Abstract
Microalgal cultivation in aquaculture wastewater (AWW) from recirculating aquaculture systems (RAS) is an approach for combined production of valuable algal biomass and AWW treatment. The growth, nutrient uptake, fatty acid (FA) profile, and tocopherol content of mixed algal cultures of Euglena gracilis with Selenastrum grown in AWWs from pikeperch (Sander lucioperca) and catfish (Clarias anguillaris) RAS were studied. The highest algal biomass (1.5 g L−1), lipid (84.9 mg L−1), and tocopherol (877.2 μg L−1) yields were achieved in sludge-amended pike perch AWW. Nutrient removal rates in experiments were 98.9–99.5 and 98.4–99.8% for NH4-N and PO4-P, and 75.4–89.2% and 84.3–95.7% for TN and TP, respectively, whereas the COD was reduced by 45.8–67.6%. Biomass EPA and DHA content met, while ARA and tocopherol content exceeded the requirements for fish feed. Algal cultivation in AWWs is a promising alternative for AWW treatment while providing a replacement for fish oil in feed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture is a fast-growing sector in food production, with an average annual growth of 3.2% and in 2014 human consumption of farmed fish outweighed consumption of wild-caught fish for the first time (FAO 2016). Due to the increase in fish farming, serious concerns have been raised regarding environmental pollution related to the aquaculture wastewater (AWW) and over-exploitation of wild fish populations used as fish-feed ingredients (Ansari et al. 2017). Environmental friendly practices in fish farming and the use of local and low trophic level biomass, e.g., microalgae for feed, may decrease the environmental impacts of fish farming (Martins et al. 2010).
Use of recirculating aquaculture systems (RASs) decreases the pollution effect of fish farming and the need for fresh water (Martins et al. 2010). Therefore, RAS technology is recognized as a future solution to develop aquaculture practices in several European countries (Badiola et al. 2012). In RASs, AWW is treated, and a portion of the effluent water is re-circulated through the aquaculture system. In comparison to flow-through culturing systems, the water exchange rate in very intensive RASs is reduced from > 50 to < 0.1 m3 kg−1 fish feed (Martins et al. 2010). Additionally, minimizing feed use (i.e., feed conversion ratio) reduces waste generation in RAS (Martins et al. 2010). However, in RASs, treatment of concentrated sludge containing suspended solids and nutrients, especially phosphorus, increase operating costs (Martins et al. 2010).
Until 1990, feed ingredients, e.g., in Norwegian salmon industry, were mainly of marine origin, whereas in 2013, the proportion of plant ingredients had increased to 70%, of which 19% were plant oils (Ytrestøyl et al. 2015). Decreased use of fish oils in feed diminishes the content of long-chain polyunsaturated fatty acids (LC-PUFAs) in the feed, especially omega-3 fatty acids (FAs). Low contents of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in particular (Sørensen et al. 2016) reduce the health benefits of farmed fish (Ytrestøyl et al. 2015). Additionally, less attention has been paid to the LC-PUFA arachidonic acid (ARA), belonging to omega-6 FAs, even though it can also be critical for fish growth (Rombenso et al. 2016; Torrecillas et al. 2017). Microalgae are a natural source of LC-PUFAs in aquatic food webs (Brett and Müller-Navarra 1997) and therefore, to avoid deterioration of the nutritional value of farmed fish, interest in replacing LC-PUFA rich fish oils with microalgae is rising (Van Hoestenberghe et al. 2016).
Furthermore, the micronutrients with antioxidative properties, such as tocopherols protecting FAs from oxidation, are important feed components (Hamre 2011; Hamre et al. 2016; Shahidi and Costa de Camargo 2016). Biologically, the most active tocopherol is α-tocopherol (generally known as vitamin E) (Ortíz et al. 2006), which is often present in high concentrations in plant oils (Schwartz et al. 2008; Hamre et al. 2016). However, it is unstable during feed processing, and storage and, therefore, supplementation of feeds with higher concentrations of α-tocopherol than minimal requirements are recommended (Hamre et al. 2016).
Among the microalgal genera, euglenoids and green algae (Chlorophyceae) are common in polluted environments and efficiently remove nutrients from wastewaters (Mustafa et al. 2012; Mahapatra et al. 2013; Tossavainen et al. 2017, 2018). The maximum reported growth rate for Euglena sp., grown in municipal wastewater, is 0.79 day−1 (on dry weight (DW) basis) (Mahapatra et al. 2013), whereas growth rates of several green algal strains grown in various synthetic wastewaters varied between 0.04 and 0.32 day−1 for Selenastrum capricornutum, 0.07 and 0.38 day−1 for Scenedesmus obliquus, and 0.04 and 0.52 day−1 for Chlorella vulgaris (Zhao et al. 2016). In our previous study (Tossavainen et al. 2017), the growth rate of Selenastrum sp., grown in various composting fluids, was 0.34–0.68 day−1. Furthermore, euglenoids are producers of LC-PUFAs (Korn 1964; Schwarzhans et al. 2015; Tossavainen et al. 2018). The most abundant FAs in green algae are palmitic acid (C16:0), oleic acid (C18:1), linoleic acid (C18:2(n-6), LA), and linolenic acid (C18:3(n-3), ALA), whereas green algae are not able to synthesize LC-PUFAs (Taipale et al. 2013; Tossavainen et al. 2017). Also, odd number FAs, not common in biological organisms are typical of euglenoids (Schwarzhans et al. 2015). Additionally, very high concentrations of tocopherols (0.3–5.1 mg g−1 or 2.0–143.6 mg L−1) (Tani and Tsumura 1989) in comparison to several other algae (158.7–184.7 μg g−1 or 0.3–5.5 mg L−1) (Tani and Tsumura 1989; Vismara et al. 2003) or common plant oils (0.32–257 mg per 100 g of oil) (Schwartz et al. 2008) are reported in euglenoids, whereas tocopherols were not found in several strains of molds and yeasts (Tani and Tsumura 1989).
