates in red algal cell walls (Miranda et al. 2013). Roseovarius can also use glycerol provided by the marine macroalga Ulva mutabilis as a carbon source (Kessler et al. 2018). Here we found the most dominant phylum in seawater was Rhodobacteraceae in response to P. haitanensis cultivation (Fig. 3). Rhodobacteraceae, belonging to Alphaproteobacteria, are key players of biogeochemical cycling and account for up to 30% of bacterial communities in pelagic environments (Simon et al. 2017). Marine Rhodobacteraceae generally function in fucoidan desulfonation and the synthesis of the plant hormones and compatible solutes (e.g., ectoin and carnitine) (Simon et al. 2017). Additionally, Flavobacteriaceae exhibited higher abundance in seawater with Pyropia cultivation compared with non-cultivation seawater (Fig. 4), and they have the capacity to use brown seaweed fucoidan, a mixture of sulfated fucose-containing polysaccharides (Sakai et al. 2002). Moreover, Jiao et al. (2010) showed that dissolved organic matter released by algae can be utilized directly by microbes, constituting a microbial loop in aquatic ecosystems. Therefore, P. haitanensis may provide a habitat and nutrients for microbes able to degrade algal polysaccharides. The seaweeds have the capacity to exude organic carbon and nutrients, whereas the seawater without seaweed cultivation contains relatively low nutrient concentrations. This might explain the strong differences in microbial communities between seawater with and without seaweed cultivation (Pregnall 1983).
In addition to eukaryotes, a dominated fungal species is Choreotrichia, which provides a key trophic link between the microbial and macroscopic components of the pelagic food web (Doherty et al. 2010). This finding indicated the important role of P. haitanensis cultivation in shaping the microbial diversity of cultivation seawater.
Differences in the microbial communities during various life stages of P. haitanensis
The annual life cycle of P. haitanensis consists of a thallus stage and a shell-living conchocelis stage (Zeng 1985; Blouin et al. 2011). These stages are remarkably distinct in morphology, longevity, ploidy, and living environment. The conchospore develops into the thallus after undergoing meiosis. Meiosis in P. haitanensis occurs during the first two divisions of the germinating conchospore. The initial four cells of a developing conchosporeling constitute a linear genetic tetrad, resulting in the formation of chimeric blades of P. haitanensis (Yan et al. 2005). In the present study, the diversity, evenness and richness of the bacteria were highest in the adult thallus stage (Fig. 6). Additionally, the microbial community was obviously different between conchocelis and thallus stages (Figs. 6, 7, and 8). Firmicutes and the SAR86 group that belong to uncultured marine γ-proteobacterial were enriched in the thallus stage (Figs. 3 and 8). Interestingly, although the relative abundance of Firmicutes is very high in seawater without sporangial cultivation, it was not detected in sporangia (Fig. 3). This indicated that Firmicutes play important roles in thallus growth and development. Firmicutes and Proteobacteria (Alphaproteobacteria and Gammaproteobacteria) are also abundant on Porphyra umbilicalis blades collected in different seasons (Miranda et al. 2013). Proteobacteria and Firmicutes are involved in the morphogenesis of the green alga Ulva by synthesizing the differentiation inducer thallusin (Matsuo et al. 2005). Roseovarius sp. MS2 is able to sense dimethylsulfoniopropionate released by Ulva and promote the subsequent Ulva development and morphogenesis (Kessler et al. 2018). Matsuo and colleagues (2003) also found that the normal morphology of Monostroma oxyspermum depends on the presence of particular bacteria and not on bacteria in general. Therefore, the present data suggested that the enriched bacteria might promote Pyropia morphogenesis.
