Abstract
Baby schema refers to physical features perceived as cute, known to trigger attention, induce positive emotions, and prompt social interactions. Given the reduced visual attention to social stimuli observed in individuals on the autism spectrum, the current study examines whether the sensitivity to baby schema is also affected. We expected that the looking time towards cute-featured stimuli would vary with symptom severity levels and would be associated with social affect. Ninety-four children (31 typically developing; 63 diagnosed with autism spectrum disorder - ASD) aged 20–83 months (M = 49.63, SD = 13.59) completed an eye-tracking visual exploration task. Autistic participants were separated into two groups based on symptom severity: children with high autism severity symptoms (HS ASD; N = 23) and low-moderate autism symptoms (LMS ASD; N = 40). Animals and neutral objects were simultaneously presented on the screen along with either human babies (condition 1) or adults (condition 2). The results indicated that visual attention oriented to cute-featured stimuli varied with autism symptom severity: only LMS and TD groups spend more time looking at cute-featured stimuli (babies; animals) than neutral objects. Moreover, children with higher severity in the social affect domain spent less time on the stimuli depicting cute than non-cute stimuli. These findings suggest that autism symptom severity and social skills are linked to variations in visual attention to cute stimuli. Implications of baby schema sensitivity are discussed in relation to the development of social competencies and play, responsiveness to robot-based interventions, as well as appraised relevance in autistic children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The baby schema (Kindchenschema) refers to a set of features described as cute (round head, big eyes, chubby cheeks, etc.) that naturally triggers interest and attention, caretaking and protection behavior (Lorenz, 1943; Yao et al., 2022). This universal and cross-cultural response to the baby schema is known as the cuteness effect (Lorenz, 1943; Luo et al., 2015; Yao et al., 2022). Cute-featured stimuli, commonly babies and animals, have high emotional content, elicit positive emotions, release oxytocin, decrease anxiety, and promote bonding, social interactions, empathy, prosocial, and play behavior (Borgi et al., 2014; Doebel et al., 2022; Golonka et al., 2023; Levinson, 2022; Takamatsu, 2023; Yao et al., 2022). They also activate dopaminergic reward systems and brain regions related to motor approach behavior, attachment, emotion processing, and theory of mind (Luo et al., 2015). From an evolutionary perspective, survival of vulnerable youngsters may depend on rapidly attracting the attention of adults (Brosch et al., 2007, 2008; Glocker et al., 2009; Lei et al., 2020), but also of other children (Glocker et al., 2009; Saxton et al., 2020). The limited research in children shows that the cuteness response emerges already around the age of two (Borgi & Cirulli, 2013; Doebel et al., 2022; Saxton et al., 2020), and that the baby schema impacts gaze allocation in 3–6 years old children (Borgi et al., 2014). One study indicated that 3- to 12-month-old infants with no sibling or nursery experience show a weak visual preference bias towards infant faces compared to child faces (Damon et al., 2021). Yet, due to the lack of strong evidence, it is unclear whether sensitivity to baby schema can emerge in young toddlers. Finally, sensitivity to baby schema nurtures relationships (e.g., bonding, affiliation) and represents an early marker of social skills.
Exploring the sensitivity to baby schema in children presenting socio-emotional difficulties, such as those on the autism spectrum, could shed light on early processes that potentially impede engagement in social interactions, motivation, and interest (Cai et al., 2019; Hadjikhani et al., 2014; O’Connor et al., 2019). An atypical perception of environmental cues has been linked to reduced helping behavior in autistic individuals (Komeda et al., 2019). Identifying whether atypical patterns extend to the baby schema could provide insight into the underlying mechanisms of social behavior and appraised relevance. For instance, the nature of attention patterns toward baby schema may indicate alterations in social reward systems or in appraisals of socio-emotional triggers that support bonding, caregiving, and positive relations.