Instead of algal monocultures, mixed cultures can have some advantages in wastewater treatment and productivity since niche differentiation of algal strains, i.e., variation in growth requirements, can result in more efficient resource allocation. This means more efficient nutrient utilization (Stockenreiter et al. 2016; Tossavainen et al. 2018) and better tolerance against invasive algal species (Godwin et al. 2018) or bacterial contaminations (Tossavainen et al. 2018). Transgressive overyielding, i.e., higher lipid productivity in mixed cultures than in most productive monocultures, has been reported in communities of at least five algal strains (Stockenreiter et al. 2016). However, among mono and mixed green algae cultures, the highest biocrude (raw material for transportation fuels) yield was found in a monoculture of Chlorella, although the biocrude yield in two or four strain mixed cultures exceeded the mean of the mixed cultures component species (Godwin et al. 2018). Also under some circumstances, biomass production in mixed cultures has been enhanced (Liu 2016). In our previous study (Tossavainen et al. 2018), we found no evidence for biomass or lipid overyielding in small-scale mixed cultures with two or three strain mixture of Euglena gracilis, Selenastrum, and Chlorella sorokiniana in comparison to the corresponding monocultures, whereas in pilot scale, lipid production was enhanced in mixed culture of E. gracilis with Selenastrum.
High investment and maintenance costs diminish the full implementation of environmentally friendly RAS technology (Badiola et al. 2012) and the commercialization of microalgae as a feed ingredient (Ansari et al. 2017). Converting nutrients from AWWs to algal biomass could decrease the costs of biomass production and AWW treatment and simultaneously simplify the AWW treatment process (Badiola et al. 2012). So far, few studies have explored the potential of algal culturing in RAS AWW treatment, or biomass and total lipid production (Halfhide et al. 2014; Ansari et al. 2017). In this study, we assessed the potential of the non-axenic mixed algal culture of Euglena gracilis with Selenastrum sp. to produce biomass and valuable FAs, particularly EPA, DHA and ARA, and tocopherols, and to simultaneously purify AWW and improve the economy of RAS. Cultivation experiments were carried out in AWWs both with and without amendment of sludge from the solids removal tanks of pike perch or catfish RASs. We hypothesized that sludge amendment (i) increases nutrient concentrations in AWWs, thus enhancing algal biomass production, (ii) nutrients are removed from AWWs, and sludge amendment AWWs, and (iii) growth medium composition affects the FA and tocopherol content in the produced biomass. We also evaluated the biochemical suitability of the produced algal biomass as a possible ingredient in commercial fish feeds.
Material and methods
RAS unit and collection of AWWs
AWWs and sludge for cultivation experiments were collected from a RAS fish pool used for growing pikeperch (Sander lucioperca) or catfish (Clarias anguillaris), and from a solids removal tank which is part of the AWW treatment in RAS (Fig. 1a). The volume of the RAS was 78 m3 (fish pool volume 65 m3), and the flow rate was 35 L s−1. The RAS included a drum filter and a flotation tank for solid (later called sludge) removal, aerobic biofilters (Rotating Bed Biofilm Reactor (RBBR), Clewer R2000, Clewer Technology Oy) for nitrification and anaerobic biofilters (RBBR, Clewer R2000, Clewer Technology Oy) for denitrification. Before recirculation to the fish tank, the water was CO2 stripped by introducing O2 produced on-site (Oxymat-O2 generator) and dissolved into the system via an oxygen tower (Kalavesi Consultants Ltd.), and then sterilized by O3 (Pacific ozone; G series, 12 g h−1) and UV (UltraAqua; MonoRay 440 w, open channel) treatment. Water consumption in fish production was very low, only 0.05 m3 kg−1 of fish. Feed load into the system was max. 75 kg d−1. Both fish species were fed with Circuit White 5 feed (Raisioagro Ltd) optimized for RAS farming. Feed conversion ratios were 1.1–1.2 for pike perch and 0.78 for catfish. Collected sludge enriched by drum filtering and flotation was usually delivered to municipal wastewater treatment.
Microalgal cultivation
The non-axenic mixed cultures of Euglena gracilis (CCAP 1224/5Z) and Selenastrum (SCCAP K-1877) were cultivated in AWWs with and without sludge amendment. We selected the strain combination instead of a monoculture on the basis of our earlier study (Tossavainen et al. 2018) showing efficient wastewater treatment capacity (NH4-N uptake), better tolerance to bacterial contaminations and more efficient lipid production in a pilot scale culture of E. gracilis with the presence of Selenastrum in early cultivation phase in comparison to monoculture of E. gracilis. Additionally, E. gracilis was chosen since it is a known producer of LC-PUFAs and has a high content of tocopherols. Cultivation media were (i) AWW from pike perch pool, (ii) sludge-amended AWW from pike perch pool, (iii) AWW from catfish pool, and (iv) sludge-amended AWW from catfish pool. AWWs without sludge-amendment were used as controls to study the influence of sludge-amendment on growth, nutrient removal, and lipid and tocopherol content.