The fungal enrichment results showed that Cryptophyta was dominant during the thallus stage, while Choanoflagellida, Cryptophyceae, Capsasporidae, and Trichocomaceae were enriched during the conchocelis stage. We also found that seawater properties had a direct positive effect on P. haitanensis eukaryotic diversity (λ = 0.66**, P < 0.01; Fig. 5b). This difference, therefore, may result from the higher water temperature in the culture ponds during industrial cultivation of conchocelis compared with the seawater temperature during oceanic cultivation of thalli. High temperature is known to improve the growth rates of marine fungi. In addition, the water exchange rate in the conchocelis culture ponds was lower than that in the ocean, and the slow flow rate in the ponds may facilitate the accumulation of fungi. Pyropia disease outbreaks always occur during conchocelis cultivation, especially in hot summers, and results in serious economic losses (Guan et al. 2013). Thus, excessive temperature changes should be prevented and the seawater should be refreshed in a timely fashion in the culture ponds to protect conchocelis from disease induced by fungi. Furthermore, the diversity, evenness, and richness of eukaryotes were the greatest in the conchospore stage, while they were very low in the sporangial stage (Fig. 6), even though conchospore are released after sporangial mature. This might occur because conchospores are single-celled algae that lack a cell wall (Fan et al. 2008), which allows epiphytic eukaryotic to easily parasitize or invade algae and even induce disease (Egan et al. 2013). For example, Olpidiopsis porphyrae utilizes encysted zoospores to infect Pyropia (Sekimoto et al. 2008). In addition, some epiphytic and endophytic fungi secrete defensive compounds to resist invasion from other microorganisms, such as terpenoids, alkaloids, and lipids, which exhibit antibacterial and antifungal activities (Rateb and Ebel 2011; Egan et al. 2013). However, the functions of eukaryotes in the alga–fungus symbiosis require further investigation.
The advantages of microbes for P. haitanensis growth and development
One topic in the macroalgal holobiont is the advantages of the presence of microbes on macroalgae. For example, the microbes produce plant growth regulators (e.g., indole-3-acetic acid and cytokinins) (Maruyama et al. 1990; Singh et al. 2011), quorum sensing molecules (Twigg et al. 2014; Singh et al. 2015), and bioactive compounds (e.g., vitamin B12) (Singh et al. 2011; Singh and Reddy 2014). Here, Bacillaceae were enriched in the seawater with Pyropia cultivation (Fig. 4). Isolates of Bacillus species from the rhizosphere of soybean (Glycine max) produce indole-3-acetic acid and stimulate increased root lengths, shoot lengths, and numbers of lateral roots in soybean seedlings (Wahyudi et al. 2011). Furthermore, Bacillus pumilus and B. licheniformis, isolated from the rhizosphere of alder (Alnus glutinosa), produce high concentrations of gibberellins to promote host plant growth (Gutiérrez-Mañero et al. 2001). A Bacillus megaterium strain promoted the growth of Arabidopsis thaliana and Phaseolus vulgaris seedlings by regulating cytokinin signal transduction (Ortíz-Castro et al. 2008). Pyropia haitanensis co-cultured with Bacillus sp. WPySW2 grew better than wild type at 20 °C (Xiong et al. 2018). The present data suggested that Bacillaceae may be applicable as plant growth and development promoters for Pyropia. Moreover, Tait et al. (2005) demonstrated that the disruption of quorum sensing (N-acylhomoserine lactone) in seawater prevents the attraction of Ulva zoospores to bacterial biofilms. Additionally, many macroalgae are rich in vitamin B12, including Pyropia (Nakamura et al. 2013), but vitamin B12 can only be synthesized by prokaryotes. Thus, a mutualistic relationship has been proposed to exist between vitamin B12-dependent algae and heterotrophic bacteria. In the present study, Firmicutes were a key factor in the interactions among different bacteria in the thallus stage (Fig. 3). Firmicutes are generally one of the most abundant bacteria on seaweed surfaces and are potential candidates as producers of vitamin B12 (Singh and Reddy 2014). Pyropia/Porphyra may obtain vitamin B12 from its abundant bacterial epiphytes (Brawley et al. 2017). Thus, the enriched microbial community in the cultivation seawater might be beneficial for the development and growth of the host seaweed (Singh et al. 2011; Singh and Reddy 2014).
Conclusion
We observed that P. haitanensis harbored phylogenetically diverse bacteria and eukaryotic organisms during the entire cultivation period. A distinct shift in the microbial community composition of P. haitanensis was observed between seawater with and without Pyropia cultivation as well as between the oceanic cultivation of thalli and industrial cultivation of conchocelis. The detected potential candidate bacteria might promote Pyropia morphogenesis and produce vitamin B12 and various plant hormones that promote the growth and development of Pyropia. The present results enhance our knowledge of the important role of seaweed cultivation in shaping the microbial diversity of cultivation seawater and the beneficial bacterial and fungal associations with seaweed.