Many studies, mostly using eye-tracking, generally indicate attenuated attention toward social stimuli during visual exploration in autistic individuals (Bast et al., 2021; Chita-Tegmark, 2016; Frazier et al., 2017). Although some studies showed that autistic children are more likely to direct their attention to non-social stimuli that represent circumscribed interests than to social stimuli (i.e., children and adults expressing happy faces in Sasson, Dichter et al., 2012; i.e., emotional faces of adults in Sasson & Touchstone, 2014), others found no differences between autistic and typically developing (TD) children in the time spent on social vs. non-social stimuli (Chita-Tegmark, 2016). These mixed results may be linked to task-related characteristics (e.g., type or quantity of social stimuli, type of interactive agent) and participant-related (e.g., profile heterogeneity, social skills, autism symptom severity) (Bast et al., 2021; Chawarska et al., 2016), and impact potential intervention outcomes. Indeed, the literature seems to suggest that the variations observed in the visual exploration patterns and preferences of social stimuli are often linked to the participants’ social skills level. For example, autistic children’s socialization and communication skills were positively linked to the time spent on dynamic social stimuli depicting children (Franchini et al., 2017). Also, the children’s overall preference for social stimuli (i.e., animals and human beings) was negatively associated with autism symptom severity (Celani, 2002). Interestingly, the performance in processing adult faces was found to be more strongly linked to autism symptom severity in the social affect domain than in the repetitive and restricted behavior (RRB) domain (Zagury-Orly et al., 2022).
Regarding attention to cute stimuli in autistic individuals, the evidence is limited. To the best of our knowledge, the only study addressing cuteness sensitivity in autism is an unpublished work (Sasson et al., 2012), in which a behavioral task was administered in a small sample of autistic adults (N = 9). The researchers observed that autistic participants perceived less the infant cuteness compared to the TD group, which would suggest the presence of an altered social reward processing. Another study showed that children with lower autism symptoms preferred robots presenting cute features, whereas children with more severe symptoms preferred humanoid-like robots resembling adults (Kumazaki et al., 2017). In a preference-based task (Prothmann et al., 2009), autistic children preferred interacting with dogs, seconded by persons (adults) and then by objects, but the small sample had a large range in intellectual capacities and symptom severity was not controlled. Another study found no differences in the preference for animals vs. inanimate objects in autistic children (Celani, 2002). A recent literature review suggested that, when looking at animals, autistic children’s eye gaze patterns are comparable to those with TD, but their visual attention is more biased toward animals than humans (Toutain et al., 2024). Nonetheless, a notable limitation in the findings presented in this article lies in the infrequent reporting of symptom severity across the referenced studies.
Despite these different results, autism profile heterogeneity should be considered when examining social attention. Sensitivity to social stimuli might be linked to symptom severity, particularly, by impairments in social skills (Franchini et al., 2017). The relationship between the social affect and RRB domains in autistic children is still unclear (Chaxiong et al., 2022). It is considered that social affect and RRB contribute independently to the autism diagnosis, present distinct developmental trajectories, and impact differentially the responsiveness to interventions (Gotham et al., 2007; Hus et al., 2014). Given their different nature and contributions to autistic behaviors and profiles, treating them separately might help to unmask the specific effects and weight that each may have on the visual processing of cute stimuli. Finally, examining visual processing of cute-featured stimuli in groups with varying severity of autism symptoms may help understand how attentional mechanisms affect social skills development.
These overreaching observations point towards an altered perception and processing of social, and perhaps cute, cues in autism, that may appear very early in development (e.g., infancy, see Chita-Tegmark, 2016). The evidence hints at the possibility that cute-featured objects may trigger distinct socio-affective responses, depending on the participants’ social skill levels. These responses could be discerned through patterns of visual attention and preference. We then may expect that autistic children exhibit distinct visual patterns when exploring cute stimuli (i.e., human babies and non-human animals) in comparison to their TD peers. Yet, the degree of dissimilarity from their TD peers may fluctuate, as indicated by existing literature, with the autism symptom severity levels, particularly within the domain of social skills. Thus, the current study aimed at examining the attention to stimuli with baby schema features (i.e., [human] babies and [non-human] animals) in autistic children with varying symptom severity (low-moderate versus high) compared to TD children. A novel eye-tracking paradigm was proposed: a brief visual exploration task including social stimuli with cute features (human babies, non-human animals) and without cute features (human adults), and inanimate non-social stimuli (neutral objects). Given the few and ambiguous findings regarding the precise age at which the cuteness response emerges, we expanded the scope of this new study to examine participants with ages between 1 and 6 years old. The study pursued a twofold goal.
