Abstract
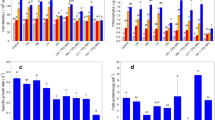
As a common pollutant, nitrite concentrations can approach 15 mg NO −2 -N L−1 in some aquatic systems. Microcystis aeruginosa blooms are common and widespread in eutrophic freshwater bodies. In this study, M. aeruginosa was exposed to nitrite concentrations ranging from 0 to 15 mg NO −2 -N L−1, and the responses of M. aeruginosa were investigated. The specific growth rates, maximum cell densities, light-saturated photosynthetic rates (Pm chla), dark respiration rates (Rd chla), and apparent photosynthetic efficiencies (αchla) showed a significant decline with nitrite concentrations increasing. Electrical conductivity and malondialdehyde contents investigation revealed cell membrane damage and apparent leakage of intracellular contents under high nitrite level conditions due to oxidative stress enhancement. Intracellular microcystin (MC)-LR content reached the highest value at 10 mg NO −2 -N L−1; however, extracellular MC-LR contents showed a continuous increase until 15 mg NO −2 -N L−1 owing to the increasing leakage of intracellular contents. These results elucidated that the high-level nitrite inhibited M. aeruginosa growth by rising oxidative stress, damaging cell membrane, and reducing photosynthesis. However, the moderate increase in nitrite concentrations promoted toxin production and release of toxin.





Similar content being viewed by others
References
Abe K, Imamaki A, Hirano M (2002) Removal of nitrate, nitrite, ammonium and phosphate ions from water by the aerial microalga Trentepohlia aurea. J Appl Phycol 14:129–134
Abe K, Matsumura I, Imamaki A, Hirano M (2003) Removal of inorganic nitrogen sources from water by the algal biofilm of the aerial microalga Trentepohlia aurea. World J Microbiol Biotechnol 19:325–328
Amé MV, Wunderlin DA (2005) Effects of iron, ammonium and temperature on microcystin content by a natural concentrated Microcystis aeruginosa population. Water Air Soil Pollut 168:235–248
Chen WM, Zhang QM, Dai SG (2009a) Effects of nitrate on intracellular nitrite and growth of Microcystis aeruginosa. J Appl Phycol 21:701–706
Chen WM, Zhang QM, Dai SG (2009b) Utilization of nitrite as a nitrogen source by Microcystis aeruginosa. J Agro Environ Sci 28(5):989–993
Codd GA (1995) Cyanobacterial toxins: occurrence, properties and biological significance. Water Sci Technol 32:149–156
Codd GA, Bell SG, Kaya K, Ward C, Beattie K, Metcalf J (1999) Cyanobacterial toxins, exposure routes and human health. Eur J Phycol 34:405–415
Downing TG, Sember CS, Gehringer MM, Leukes W (2005) Medium N:P ratios and specific growth rate comodulate microcystin and protein content in Microcystis aeruginosa PCC7806 and M. aeruginosa UV027. Microb Ecol 49:468–473
Durner J, Klessig DF (1999) Nitric oxide as a signal in plants. Curr Opin Plant Biol 2:369–374
Eddy FB, Williams EM (1987) Nitrite and fresh water fish. J Chem Ecol 3:1–38
Geng H, Xie P, Xu J (2006) Effect of toxic Microcystis aeruginosa PCC7820 in combination with a green alga on the experimental population of Brachionus calyciflorus and B. rubens. Bull Environ Contam Toxicol 76:963–969
Glass C, Silverstein J (1998) Denitrification kinetics of high nitrate concentration water: pH effect on inhibition and nitrite accumulation. Wat Res 32(3):831–839
Gong Y, Chou HN, Tu CD, Liu X, Liu J, Song L (2009) Effects of arsenate on the growth and microcystin production of Microcystis aeruginosa isolated from Taiwan as influenced by extracellular phosphate. J Appl Phycol 21:225–231
Henley WJ (1993) Measurement and interpretation of photosynthetic light-response curves in alga in the context of photoinhibition and diel changes. J Phycol 29:729–739
Huang CY, Chen JC (2002) Effects on acid-base balance, methamoglobinemia and nitrogen excretion of Europian eel after exposure to elevated ambient nitrite. J Fish Biol 61:712–725
Jang MH, Ha K, Lucas MC, Joo GJ, Takamura N (2004) Changes in microcystin production by Microcystis aeruginosa exposed to phytoplanktivorous and omnivorous fish. Aquat Toxicol 68:51–59