References
Armstrong E, Yan L, Boyd KG, Wright PC, Burgess JG (2001) The symbiotic role of marine microbes on living surfaces. Hydrobiologia 461:37–40
Beleneva IA, Zhukova NV (2006) Bacterial communities of some brown and red algae from Peter the Great Bay, the Sea of Japan. Microbiology 75:348–357
Bell W, Mitchell R (1972) Chemotactic and growth responses of marine bacteria to algal extracellular products. Biol Bull 143:265–277
Blouin NA, Brodie JA, Grossman AC, Xu P, Brawley SH (2011) Porphyra: a marine crop shaped by stress. Trends Plant Sci 16:29–37
Brawley SH, Blouin NA, Ficko-Blean E, Wheeler GL, Lohr M, Goodson HV, Jenkins JW, Blaby-Haas CE, Helliwell KE, Chan CX, Marriage TN, Bhattacharya D, Klein AS, Badis Y, Brodie J, Cao Y, Collén J, Dittami SM, Gachon CMM, Green BR, Karpowicz SJ, Kim JW, Kudahl UJ, Lin S, Michel G, Mittag M, Olson BJSC, Pangilinan JL, Peng Y, Qiu H, Shu S, Singer JT, Smith AG, Sprecher BN, Wagner V, Wang W, Wang ZY, Yan J, Yarish C, Zäuner-Riek S, Zhuang Y, Zou Y, Lindquist EA, Grimwood J, Barry KW, Rokhsar DS, Schmutz J, Stiller JW, Grossman AR, Prochnik SE (2017) Insights into the red algae and eukaryotic evolution from the genome of Porphyra umbilicalis (Bangiophyceae, Rhodophyta). Proc Natl Acad Sci U S A 114:E6361–E6370
Burke C, Thomas T, Lewis M, Steinberg P, Kjelleberg S (2011) Composition, uniqueness and variability of the epiphytic bacterial community of the green alga Ulva australis. ISME J 5:590–600
China Fishery Bureau, Fishery production (2018) China fishery statistical yearbook (in Chinese). Chinese Agriculture Express
Cole JJ (1982) Interactions between bacteria and algae in aquatic ecosystems. Annu Rev Ecol Syst 13:291–314
de Mesquita MMF, Crapez MAC, Teixeira VL, Cavalcanti DN (2019) Potential interactions bacteria-brown algae. J Appl Phycol 31:867–883
Dittami S, Arboleda E, Auguet JC, Bigalke A, Briand E, Cárdenas P, Cardini U, Decelle J, Engelen A, Eveillard D, Gachon CMM, Griffiths S, Harder T, Kayal E, Kazamia E, Lallier FH, Medina M, Marzinelli EM, Morganti T, Pons LN, Pardo S, Valverde J, Saha M, Selosse M, Skillings D, Stock W, Sunagawa S, Toulza E, Vorobev A, Leblanc C, Not F (2019) A community perspective on the concept of marine holobionts: state-of-the-art, challenges, and future directions. PeerJ arXiv: 1907.05017
Doherty M, Tamura M, Vriezen JA, McManus GB, Katz LA (2010) Diversity of Oligotrichia and Choreotrichia ciliates in coastal marine sediments and in overlying plankton. Appl Environ Microbiol 76:3924–3935
Egan S, Harder T, Burke C, Steinberg P, Kjelleberg S, Thomas T (2013) The seaweed holobiont: understanding seaweed–bacteria interactions. FEMS Microbiol Rev 37:462–476
Eisenhauer N, Bowker MA, Grace JB, Powell JR (2015) From patterns to causal understanding: structural equation modeling (SEM) in soil ecology. Pedobiologia 58:65–72
Fan XL, Wang GC, Li DM, Xu P, Shen SD (2008) Study on early-stage development of conchospore in Porphyra yezoensis Ueda. Aquaculture 278:143–149
Farooq A, Francesca M (2007) Microbial structuring of marine ecosystems. Nat Rev Microbiol 5:782
Gai S, Zhang W (2016) Microbiota shell-boring conchocelis of Pyropia yezoensis determined by the next-generation sequencing. Oceanol Limnol Sin 47:990–996 (in Chinese)
Goecke F, Labes A, Wiese J, Imhoff JF (2010) Chemical interactions between marine macroalgae and bacteria. Mar Ecol Prog Ser 409:267–299
Guan X, Li J, Zhang Z, Li F, Yang R (2013) Characterizing the microbial culprit of white spot disease of the conchocelis stage of Porphyra yezoensis (Bangiales, Rhodophyta). J Appl Phycol 25:1341–1348