First, we investigated the link between the visual patterns of cute stimuli and autism symptoms. We expected that the attentional bias towards cuteness-configured stimuli would vary across groups and depend on the autism symptom severity. Consequently, we assessed the percentage of looking time spent (i.e., fixation percentage) on the areas of interest (AOIs), a widely-used eye-tracking parameter (Chita-Tegmark, 2016). We hypothesized that only children with lower symptom severity and TD peers would allocate more time to cute social stimuli (animals, babies), compared to non-cute social stimuli (adults and/or neutral objects). We expected then that the visual attention bias towards cute stimuli is reduced in children with higher autistic symptoms compared to the other two groups. We also explored the engagement with the stimuli (i.e., average fixation duration) as well as the initial orientation (i.e., time to first fixation) on AOIs.
Second, we examined the link between the sensitivity to cuteness, further labeled as the baby sensitivity index (percentage of looking time spent on all “cute” stimuli versus all “non-cute” stimuli), and symptom severity in the two core domains described in the autism spectrum disorder (ASD) diagnosis (i.e., social affect and RRB). A second baby sensitivity index was calculated specifically on human stimuli (percentage of looking time spent on babies as “cute” humans versus adults as “non-cute” humans) and tested for links with the symptom severity in the social affect and RRB. We expected that increased attention towards cute over non-cute stimuli and babies over adults, respectively, is linked to social affect only.
Methods
Participants
Participants included in the current study are part of the a longitudinal autism cohort, that started in 2012 (Geneva Autism Cohort), in which children are invited for assessments every six months for two years, described elsewhere (Franchini et al., 2017; Robain et al., 2020). Children on the autism spectrum were recruited through French and English-speaking parent associations and clinical centers. TD peers were recruited through local announcements and word-of-mouth. Parents or legal guardians gave their written informed consent for participation. The study was approved by the local ethics committee.
Inclusion criterion for all participants was an age of 1 to 6 years. Moreover, the autistic children were required to satisfy DSM 5 criteria for Autism Spectrum Disorder (ASD; American Psychiatric Association, 2013), and have their clinical diagnosis confirmed using the ADOS (Autism Diagnostic Observation Schedule-Generic; ADOS-G; Lord et al., 2000, Autism Diagnostic Observation schedule, second edition; ADOS-2; 2012; described in the section below). Finally, the TD children were screened for any developmental concerns, autism symptoms (see Table 1), and/or history of ASD in first-degree relatives.
A total of 164 children completed the eye-tracking task. Seventy children (42%) were excluded for attending each of the six frames presented during the visual exploration eye-tracking task for less than 50% of the exposition time. The threshold was chosen based on previous eye-tracking data preparation used in research with autistic toddlers (Pierce et al., 2011, 2016). To our knowledge, no compromise exists yet regarding a viewing time threshold in research on autistic children. The final sample retained for analyses included 63 autistic and 31 TD children.
Based on the overall ADOS total calibrated severity score (CSS) and the CSS classification and cutoffs indicated in the ADOS-2 manual (Gotham et al., 2009; Hus et al., 2014; Lord et al., 2012), two ASD subgroups were distinguished within the autistic group. The first subgroup included participants with high severity autism symptoms (HS ASD; N = 23) who have obtained a total severity score ranging from 8 to 10. The second subgroup (i.e., participants having low to moderate autism symptoms; LMS ASD) included participants with scores indicating low (score ranges 3–4; N = 5), and moderate severity autism symptoms (score range 5–7; N = 35). The two groups (HS ASD and LMS ASD) were significantly different only on the ADOS social affect CSS (t(61) = 7.80, p < .001). There were no other significant differences (p > .05) between the two ASD groups regarding measures of RRB CSS, adaptive behavior (Vineland Adaptive Behavior Schedule-second edition; VABS-II; Sparrow et al., 2005), nor cognitive skills (Mullen Scales of Early Learning; MSEL; Mullen, 1995) (See Table 1). The three groups (HS ASD, LMS ASD, and TD) did not significantly differ in gender (χ2 = 3.01, p = .22) or age (F(2,94) = 1.76, p = .18).
For a description of participants’ socio-demographic characteristics (age, gender, and parents’ education level), and scores on autism symptoms, adaptive behavior, and cognitive skills, see Table 1. Following previous research (Wood de Wilde et al., 2023), we used the parents’ educational level (highest educational level attained among the two parents) to depict the socio-economic background of our participants. The educational level of parents was summarized along three categories: elementary/primary school, high school, and university. Parents were also asked to provide information regarding their nationality(ies) and the language(s) spoken to the child at home. The present sample illustrates the multicultural diversity of the recruited participants: 34 different nationalities and 17 different spoken languages, which is representative of the geographic region in which the study took place (see Wood de Wilde et al., 2023). Among the 34 nationalities, 13 are European and the 21 left are African, North and South American, Asian, and Australian.