The experiments were carried out with three replicates in 2-L borosilicate bottles equipped with aeration, degassing, and harvesting pipes in a growth chamber (SANYO growth cabinet MLR-350 H; 294L). The light/dark cycle used was 16L:8D, the photon flux density was 250 μmol photons m−2 s−1 (Li-Cor 190R Quantum Sensor and LI-1400 Light Sensor Logger, Li-Cor, USA), and the temperature was set to 20 °C. Cultures were fed with 0.5 L min−1 of 2% CO2 (purity 99.8%) in moist air. For moisturizing and mixing of supplied gases, compressed air (10 L min−1, Hailea 318 air compressor) and CO2 (0.2 L min−1) were pumped through the water phase in a separate flask before injection into the cultivation bottles. Filters (PTFE membrane filters, Acro37 TF Vent) were connected to the gassing tubes to avoid contamination.
Before starting the experiments, cultivation bottles were filled with 1.6 L of AWWs with or without sludge. In sludge-amended cultures, 43 mL of sludge was added. Before adding to the culturing bottles, the sludge batches were homogenized by shaking. Equal amounts (0.04 g DW) of algal pre-cultures grown in EG-medium (http://www.ccap.ac.uk/media/documents/EG.pdf) were inoculated to the culturing bottles.
Sampling and harvesting
Algal growth was measured by determination of DW. Samples for algal DW determination were taken immediately after inoculation of the algae to the culturing bottles and during the early stationary phase (days 1–6 depending on culture) and after that during the exponential and late stationary phase. Culture pH was measured on each sampling day. Cultivation time was 13 days for AWW grown cultures and 14 days for sludge-amended AWW grown cultures. Survival of strains was checked from samples taken during the growth. Samples for nutrient analysis were taken at the beginning of the experiments, twice during the growth phase, and at the end of the cultivation period. For FA and tocopherol extraction and analysis, biomass from the last cultivation day when the cultures were in late stationary phase was collected by centrifugation (4000 rpm, 10 min) (Multifuge 1 S-R, Kendro Laboratory Products) and the biomass was stored frozen (− 70 °C) and freeze-dried before extraction.
Analytical methods
DW was determined by filtering samples on pre-dried (120 °C, 4 h) filters (GF/C, Ø 47 mm, Whatman) and drying the filters (120 °C, overnight) before weighing (Tredici and Zittelli 1998). The filtrate from DW determination was collected for nutrient analysis. Specific growth rates (day−1 base on DW) during the exponential growth phase were calculated using the equation μ = Ln (DW1 / DW0) / (t1 − t0), where DW0 and DW1 are the DW biomass at the beginning (t0) and end (t1) of the exponential growth. Total nitrogen (TN), ammonium (NH4-N), total phosphorus (TP), phosphate (PO4-P), and chemical oxygen demand (COD) were analyzed using Hach Lange kits (Hach Lange, Germany), a DR 2800TM spectrophotometer (Hach Lange) and a HT 200S high temperature thermostat heating block (Hach Lange). Initial nutrient and COD concentrations were determined before algal inoculation. Survival of strains was checked microscopically, and the cell concentrations were counted from the samples taken after the inoculation and from the end of the cultivation using Lund chambers (Lund 1959). Samples taken for microscopy were stained with acidic Lugol’s solution, and for reliable cell counts, at least 100 cells were counted.
Lipids were extracted from 0.1 g homogenized freeze-dried samples with ethanol using accelerated solvent extraction (Dionex ASE-200, Dionex Corporation, USA). The samples were mixed with Ottawa sand (Fisher P/N 23-3) and extracted in 11-mL extraction cells at 1500 psi and 125 °C with a 5 min heating time and one 11 min extraction cycle. The extracts were evaporated into dryness and re-dissolved in 10 mL of a heptane-isopropanol mixture (3 + 2, v/v). Aliquots were taken for FA and tocopherol analyses.
FAs were analyzed as methyl esters by gas chromatography using an internal standard method (nonadecanoic acid methyl ester) (Damerau et al. 2014). α-, β-, γ-, and δ-tocopherols were analyzed by normal phase liquid chromatography with fluorescence detection using an external standard method (Schwartz et al. 2008). Total fatty acid (TFA) content was calculated as the sum of the analyzed FAs as methyl esters, and the total tocopherol content was calculated as the sum of analyzed tocopherols.
Statistics
Differences (p < 0.05) in biomass production (DW) and total lipid, EPA, DHA, ARA, and total tocopherol contents and yields were statistically tested (SPSS Statistics, version 24, IBM USA). All the statistics, except testing the yields of DHA and ARA, were performed with one-way ANOVA and post hoc Tukey’s HSD test. Because of heterogeneity of variances (Levene statistic, homogeneity of variances test), differences in DHA and ARA yields were tested with Kruskal-Wallis test, and Mann-Whitney U test was used in the pairwise comparison. The significance level in all tests was p < 0.05. Results of the non-parametric tests were Bonferroni corrected.