Gutiérrez-Mañero FJ, Ramos-Solano B, Probanza AN, Mehouachi J, Tadeo FR, Talon M (2001) The plant-growth-promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiol Plant 111:206–211
Hartmann M, Widmer F (2006) Community structure analyses are more sensitive to differences in soil bacterial communities than anonymous diversity indices. Appl Environ Microbiol 72:7804–7812
Hehemann JH, Correc G, Thomas F, Bernard T, Barbeyron T, Jam M, Helbert W, Michel G, Czjzek M (2012) Biochemical and structural characterization of the complex agarolytic enzyme system from the marine bacterium Zobellia galactanivorans. J Biol Chem 287:30571–30584
Jiao N, Herndl GJ, Hansell DA, Brenner R, Kattner G, Wilhelm SW, Kirchman DL, Weinbauer MG, Luo T, Chen F, Azam F (2010) Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean. Nat Rev Microbiol 8:593–599
Kent M (2011) Vegetation description and data analysis: a practical approach. Wiley, Hokoben
Kessler RW, Weiss A, Kuegler S, Hermes C, Wichard T (2018) Macroalgal–bacterial interactions: role of dimethylsulfoniopropionate in microbial gardening by Ulva (Chlorophyta). Mol Ecol 27:1808–1819
Lemay MA, Martone PT, Keeling PJ, Burt JM, Krumhansl KA, Sanders RD, Parfreyet LW. (2018) Sympatric kelp species share a large portion of their surface bacterial communities. Environ Microbiol 20(2):658–670
Marshall K, Joint I, Callow ME, Callow JA (2006) Effect of marine bacterial isolates on the growth and morphology of axenic plantlets of the green alga Ulva linza. Microb Ecol 52:302–310
Maruyama A, Maeda M, Simidu U (1990) Distribution and classification of marine bacteria with the ability of cytokinin and auxin production. Bull Jap Soc Microb Ecol 5:1–8
Matsuo Y, Imagawa H, Nishizawa M, Shizuri Y (2005) Isolation of an algal morphogenesis inducer from a marine bacterium. Science 307:1598
Miranda LN, Hutchison K, Grossman AR, Brawley SH (2013) Diversity and abundance of the bacterial community of the red macroalga Porphyra umbilicalis: did bacterial farmers produce macroalgae? PLoS One 8:e58269
Nakamura Y, Sasaki N, Kobayashi M, Ojima N, Yasuike M, Shigenobu Y, Satomi M, Fukuma Y, Shiwaku K, Tsujimoto A, Kobayashi T, Nakayama I, Ito F, Nakajima K, Sano M, Wada T, Kuhara S, Inouye K, Gojobori T, Ikeo K (2013) The first symbiont-free genome sequence of marine red alga, Susabi-nori (Pyropia yezoensis). PLoS One 8:e57122
Ortíz-Castro R, Valencia-Cantero E, López-Bucio J (2008) Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal Behav 3:263–265
Pregnall AM (1983) Release of dissolved organic carbon from the estuarine intertidal macroalga Enteromorpha prolifera. Mar Biol 73:37–42
Rateb ME, Ebel R (2011) Secondary metabolites of fungi from marine habitats. Nat Prod Rep 28:290–344
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351
Sakai T, Kimura H, Kato I (2002) A marine strain of Flavobacteriaceae utilizes brown seaweed fucoidan. Mar Biotechnol 4:399–405
Sekimoto S, Yokoo K, Kawamura Y, Honda D (2008) Taxonomy, molecular phylogeny, and ultrastructural morphology of Olpidiopsis porphyrae sp. nov. (Oomycetes, straminipiles), a unicellular obligate endoparasite of Bangia and Porphyra spp. (Bangiales, Rhodophyta). Mycol Res 112:361–374
Shen M, Yang R, Luo Q, Wang S, Ren J (2013) Microbial diversity of Pyropia haitanensis phycosphere during cultivation. Acta Microbiol Sin 53:1087–1102 (in Chinese)
Simon M, Scheuner C, Meier-Kolthoff JP, Brinkhoff T, Wagner-Döbler I, Ulbrich M, Klenk HP, Schomburg D, Petersen J, Göker M (2017) Phylogenomics of Rhodobacteraceae reveals evolutionary adaptation to marine and non-marine habitats. ISME J 11:1483–1499
Singh RP, Reddy CRK (2014) Seaweed–microbial interactions: key functions of seaweed-associated bacteria. FEMS Microbiol Ecol 88:213–230