Measures
Autism Symptoms
The ADOS is a semi-structured assessment measuring the overall symptomatology levels and the presence of autism symptoms across two domains: social affect and RRB (Lord et al., 2000, 2012). In general, social affect items refer to communication and reciprocal social interaction skills. RRB items refer, for example, to stereotyped/idiosyncratic use of words/phrases, unusual sensory interest, mannerisms, and excessive interest in specific topics. To facilitate the comparison of scores from ADOS-G and ADOS-2 versions, the raw scores were transformed into standardized CSS according to previously published procedures (Esler et al., 2015; Gotham et al., 2007, 2009; Hus et al., 2014). Three scores were obtained: an overall total CSS, the social affect CSS, and the RRB CSS. The CSS ranges on a 10-point scale. Based on the theoretical cutoffs and labels from ADOS-2 manual instructions (Lord et al., 2012), a quantitative assessment of autism symptom severity using the CSS classified participants’ total scores as following: 1–2 as minimal to no evidence, 3–4 as low severity, 5–7 as moderate severity and 8–10 as high severity.
Adaptive Behavior
Children’s adaptive behavior was assessed using the VABS-II (Sparrow et al., 2005), which is a semi-structured standardized parent report interview. Standardized scores were obtained for the following domains: Communication, Daily Living, Socialization Skills, and Motor Skills. An Adaptive Behavior composite score was calculated to obtain an overall measure of adaptive functioning.
Cognitive Skills
Participants’ developmental levels were measured using MSEL (Mullen, 1995). An early learning composite score (M = 100, SD = 15) was calculated based on four cognitive domains: fine motor, visual reception, receptive language, expressive language scales. The score provides a measure of general cognitive functioning.
Stimuli and Procedure
Our novel eye-tracking Visual Exploration Task (VET) and procedure were inspired by Sasson and colleagues (2008). First, 120 colored static stimuli (i.e., 15 human adults, 15 human babies, 30 animals, and 60 neutral objects) were selected using several sources. The human stimuli were a mix of faces and the upper body of people. Baby faces were selected from the freely available copyright protected Children Affective Facial Expression set (LoBue, 2014; LoBue & Thrasher, 2015). Adult faces were selected from the freely available copyright protected resource of Chicago Faces Database (Ma et al., 2015). All human stimuli were depicting neutral faces (to exclude any potential emotional attention biases). The animals (cats and dogs only) and neutral objects were retrieved from Google search engine. Age of animals was not controlled. The neutral objects were selected from domains that are not known to represent common circumscribed interest of individuals on the autism spectrum (i.e., these objects are referred to as low autism-interest stimuli such as furniture, bathroom and kitchen objects, office supplies, etc.; see Sasson et al., 2008).
Second, to examine the emotional content of the stimuli (see Pool et al., 2016) for the study goal, forty-four adults aged 21–49 years (27 females, 15 males; Mage = 29.19, SDage = 9.37) have rated the selected images in terms of cuteness and pleasantness, on a 5-point Likert scale (1= “not cute at all”/ “very unpleasant” and 5= “very cute”/ “very pleasant”) in an online survey. When comparing stimuli depicting animals to human babies, there were no significant differences in terms of cuteness, nor valence. Animals and human babies were rated significantly cuter and more pleasant than adults and neutral objects (p < .001). Adults were rated significantly cuter and more pleasant than neutral objects (p < .001). Therefore, the emotional content was found higher in stimuli depicting babies and animals compared to adults and neutral objects, as well as in stimuli depicting adults compared to neutral objects (see Table 2). All rated stimuli were retained for the task.
Next, the 120 stimuli were randomly distributed on six frames on a white background. Each frame contained 20 visual stimuli from the three different categories: ten neutral objects, five animals, and five humans (human adults in Condition 1 and human babies in Condition 2). Although stimuli originated from several sources, luminosity differences between stimuli were expected to have a low impact on attention given the simultaneous presentation of twenty stimuli on the screen. To control for perceptually driven attention biases, patterns on clothes were avoided and saturation was harmonized. Human gender (female, male), ethnicities, animal species (cats, dogs), the type of neutral objects, and the form of stimuli depicting beings (standing persons, face only) were balanced across frames.