Results
Algal growth and nutrient removal
Based on the optimal N/P ratio (molar ratio, N16/P1) (Redfield 1958) in algal biomass, nutrient ratios in both AWWs without sludge amendment were close to algal requirements (N15/P1), whereas the sludge amendment resulted in higher relative amount of P (N12/P1 in sludge-amended pike perch AWW and N11/P1 in sludge-amended catfish AWW). The higher initial nutrient concentrations in the sludge-amended AWWs (Table 1) enhanced the algal biomass growth. The highest biomass content (1.5 g L−1) was reached in the sludge-amended pike perch AWW (p < 0.05) (Fig. 2) with the highest TN and TP concentrations (34.4 and 6.1 mg L−1) (Table 1). In the sludge-amended catfish AWW, the algal biomass was 1.0 g L−1, whereas it was only half of that (0.47 g L−1) in cultures grown in pikeperch AWW and lowest (p < 0.05) in catfish AWW grown culture (0.08 g L−1). The specific growth rates (mean ± SD) during the exponential growth phase (days 1–7 in pikeperch AWWs and 4–12 in AWWs with sludge amendment) were 0.27 day−1 ± 0.04 in pikeperch AWWs, 0.33 day−1 ± 0.07 in sludge-amended pike perch AWWs, and 0.38 day−1 ± 0.21 in sludge-amended catfish AWWs. In catfish AWWs without sludge-amendment, the experiment resulted in negative growth rates. In all cultures, pH values were between 7.05 and 7.50 throughout cultivation. At the end of cultivation, only E. gracilis was present in pikeperch AWW, and it was the strongly dominating strain also in other cultures, where the cell densities of Selenastrum at the end of the cultivation were under reliable counting limits (see “Analytical Methods”). Cell densities of E. gracilis and Selenastrum after inoculation and the cell densities of E. gracilis at the end of the experiments are given in supplementary material (Online Resource 1).
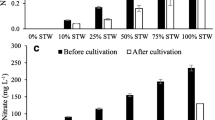
Bioavailable nutrients were consumed from all cultivations during the early phase of the experiment, and finally, in all cases, 98.9–99.5% and 98.4–99.8% of NH4-N and PO4-P were removed, respectively (Table 1). Almost complete removal of NH4-N and PO4-P was reached during the first four to six (NH4-N) and four (PO4-P) cultivation days (Fig. 3b, d). TN and TP removal was 75.4–89.2% and 84.3–95.7%, respectively (Table 1, Fig. 3a, c). The COD reduction (Table 1, Fig. 3e) was higher (67.5%) in cultures grown in sludge-amended catfish AWW than in other AWWs (43.8–53.4%).
a–e Nutrient and COD reduction (mg L−1) (mean ± SD) in the algal cultivation experiments in pikeperch (PP) and catfish (CF) aquaculture wastewaters (AWWs) with and without sludge (S) amendment (n = 3). PP AWW (open square), PP AWW + S (closed square), CF AWW (open triangle), and CF AWW + S (closed triangle)
FA and tocopherol composition in algal biomass
In biomass, total lipid, EPA, and DHA contents (70.3 mg g−1, 7.6 mg g−1, and 4.5 mg g−1) were the highest (p < 0.05) in cultures grown without sludge amendment in pikeperch AWW (Table 2). In cultures grown in catfish AWW without sludge amendment and in sludge-amended pike perch AWW, biomass lipid contents were comparable (56.5 and 55 mg g−1) (p > 0.05), and higher than in cultures grown in sludge-amended catfish AWW (43.6 mg g−1) (p < 0.05). In pike perch and catfish AWW grown cultures, the contents of EPA were comparable and higher (7.6 and 6.2 mg g−1) (p < 0.05) than in sludge-amended pike perch and catfish AWW grown cultures (4.7 and 3.7 mg g−1). The DHA content was highest in pikeperch AWW grown culture (4.5 mg g−1) (p < 0.05), lowest in sludge-amended catfish AWW (2.3 mg g−1) and comparable in cultures grown in sludge-amended pike perch AWW and catfish AWW (3.2 and 3.3 mg g−1). The ARA content was higher in both AWW grown cultures without sludge amendment (4.3 and 4.0 mg g−1 in pike perch and catfish AWWs) than in sludge-amended cultures (3.0 and 2.4 mg g−1 in pike perch and catfish AWWs) (p < 0.05). Almost half of the total lipids in both sludge-amended algae cultures were PUFAs (48.9% and 49.2% in sludge-amended pike perch and catfish AWWs, respectively), whereas in AWWs without sludge amendment, PUFA contribution to all FAs was 56.7% in pike perch and 60.1% in catfish AWW. The proportions of EPA, DHA, and ARA in TFAs in cultures were 8.2–11.2%, 5.3–6.4%, and 5.4–7.3%, respectively. Since the E. gracilis was the dominating strain in all cultures, FA composition reflected the FA composition of E. gracilis.
However, higher biomass yield (Fig. 2) in sludge-amended pikeperch AWW resulted in the highest lipid production (84.9 mg L−1) (p < 0.05) as well as higher yields of ARA, EPA, and DHA (4.6, 2.3, and 5.0 mg L−1) (Table 2) than in other cultures, although the difference was statistically significant only for EPA. Generally, in total lipids, the proportion (% of TFAs) of all PUFAs as well as ARA, EPA, and DHA were higher and the proportion of saturated FAs (SAFAs) lower in cultures grown in AWWs without sludge amendment than in sludge-amended cultures (Table 2).