Singh RP, Bijo AJ, Baghel RS, Reddy CRK, Jha B (2011) Role of bacterial isolates in enhancing the bud induction in the industrially important red alga Gracilaria dura. FEMS Microbiol Ecol 76:381–392
Singh RP, Baghel RS, Reddy CRK, Jha B (2015) Effect of quorum sensing signals produced by seaweed-associated bacteria on carpospore liberation from Gracilaria dura. Front Plant Sci 6:117
Su JQ, Wei B, Ou-Yang WY, Huang F-Y, Zhao Y, Xu H-J, Zhu Y-G (2015) Antibiotic resistome and its association with bacterial communities during sewage sludge composting. Environ Sci Technol 49:7356–7363
Tait K, Joint I, Daykin M, Milton DL, Williams P, Camara M (2005) Disruption of quorum sensing in seawater abolishes attraction of zoospores of the green alga Ulva to bacterial biofilms. Environ Microbiol 7:229–240
Twigg MS, Tait K, Williams P, Atkinson S, Cámara M (2014) Interference with the germination and growth of Ulva zoospores by quorum-sensing molecules from Ulva-associated epiphytic bacteria. Environ Microbiol 16:445–453
van der Loos LM, Eriksson BK, Salles JF (2019) The macroalgal holobiont in a changing sea. Trends Microbiol 7:635–650
Vilo C, Dong Q (2012) Evaluation of the RDP classifier accuracy using 16S rRNA gene variable regions. Metagenomics 1:1–5
Wahyudi AT, Astuti RP, Widyawati A, Mery A, Nawangsih AA (2011) Characterization of Bacillus sp. strains isolated from rhizosphere of soybean plants for their use as potential plant growth for promoting rhizobacteria. J Microbiol Antimicrob 3:34–40
Xie X, He Z, Hu X, Yin H, Liu X, Yang Y (2017) Large-scale seaweed cultivation diverges water and sediment microbial communities in the coast of Nan’ao Island, South China Sea. Sci Total Environ 598:97–108
Xiong Y, Yang R, Sun X, Yang H, Chen H (2018) Effect of the epiphytic bacterium Bacillus sp. WPySW2 on the metabolism of Pyropia haitanensis. J Appl Phycol 30:1225–1237
Yan XH, Li L, Aruga Y (2005) Genetic analysis of the position of meiosis in Porphyra haitanensis Chang et Zheng (Bangiales, Rhodophyta). J Appl Phycol 17:467–473
Yan Y, Yang H, Tang L, Li J, Mao Y, Mo Z (2019) Compositional shifts of bacterial communities associated with Pyropia yezoensis and surrounding seawater co-occurring with red rot disease. Front Microbiol 10:1666
Zeng C (1985) Seaweed aquaculture. Shanghai Scientific & Technical Publishers, Shanghai
Zhu Y, Wu M, Gao N, Chu W, Wang S (2016) Impacts of nitrate and electron donor on perchlorate reduction and microbial community composition in a biologically activated carbon reactor. Chemosphere 2016:134–143
Acknowledgments
We thank the Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac) for editing the English text of a draft of this manuscript.
Funding
This work was supported by “National key R&D Program of China (Grant No: 2018YFD0900106 and 2018YFD0901500), Fujian Province Science and Technology Major Project (2019NZ08003)” and supported by “China Agriculture Research System (Grant No: CARS-50).”
Author information
Authors and Affiliations
Contributions
C.T.X, W.L.W, and L.W conceived and designed the experiment. C.T. X, W.L.W, and L.W performed the experiments and data analysis. C.T.X and C.S.C contributed by planning, supervising, and financing the work. K. X, D.H.J, and Y. X helped to prepare the materials and reagents. W.L.W, L. W, and C.T.X drafted and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, W., Wu, L., Xu, K. et al. The cultivation of Pyropia haitanensis has important impacts on the seawater microbial community. J Appl Phycol 32, 2561–2573 (2020). https://doi.org/10.1007/s10811-020-02068-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02068-6