Half of the frames (three out of a total of six) included human adult stimuli (condition 1), while the other half included human baby stimuli (condition 2) (see reconstruction of frames with freely available images in Fig. 1). To avoid biases and increase randomization, two versions of the VET were created (A and B). The second version (B) was built using the same 120 stimuli from version A and six new frames were built by obtaining new combinations of the stimuli. The stimuli ratio on each frame was maintained, but their positions on the frame and across the frames were changed.
The left image represents an example of a frame adults-animals-neutral (condition 1). The right image represents an example of a frame babies-animals-neutral (condition 2). Note: This is a reconstruction with freely available images with no copyright restrictions, as the images used in the original task are partially copyright protected
The six frames were presented randomly for 10 s each (the total duration of each 3-frame condition was 30 s). Each frame was preceded by a 500-millisecond fixation target. Overall, the VET lasted 63 s.
The task was implemented in Tobii Studio Software, version 3.2.3, and administered on a Tobii TX300 eye-tracker (Tobii, Sweden, www.tobii.com) with 23-inch display, 1920 × 1200 resolution (72 dpi), and a sampling rate of 300 Hz. Participants were positioned at approximately 60 cm from the screen. Raw data were filtered using the I-VT filter (default parameters, minimal fixation duration threshold set at 60 milliseconds) (Olsen, 2012). A nine-point calibration procedure was completed before showing the stimuli. To ensure a satisfactory eye gaze data collection, the calibration was performed in operator paced-mode. If any of the 9 calibration points was missed or showed error vectors, the calibration was repeated. The task was administered uniquely if all the calibration points were satisfactory (no error vectors). In each of the two conditions, the stimuli were divided in three AOIs: humans, animals, and neutral objects.
Participants were randomly assigned to version A or B and instructed to freely look at the screen. For each participant, we collected the fixation percentage (calculated as the sum of fixation durations on a particular AOI divided by the total time of stimuli exposure, which was 30 s per each condition). Additionally, the average fixation duration and the time to the first fixation on the AOI were examined.
A Baby Sensitivity Index (BSI) was calculated as the difference between the fixation percentage on all cute stimuli (babies, animals) and the fixation percentage on all non-cute stimuli (adults, neutral objects). A high score indicated a preference towards cute stimuli and a low score indicated a preference towards non-cute stimuli. Second, a Human Baby Sensitivity Index (Human BSI) was calculated as the difference between the fixation percentage on babies and the fixation percentage on adults. A high score indicated a preference towards babies and a low score indicated a preference towards adults.
Data Analysis
First, to investigate the differences between groups related to each AOI from each condition, repeated measure MANOVAs and post-hoc t-test analyses were run in IBM Statistics version 26. Based on our hypotheses and study design, significant three-way interactions were expected to be driven by greater visual biases of the TD and LMS ASD groups towards cute stimuli within and between conditions, compared to the HS ASD group. The 3 × 2 × 3 repeated measure MANOVAs were separately run on each of the eye-tracking parameters: fixation percentage, average fixation duration, and time to first fixation. Each MANOVA was conducted using a 3-level between-group variable (group: HS ASD, LMS ASD, and TD), a 2-level within-group variable (Type of Frame: Condition 1 - “Human Adult” and Condition 2 - “Human Baby”), and a 3-level within-group variable (Stimuli: humans, animals, neutral objects). Wilks’s lambda F-test statistics are further reported. The post-hoc independent samples t-tests looked into differences between groups on each stimulus type. The paired samples t-tests were run to identify differences within group on the following relevant comparisons: adults versus babies (between conditions), adults versus animals (condition 1), adults versus neutral objects (condition 1), animals versus neutral objects (condition 1), babies versus animals (condition 2), babies versus neutral objects (condition 2), animals versus neutral objects (condition 2). To control for false discovery rates (FDR) in multiple comparisons, we have applied Benjamini and Hochberg’s procedure (1995) to control for the proportion of false discoveries using the MATLAB function fdr_bh.m (Groppe, 2015). The Benjamini and Hochberg’s FDR correction procedure adjusted the p-value threshold at p < .012. The visual graphs were created in Graph Pad Prism, version 8.4.3. To examine the association between BSIs and symptom severity (social affect and RRB), two separated regression analyses were conducted and illustrated graphically using R Studio 1.4.1103.