Tocopherols consisted of α- and γ-tocopherol, whereas β- and δ-tocopherols were not detected. As in the case of FAs, the total tocopherol content was higher (p < 0.05) in pike perch and catfish AWWs without sludge amendment (1358 and 1102 μg g−1) (Fig. 4a) than in the corresponding sludge-amended cultures, where the content of tocopherols was approximately 50% lower (580 and 545 μg g−1). The tocopherol yield was highest in the sludge-amended pike perch AWW grown culture, 877.2 μg L−1, although there was no significant difference in comparison to cultures grown in pikeperch AWW (634.2 μg L−1) or in sludge-amended catfish AWW (550.3 μg L−1) (Fig. 4b). In sludge-amended cultures in pike perch and catfish AWWs, α-tocopherol made up 78% and 72% of total tocopherol, while in the cultures without sludge amendment, the proportions of α-tocopherol were lower, 66% and 68%.
a-b α- and γ-tocopherol. a Concentrations (μg g−1) and b yields (μg L−1) (mean ± SD) at the end of the algal cultivation experiments in pikeperch (PP) and catfish (CF) aquaculture wastewaters (AWWs) with and without sludge (S) amendment (n = 3). Black column = α-tocopherol, white column = γ-tocopherol
Discussion
As hypothesized, higher nutrient concentrations in sludge amendment AWWs enhanced the algal growth. Generally, biomass production was weaker in the cultures grown in catfish versus pikeperch AWWs, but the mean growth rates were near to similar in both sludge-amended cultures. The biomass yield in most efficiently grown cultures in sludge-amended pikeperch AWW was comparable to tilapia AWW grown cultures of Scenedesmus obliquus or Chlorella sorokiniana, but lower than in an Ankistrodesmus falcatus culture (Ansari et al. 2017). Based on our results and the earlier study in tilapia, AWWs indicate that several types of AWWs are suitable for algal cultivation.
As presumed, nutrients especially NH4-N and PO4-P were efficiently removed from cultivation media. Efficient growth in AWW grown cultures without sludge-amendment stopped a few days after the bioavailable nutrients were removed, which indicates nutrient limitation. This was in contrast to the sludge-amended cultures, in which efficient growth continued for almost 1 week after most of the bioavailable NH4-N and PO4-P were exhausted. This was probably a consequence of the more efficient nutrient uptake at the beginning of the experiments and further survival with the stored intracellular nutrients. Luxury uptake of P is a well-known phenomenon among algae (Zhu et al. 2015), whereas under N deficiency, cellular metabolism is switched towards the non-N containing compounds such as lipids and starch (Geider and La Roche 2002). Additionally, NH4-N concentration in the sludge-amended pikeperch AWWs increased rapidly during the first four cultivation days. Transformation of organic or inorganic N to readily bioavailable NH4-N might explain the most efficient biomass production in that culture. NH4-N release to cultivation medium was most probably a consequence of bacterial degradation of organic compounds in sludge, also shown earlier in non-axenic algal cultures grown in organic N containing medium (Berman and Chava 1999). Earlier studies have demonstrated efficient removal of nutrients by algal cultivation in tilapia (Ansari et al. 2017), flounder (Guo et al. 2013), and catfish (Nasir et al. 2015) AWWs. This indicates that algae could be used in the treatment of several types of AWWs. In tilapia AWWs (Ansari et al. 2017), N supplementation enhanced algal biomass production. Our results showed that sludge supplementation to AWWs provides a good source of nutrients for algal growth and additional nutrients are not needed.
More efficient COD reduction in cultures grown in the sludge-amended medium may have been a consequence of higher bacterial concentrations originating from the added non-sterilized sludge. Fast COD removal in algal-bacterial co-cultures in AWW, as well as other wastewaters, has been shown earlier (Halfhide et al. 2014; Tossavainen et al. 2017). However, mixotrophic uptake of organic substrates in E. gracilis and green algae has also been reported (Yamane et al. 2001; Kim et al. 2013), which suggests that contingently E. gracilis and Selenastrum contributed to COD removal.
Nutrient or COD concentrations alone do not explain the differences in biomass production between the cultures. As a result of the less efficient feed conversion by pike perch compared to catfish, there were presumably higher amounts of algal-available micro-nutrients and minerals in AWW and sludge from pike perch farming. Efficient nutrient and COD removal from AWWs indicates that the integration of an algal photobioreactor (PBR) to a RAS could reduce the need for AWW treatment. Our results in sludge-amended cultures further suggest that using algae as a biofilter, the filtration and flotation stages in the AWW treatment process could be at least partly bypassed which would improve the economic feasibility of RAS by reducing AWW treatment costs while also producing valuable algal biomass. However, the volume of AWW produced in large-scale RAS is quite high, and a more practical alternative for algal biomass production could be a separate algal cultivation unit utilizing water, containing sludge collected by flotation. A RAS equipped with an optimized algal AWW treatment could potentially generate water, which is clean enough for recirculation or release without further treatment. A simplified chart for a proposed integrated PBR-RAS unit is given in Fig. 1a, b.
The biochemical composition of algal biomass varied in different AWWs. In our study, in cultures without sludge-amendment, the higher cellular FA and tocopherol content probably resulted from lower initial nutrient concentrations in AWWs. Enhanced FA production of algae under nutrient limitation, especially N limitation, is a general phenomenon reported in several studies (Schüler et al. 2017) including E. gracilis (Regnault et al. 1995). Additionally, lowered NO3-N concentration has been shown to increase the α-tocopherol content in Nannochloropsis oculata biomass (Durmaz 2007). Contradictory, only P limitation had a positive influence on α-tocopherol contents of Chlorella vulgaris, Phaeodactylum tricornutum, and Tetraselmis suecica (Goiris et al. 2015), whereas α-tocopherol production of T. suecica was enhanced in nutrient-replete conditions (Carballo-Cárdenas et al. 2003). However, we did not analyze the biomass nutrient contents in the end of the cultivation, and thus, it is unclear, whether the algae were limited by a single nutrient or by both nutrients. The low N/P ratios and possibility of luxury uptake of phosphorus (Zhu et al. 2015) indicate N rather than P limitation in both cultures. Lower N/P ratios in sludge amended AWWs potentially resulted in more prominent N deprivation. However, differences in nutrient concentrations do not explain the differences in algal tocopherol and FA contents in cultures using AWW from different fish species. Unidentified fish species related factors, such as fish excretion or non-utilized mineral feed residues influenced lipid and tocopherol metabolism. Additionally, the higher proportion of α-tocopherol in sludge-amended cultures can be at least partly directly explained by the composition of aquaculture waste; sludge contains fish excrement and feed residue, both of which can be assumed to be rich in α-tocopherol.