Jang MH, Ha K, Jung JM, Lee YJ, Takamura N (2006) Increased microcystin production of Microcystis aeruginosa by indirect exposure to nontoxic cyanobacteria: potential role in the development of Microcystis bloom. Bull Environ Contam Toxicol 76:957–962
Jensen FB (2003) Nitrite disrupts multiple physiological functions in aquatic animals. Comp Biochem Physiol, A 135:9–24
Kaebernick M, Neilan BA (2001) Ecological and molecular investigations of cyanotoxin production. FEMS Microbiol Ecol 35:1–9
Lamattina L, Garcia-Mata C, Graziano M et al (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 54:109–136
Lewis WM, Morris DP (1986) Toxicity of nitrite to fish: a review. Trans Am Fish Soc 115:183–195
Loranger C, Carpentier R (1994) A fast bioassay for phytotoxicity measurements using immobilized photosynthetic membranes. Biotech Bioengin 44:178–183
Masser MP, Rakocy J, Losordo TM (1999) Recirculating aquaculture tank production systems: management of recirculating systems. SRAC Publ 452. Southern Regional Aquaculture Center, Auburn, 11 pp
Nijhof M, Klapwijk A (1995) Diffusional transport mechanisms and biofilm nitrification characteristics influencing nitrite levels in nitrifying trickling filter effluents. War Res 29(10):2287–2292
Oncel I, Yurdakulol E, Keles Y et al (2004) Role of antioxidant defense system and bio-chemical adaptation on stress tolerance of high mountain and steppe plants. Acta Oecol Int J Ecol 26(3):211–218
Pflugmacher S, Codd GA, Steinberg CEW (1999) Effects of the cyanobacterial toxin microcystin-LR on detoxication enzymes in aquatic plants. Environ Toxicol 14:111–115
Purczeld P, Chan CJ, Portis JR, Heldt HW, Heber U (1978) The mechanism of the control of carbon fixation by the pH in the chloroplast stroma: studies with nitrite mediated proton transfer across the envelope. Biochim Biophys Acta 51:488–498
Razumov VA, Tyutyunova FI (2001) Nitrite contamination of the Moskva River: causes and effects. Water Res 28:324–334
Rivasseau C, Martins S, Hennion MC (1998) Determination of some physicochemical parameters of microcystins (cyanobacterial toxins) and trace level analysis in environmental samples using liquid chromatography. J Chromatogr A 799:155–169
Sahay A, Jajoo A, Singh P, Bharti S (2006) Nitrite regulates distribution of excitation energy between the two photosystems by causing state transition. Plant Physiol Biochem 44:7–12
Shimazaki K, Yu SW, Sakaki T et al (1992) Differences between spinach and kidney bean plants in terms of sensitivity to fumigation with NO2. Plant Cell Physiol 33:267–273
Spiller H, Boger P (1977) Photosynthetic nitrite reduction by dithioerythritol and the effect of nitrite on electron transport in isolated chloroplasts. Photochem Photobiol 26:397–402
Stemler A, Murphy JB (1985) Bicarbonate reversible and irreversible inhibition of PS II by monovalent anions. Plant Physiol 77:974–977
Westhuizen AJV, Eloff JN (1985) Effect of temperature and light on the toxicity and growth of the blue-green alga Microcystis aeruginosa (UV-006). Planta 163:55–59
White SH, Duivenvoorden LJ, Fabbro LD (2005) A decision-making framework for ecological impacts associated with the accumulation of cyanotoxins (cylindrospermopsin and microcystin). Lake Reserv Manage 10:25–37
Yang S, Wang J, Cong W, Cai Z, Ouyang F (2004) Utilization of nitrite as nitrogen source by Botryococcus braunii. Biotechnol Lett 26:239–243
Zhang WH (2000) Determination of MAD content in plant tissue. In: Li HS (ed) Principles and techniques of plant physiological biochemical experiment. Higher Education Press, Beijin, pp 260–261
Zhang TT, Zheng CY, Hu W, Xu WW, Wang HF et al (2010) The allelopathy and allelopathic mechanism of phenolic acids on toxic Microcystis aeruginosa. J Appl Phycol 22:71–77
Acknowledgements
This work was supported by the corporation project between Nankai University and Tianjin University funded by the Educational Ministry of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, W., Liu, H., Zhang, Q. et al. Effect of nitrite on growth and microcystins production of Microcystis aeruginosa PCC7806. J Appl Phycol 23, 665–671 (2011). https://doi.org/10.1007/s10811-010-9558-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-010-9558-y