Results
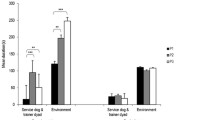
Fixation Percentage
The 3 × 2 × 3 repeated measure MANOVA showed a significant three-way interaction (F(4,180) = 3.68, p = .007, partial η2 = .08). The interaction remained significant when age was introduced as covariate (F(4,178) = 3.34, p = .012, partial η2 = .07). A total of 39 comparisons (independent and paired samples t-tests) in which we expected differences were run.
Next, between-group post-hoc t-tests revealed that the HS ASD group spent significantly more time on adults (condition 1) than the LMS ASD group (t(61) = 2.87, p = .006), a result which remained significant after applying the Benjamini and Hochberg’s correction procedure.
Within-group post-hoc t-tests showed that LMS ASD and TD groups spent more gaze time on stimuli depicting babies than those depicting adults (t(39) = 2.51, p = .016, respectively t(30) = 2.12, p = .042), whereas the HS ASD group spent more time on adults than babies (t(22) = -2.31, p = .031). However, after applying the Benjamini and Hochberg’s correction procedure, none of these comparisons reached the significance threshold.
In condition 1, the TD group and the HS ASD group spent significantly more time on adults rather than neutral stimuli (t(30) = 3.36, p = .002, respectively, t(22) = 4.35, p < .001), which was not significant in the LMS ASD group (t(39) = 1.98, p = .055). However, the TD group and the LMS ASD spent significantly more time looking at animals rather than neutral stimuli (t(30) = 3.80, p = .001 and t(39) = 3.45, p = .001, respectively). The significant results were maintained after applying the Benjamini and Hochberg’s corrected threshold. In the HS ASD, the initial result indicating that the group spent significantly more time on animals than neutral stimuli (t(22) = 2.31, p = .031) did not survive the corrected threshold.
In condition 2, the LMS ASD group spent significantly more time on babies and animals compared to neutral stimuli (t(39) = 2.70, p = .010, respectively, t(39) = 2.68, p = .011). The same pattern was found in the TD group (t(30) = 4.64, p < .001 and t(30) = 3.72, p = .001, respectively). These results were also maintained after applying the Benjamini and Hochberg’s corrected threshold. Within-group differences were not reproduced in the HS ASD group (t(22) = 0.88, p > .05, and t(22) = 1.39, p > .05 respectively). See Fig. 2; Table 3.
Additionally, to better understand the role of age for this particular significant result, Pearson correlations were run to explore potential associations between age and fixation percentages on each of the AOIs, in each group. Therefore, 18 Pearson correlations were run, setting a Bonferroni corrected p-value threshold of .002. The only significant correlation was a negative weak association between age and fixation percentage on stimuli depicting babies in the HS ASD (r = -.47, p = .025), which did not survive the Bonferroni correction.
Average Fixation Duration
A 3 × 2 × 3 repeated measure MANOVA showed no significant three-way interaction, F(4,180) = 2.12, p = .081, partial η2 = .05.
Time to First Fixation
A 3 × 2 × 3 repeated measure MANOVA showed no significant three-way interaction (F(4,180) = 0.47, p = .76, partial η2 = .01), but only a significant main effect of the stimuli (F(2,90) = 17.34, p < .001, partial η2 = .28). On average, across frames, the time to first fixation on stimuli depicting humans was shorter than compared to animals and neutral objects.
Baby Sensitivity Index
First, social affect significantly predicted the BSI in the entire sample: B = -2.74, β = -.22, t(91) = -2.10, p = .04. Social affect scores explained a small proportion of variance in BSI: adjusted R2 = .04. When age was introduced in the regression model, the model was still significant (p = .04, adjusted R2 = .05) and social affect remained the unique significant predictor: B = -3.19, β = -.25, t(90) = -2.39, p = .02. With the HS ASD group removed from the sample, social affect is no longer a significant predictor of BSI: B = -2.95, β = -.19, t(68) = -1.56, p = .12; adjusted R2 = .02). With the TD group removed from the sample, the social affect is not anymore predicting the BSI (B = -3.97, β = -.22, t(61) = -1.75, p = .09; adjusted R2 = .03) in autistic participants (HS and LMS ASD groups combined). RRB did not significantly predict BSI: B = -.91, β = -.10, t(91) = -0.92, p = .36; adjusted R2 = -.002). When the three groups were taken separately, a significant result was found only within the LMS ASD group in relationship with social affect (p = .048). See Fig. 3.