At the end of the experiments, E. gracilis was the dominating strain in all cultures. Optimal pH range for E. gracilis is 2.5–7.0 (Olaveson and Nalewajko 2000), whereas for example to the green alga Chlorella sorokiniana, optimum is 6 but it can grow also in alkaline conditions (pH 9) (Qiu et al. 2017). Results here support our earlier assumption that pH values near to neutral result in the dominance of E. gracilis over Selenastrum (Tossavainen et al. 2018). Because of the resulting dominance, the biochemical composition in the produced biomass reflected the metabolic compounds of E. gracilis. Biomass for FA analysis was harvested soon after the efficient growth was ceased. Typically, under optimal growth conditions and in the exponential growth phase, the proportion of membrane lipids rich in PUFA and LC-PUFA in total FAs is high, whereas synthesis of storage lipids containing SAFAs and monounsaturated fatty acids (MUFAs) is activated under nutrient starvation in the stationary growth phase (Hodgson et al. 1991). A gradual decrease in LC-PUFA content during the growth has been shown earlier in a monoculture of E. gracilis (Schwarzhans et al. 2015; Tossavainen et al. 2018). However, in our study, the TFA, PUFA, and LC-PUFA contents were higher in AWW cultures grown without sludge-amendment which indicates accumulation of storage lipids but also the higher relative amount of membrane lipids than in sludge-amended cultures. Thus, higher SAFA and lower PUFA content in our sludge-amended cultures can be explained by low N and high organic carbon content in the growth medium rather than the growth phase. These conditions can induce wax ester synthesis in E. gracilis (Regnault et al. 1995) resulting in undesirably high SAFA content. However, as shown here and elsewhere (Tossavainen et al. 2017), the high biomass production results in a higher total lipid yield and thus, also higher LC-PUFA and tocopherol yields.
In our algal cultures, EPA and DHA contents were close to or higher than in general required for fish feeds and exceeded the required proportions of ARA and α-tocopherol. Requirements for EPA, DHA, and ARA in feed vary according to fish species, life stage, and growth environment (Rombenso et al. 2016). Sørensen et al. (2016) indicated that up to 6% of fishmeal in feed for Atlantic salmon (Salmo salar) could be substituted with the microalga P. tricornutum. Required proportions of EPA, DHA, and ARA in study of Sørensen et al. (2016) were 8.0–9.3%, 9.1–10.9%, and 0.3–0.5%. Feeding experiments with California Yellowtail (Seriola dorsalis) juveniles indicated that DHA and ARA are critical LC-PUFAs to maximize growth and that supplementing a soybean-oil-based diet with 1.2% EPA, 1.2% DHA, and 0.2% ARA is sufficient to meet LC-PUFA requirements (Rombenso et al. 2016), whereas the corresponding values in our cultures were 8.6–11.2%, 5.3–6.4%, and 5.4–7.3%. According to feeding experiments of Atlantic salmon, α-tocopherol supplementation of 150 mg kg−1 in the feed is recommended (Hamre et al. 2016). Amounts of α-tocopherol in our cultures were 2.7–6-fold higher than the recommended dose. Now feed is supplemented with α-tocopherol in a synthetic form (α-tocopheryl acetate) (Hamre 2011), but interest in natural vitamin E supplements is increasing (Afonso et al. 2013).
To our knowledge, this is the first time, when RAS AWWs from pikeperch and catfish farming were used for algal cultivation and our approach to enrich AWWs with solids from sludge removal tank is unique. The high content of LC-PUFAs and tocopherols in the algal biomasses produced in our experiments indicate that AWW-grown mixed cultures of E. gracilis with Selenastrum are a promising substitute for at least part of the fish oil used in feed. Additionally, earlier studies have shown the high protein content of E. gracilis and green algae (Becker 2007). However, at the end of the experiments, biomass in our cultures consisted mostly of E. gracilis biomass, and further investigations are needed to clarify the possible advantages of mixed algal cultivation in AWWs. Furthermore, characterization of the whole biomass composition is necessary for the evaluation of biomass suitability and safety for feed use.
Conclusions
This study showed that AWWs from RAS units used for growing pikeperch and catfish are suitable for algal biomass production and that sludge amendment to the AWWs increases algal biomass production. Efficient reduction of nutrients and COD from AWWs in mixed cultures of E. gracilis and Selenastrum indicates that AWW treatment can be enhanced by integrating algal culturing units with RAS units. EPA and DHA contents in the biomass were comparable to fish feeds containing fish and plant oils, and the ARA content exceeded the minimal requirements for feed. Tocopherol contents were superior to common plant oils.