Correlations between overall baby sensitivity index (cute versus non-cute stimuli) and social affect and repetitive and restricted behavior calibrated severity scores based on the ADOS across the entire sample (N = 94). Note: X-axis origin = 1. While the groups are color-coded in the graph, the grouping variable was not included the regression model
Second, social affect significantly also predicted the human BSI in the entire sample: B = -.97, β = -.24, t(91) = -2.32, p = .02. Social affect scores explained a small proportion of variance in BSI: adjusted R2 = .05. When age was introduced in the regression model, social affect remained the unique significant predictor: B = -.94, β = -.23, t(90) = -2.19, p = .03; adjusted R2 = .04. With the HS ASD group removed from the sample, social affect is no longer a significant predictor of BSI (B = .10, β = .2, t(68) = 0.17, p = .87). With the TD group removed from the sample, the social affect remained a significant predictor of BSI (B = -1.58, β = -.28, t(61) = -2.28, p = .03; adjusted R2 = .06) in autistic participants (HS and LMS ASD groups combined). RRB did not significantly predict BSI: B = -.36, β = -.12, t(91) = -1.13, p = .26; adjusted R2 = .003. See Fig. 4. When the three groups were taken separately, no significant results were found within each group (p > .05). As for the overall BSI, these changes occurring when one group is removed or when they are analyzed separately are likely because of the low variance (See Figs. 3 and 4 in this manuscript, and Fig. S4 in the supplementary material).
Correlations between human baby sensitivity index and social affect and repetitive and restricted behavior calibrated severity scores based on the ADOS across the entire sample (N = 94). Note: X-axis origin = 1. While the groups are color-coded in the graph, the grouping variable was not included the regression model
Discussion
The current study examined whether autism symptom severity in young children is associated with visual exploration of cute social stimuli using a novel eye-tracking task. The task triggered differential visual patterns in relation to the heterogeneity of ASD profiles, supporting the potential of eye-tracking to study autistic traits (Mastergeorge et al., 2021).
As expected, comparisons following the three-way significant interaction suggest that only children with lower autistic symptoms (LMS ASD) and typically developing (TD) children spent significantly more time on cute stimuli than neutral objects. LMS ASD and TD groups looked more at animals than at neutral objects (condition 1: adults-animals-neutral), and more at babies and animals than at neutral objects (condition 2: babies-animals-neutral). Although TD participants showed a substantial visual bias towards cute stimuli, they looked significantly more at adults than neutral objects in condition 1, similar to HS ASD group.
Next, no significant interactions were found regarding children’s engagement with stimuli (average fixation duration), nor their initial fixations. All children oriented their initial fixations to stimuli depicting humans significantly faster than to the other stimuli. This result adds to the contradictory evidence about initial orientations in autism (Wang et al., 2020; Wilson et al., 2010). Given that differences in brain network specialization may occur with age (Johnson, 2014), longitudinal studies could examine whether groups gaps may latterly appear.
Finally, the findings indicate a significant correlation between baby sensitivity indexes and autism symptom severity in social affect, but not repetitive and restricted behaviors: the more social difficulties children had, the less fixation percentage they allocated to cute stimuli compared to non-cute stimuli, and to babies compared to adults, respectively.
Overall, our results indicate that autistic symptoms account for the visual patterns toward cute stimuli. Consistent with previous studies (Franchini et al., 2017; Zagury-Orly et al., 2022), social visual exploration in autistic children seems related to symptom severity, particularly to social affect. Social difficulties thus impact not only the preference towards robots (Kumazaki et al., 2017) and face processing (Zagury-Orly et al., 2022) but also visual attention towards cute-featured stimuli. Reduced attraction to baby schema in children with higher autism symptom severity may be negatively linked to affiliative interactions, such as play (e.g., fewer shared experiences, atypical toy play, increased solitary play) and impact, consequently, their socio-emotional development (Elbeltagi et al., 2023; Golonka et al., 2023; Zaharia et al., 2022). Indeed, more social difficulties with peers are reported in children with high autistic levels (Sari et al., 2021). Furthermore, in contrast to previous findings (Grandgeorge et al., 2016), no significant preference for animals over humans was revealed in autistic children. Thus, it may be worthwhile to consider these findings for the design of agents used in interventions for autistic children (e.g., dolls, virtual agents, robots; e.g., Stallmann et al., 2022; Yao et al., 2022) and their implications for occupational, play, and animal-assisted therapies. Considering the unexpected lack of a significant preferential distinction between adults (non-cute) and animals (cute) in Condition 1 within both LMS ASD and TD groups, this finding contributes to the mixed results found in the literature. Descriptively, TD and LMS ASD groups show a higher fixation percentage on animals than adults, but the difference does not reach statistical significance. We posit that stimuli featuring adults serve as significant competitors to cute-featured stimuli, and their potential relevance to participants lies in the depiction of caregiving figures that are crucial for one’s survival and response to primary needs.