References
Afonso C, Bandarra NM, Nunes L, Cardoso C (2013) Tocopherols in seafood and aquaculture products. Crit Rev Food Sci 56:128–140
Ansari FA, Singh P, Guldhe A, Bux F (2017) Microalgal cultivation using aquaculture wastewater: integrated biomass generation and nutrient remediation. Algal Res 21:169–177
Badiola M, Mendiola D, Bostock J (2012) Recirculating aquaculture systems (RAS) analysis: main issues on management and future challenges. Aquac Eng 51:26–35
Becker EW (2007) Micro-algae as a source of protein. Biotechnol Adv 25:207–210
Berman T, Chava S (1999) Algal growth on organic compounds as nitrogen sources. J Plankton Res 21:1423–1437
Brett M, Müller-Navarra D (1997) The role of highly unsaturated fatty acids in aquatic food web processes. Freshw Biol 38:483–499
Carballo-Cárdenas EC, Tuan PM, Janssen M, Wijffels R (2003) Vitamin E (α-tocopherol) production by the marine microalgae Dunaliella tertiolecta and Tetraselmis suecica in batch cultivation. Biomol Eng 20:139–147
Damerau A, Moisio T, Partanen R, Forssell P, Lampi A-M, Piironen V (2014) Interfacial protein engineering for spray-dried emulsions—part II: oxidative stability. Food Chem 144:57–64
Durmaz Y (2007) Vitamin E (α-tocopherol) production by the marine microalgae Nannochloropsis oculata (Eustigmatophyceae) in nitrogen limitation. Aquaculture 272:717–722
FAO (2016) The state of world fisheries and aquaculture (2016) contributing to food security and nutrition for all. FAO, Rome, p 14
Geider R, La Roche J (2002) Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis. Eur J Phycol 37:1–17
Godwin MH, Lashaway AR, Hietala DC, Savage PE, Cardinale BJ (2018) Biodiversity improves the ecological design of sustainable biofuel systems. GCB Bioenergy 10:752–765
Goiris K, Van Colen W, Wilches I, León-Tamarizc F, De Cooman L, Muylaertb K (2015) Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Res 7:51–57
Guo Z, Liu Y, Guo H, Yan S, Mu J (2013) Microalgae cultivation using aquaculture wastewater as growth medium for biomass and biofuel production. J Environ Sci 25:85–88
Halfhide T, Åkerstrøm A, Lekang OI, Gislerød HR, Ergas SJ (2014) Production of algal biomass, chlorophyll, starch and lipids using aquaculture wastewater under axenic and non-axenic conditions. Algal Res 6:152–159
Hamre K (2011) Metabolism, interactions, requirements and functions of vitamin E in fish. Aquac Nutr 17:98–105
Hamre K, Sissener NH, Lock E-J, Olsvik PA, Espe M, Torstensen BE, Silva J, Johansen J, Waagbø R, Hemre G-I (2016) Antioxidant nutrition in Atlantic salmon (Salmo salar) parr and post-smolt, fed diets with high inclusion of plant ingredients and graded levels of micronutrients and selected amino acids. Peer J 8(4):e2688
Hodgson PA, Henderson JR, Sargent JR, Leftley JW (1991) Patterns of variation in the lipid class and fatty acid composition of Nannochloropsis oculata (Eustigmatophyceae) during batch culture. J Appl Phycol 3:169–181
Kim S, Park J, Cho Y-B, Hwang S-J (2013) Growth rate, organic carbon and nutrient removal rates of Chlorella sorokiniana in autotrophic, heterotrophic and mixotrophic conditions. Bioresour Technol 144:8–13
Korn ED (1964) The fatty acids of Euglena gracilis. J Lipid Res 5:352–362
Liu J (2016) Interspecific biodiversity enhances biomass and lipid productivity of microalgae as biofuel feedstock. J Appl Phycol 28:25–33
Lund JWG (1959) A simple counting chamber for nannoplankton. Limnol Oceanogr 4:57–65
Mahapatra DM, Chanakya HN, Ramachandra TV (2013) Euglena sp. as a suitable source of lipids for potential use as biofuel and sustainable wastewater treatment. J Appl Phycol 25:855–865
Martins CIM, Eding EH, Verdegem MCJ, Heinsbroek LTN, Schneider O, Blancheton JP, Rogued’ Orbcastel E, Verreth JAJ (2010) New developments in recirculating aquaculture systems in Europe: a perspective on environmental sustainability. Aquac Eng 43:83–93
Mustafa E-M, Phang S-M, Chu WL (2012) Use of an algal consortium of five algae in the treatment of landfill leachate using the high-rate algal pond system. J Appl Phycol 24:953–963
Nasir NM, Bakar NSA, Lananan F, Hamid SHA, Lam SS, Jusoh A (2015) Treatment of African catfish, Clarias gariepinus wastewater utilizing phytoremediation of microalgae, Chlorella sp. with Aspergillus niger bio-harvesting. Bioresour Technol 190:492–498
Olaveson MM, Nalewajko C (2000) Effects of acidity on the growth of two Euglena species. Hydrobiologia 433:39–56
Ortíz CML, Moya P, Navarro VB (2006) A rapid chromatographic method for simultaneous determination of β-sitosterol and tocopherol homologues in vegetable oils. J Food Compos Anal 19:141–149
Qiu R, Gao S, Lopez PA, Ogden KL (2017) Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Res 28:192–199
Redfield AC (1958) The biological control of chemical factors in the environment. Am Sci 46:205–221
Regnault A, Chervin D, Chammai A, Piton F, Calvayrac R, Mazliak P (1995) Lipid composition of Euglena gracilis in relation to carbon-nitrogen balance. Phytochem 40:725–733
Rombenso AN, Trushenski JT, Jirsa D, Drawbridge M (2016) Docosahexaenoic acid (DHA) and arachidonic acid (ARA) are essential to meet LC-PUFA requirements of juvenile California yellowtail (Seriola dorsalis). Aquaculture 463:123–134
Schüler LM, Schulze PSC, Pereira H, Barreira L, León R, Varela J (2017) Trends and strategies to enhance triacylglycerols and high-value compounds in microalgae. Algal Res 25:263–273
Schwartz H, Ollilainen V, Piironen V, Lampi A-M (2008) Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. J Food Compos Anal 21:152–161
Schwarzhans J-P, Cholewa D, Grimm P, Beshay U, Risse J-M, Friehs E, Flaschel E (2015) Dependency of the fatty acid composition of Euglena gracilis on growth phase and culture conditions. J Appl Phycol 27:1389–1399
Shahidi F, Costa de Camargo A (2016) Tocopherols and tocotrienols in common and emerging dietary sources: occurrence, applications and health benefits. Int J Mol Sci 17:1745
Sørensen M, Berge GM, Reitanc KI, Ruyter B (2016) Microalga Phaeodactylum tricornutum in feed for Atlantic salmon (Salmo salar)—effect on nutrient digestibility growth and utilization of feed. Aquaculture 460:116–123
Stockenreiter M, Haupt F, Seppälä J, Tamminen T, Spilling K (2016) Nutrient uptake and lipid yield in diverse microalgal communities grown in wastewater. Algal Res 15:77–82
Taipale S, Strandberg U, Peltomaa E, Galloway AWE, Ojala A, Brett MT (2013) Fatty acid composition as biomarkers of freshwater microalgae: analysis of 37 strains of microalgae in 22 genera and in seven classes. Aquat Microb Ecol 71(2):165–178
Tani Y, Tsumura H (1989) Screening for tocopherol-producing microorganisms and α-tocopherol production by Euglena gracilis Z. Ag Biol Chem 53:305–312
Torrecillas S, Román L, Rivero-Ramírez F, Caballero MJ, Pascual C, Robaina L, Izquierdo MS, Acosta F, Montero D (2017) Supplementation of arachidonic acid rich oil in European sea bass juveniles (Dicentrarchus labrax) diets: effects on leucocytes and plasma fatty acid profiles, selected immune parameters and circulating prostaglandins levels. Fish Shellfish Immunol 64:437–445
Tossavainen M, Nykänen A, Valkonen K, Ojala A, Kostia S, Romantschuk M (2017) Culturing of Selenastrum on diluted composting fluids; conversion of waste to valuable algal biomass in presence of bacteria. Bioresour Technol 238:205–213
Tossavainen M, Katyal Chopra N, Kostia S, Valkonen K, Sharma AK, Sharma S, Ojala A, Romantschuk M (2018) Conversion of biowaste leachate to valuable biomass and lipids in mixed cultures of Euglena gracilis and chlorophytes. Algal Res 35:76–84
Tredici MR, Zittelli GC (1998) Efficiency of sunlight utilization: tubular versus flat photobioreactors. Biotechnol Bioeng 57:187–197
Van Hoestenberghe S, Fransman C-A, Luyten T, Vermeulen D, Roelants I, Buysens S, Goddeeris BM (2016) Schizochytrium as a replacement for fish oil in a fishmeal free diet for jade perch, Scortumbarcoo (McCulloch & Waite). Aquac Res 47:1747–1760
Vismara R, Vesti S, Kusmic C, Barsanti L, Gualtieri P (2003) Natural vitamin enrichment of Artemia salina fed freshwater and marine microalgae. J Appl Phycol 15:75–80
Yamane Y, Utsunomiya T, Watanabe M, Sasaki K (2001) Biomass production in mixotrophic culture of Euglena gracilis under acidic condition and its growth energetics. Biotechnol Lett 23:1223–1228
Ytrestøyl T, Aas TS, Åsgård T (2015) Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway. Aquaculture 448:365–374
Zhao Y, Ge Z, Lui H, Sun S (2016) Ability of different microalgae species in synthetic high-strength wastewater treatment and potential lipid production. J Chem Technol Biotechnol 91:2888–2895
Zhu S, Wang Y, Xu J, Shang C, Wang Z, Xu J, Yuan Z (2015) Luxury uptake of phosphorus changes the accumulation of starch and lipid in Chlorella sp under nitrogen depletion. Bioresour Technol 198:165–171
Acknowledgements
Kaisa Haukka gave her constructive comments to the manuscript, and John Allen checked the language.
Funding
Open access funding provided by University of Helsinki including Helsinki University Central Hospital. This work was supported by the Finnish Funding Agency for Innovation (Tekes, Algomeg-project) and the European Regional Development Fund (ERDF) (Häme, Päijät-Häme and Uusimaa regions, Levarbio-project, project code A71035). Additional funding was obtained from TES (Finnish Foundation for Technology Promotion) (Gasum Gas Fund). Kalavesi Consultants Ltd. and Clewer Technology Oy provided AWW from their RAS facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Online Resource 1
(XLSX 12 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tossavainen, M., Lahti, K., Edelmann, M. et al. Integrated utilization of microalgae cultured in aquaculture wastewater: wastewater treatment and production of valuable fatty acids and tocopherols. J Appl Phycol 31, 1753–1763 (2019). https://doi.org/10.1007/s10811-018-1689-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1689-6