Given that relevance typically drives attention to baby faces (Brosch et al., 2007, 2008; Pool et al., 2016), these visual patterns may also be explained by group differences in the appraisal of cute stimuli. As alterations in the perception of relevant social targets (Chawarska et al., 2016) and in the appraisal of negative emotions (Sharma et al., 2014) may occur in autistic individuals, our findings suggest that autistic symptom levels could impact the appraised relevance. Considering that little is known about appraisal in atypical development and that children’s emotional experience might differ from adult research (Walle & Özden, 2024), the present study may suggest that cute stimuli have lower relevance for the children in the HS ASD group. Eventually, our findings may be partially explained by alterations in social reward processing in autism, which are also expected to depend on the symptom severity (Bottini, 2018).
Furthermore, it has been claimed that the baby schema does not only induce the classical positive cuteness effect but also signals vulnerability and approachability (Sanefuji et al., 2007). This could induce an overarousal in certain autistic individuals, provoking personal distress and willingness to divert attention (Hadjikhani et al., 2014; Sanefuji et al., 2007). Consequently, cute-featured stimuli may lead to avoidance, possibly explaining the decreased time spent on cute stimuli in the HS ASD group. Therefore, for individuals with severe autistic symptoms, cute objects may represent another set of positive stimuli triggering atypical visual patterns, altering valence perception and disrupting, in such, the pleasant emotional experience (Antezana et al., 2022; Jacques et al., 2022; Zaharia et al., 2021).
Limitations and Future Perspectives
Several limitations should be considered. First, the familiarity and previous exposure to the variety of cute-featured stimuli that children encounter throughout development (e.g., younger, or same-age siblings or peers, animals, dolls, cartoons; Damon et al., 2021; Saxton et al., 2020) may intertwine with social skills and attention. Although it is suggested that having siblings can increase the cuteness response in TD participants (Luo et al., 2015; Yao et al., 2022), a precise assessment of all the environmental elements potentially impacting their cuteness responses may be unattainable. Second, it is important to conduct such studies considering diverse cultural and socio-economic backgrounds in both autistic and TD groups (see Wood de Wilde et al., 2023). For instance, the “cute” culture (also known as “kawaii”) and anthropomorphism are widespread in Japan and being frequently exposed to them could lead to different visual preferences and patterns in the exploration of cute versus non-cute, and/or human versus non-human stimuli (Atherton et al., 2023). Next, although age was not found as a moderator in our analyses, future research should investigate these observations in age-matched groups to exclude any potential age-related variability in the gaze patterns (Dalrymple et al., 2018). Also, extending future research on cuteness in children above 6 years old, combined with their self-reported ratings, would help confirm the presence of a cuteness response and clarify the exact nature of differences between the groups. Moreover, the current study implemented a categorical approach to separate autistic participants into groups supported by ADOS-2 manual instructions and theoretical categorizations and the lack of density for some value ranges (see Fig. S4, supplementary material). Hence, the present data adds to the dimensional versus categorical debate around the nature of autism (Lefort-Besnard et al., 2020; Roberts et al., 2018). For instance, a recent brain study suggests the possibility of a reconciliation between these two approaches (Tang et al., 2020). For a more comprehensive understanding of heterogeneity, future studies should incorporate larger samples of autistic individuals. Another limitation is that the cuteness conceptualization used in this study is limited to only one facet of a multidimensional model (Doebel et al., 2022). Hence, the processing of other cute attributes (e.g., sounds, behavior; Golonka et al., 2023; Kringelbach et al., 2016) in autistic children should be further investigated. Worthy to note, although evaluated as cute, the age of animals was not controlled in the current study. To better understand these results and the generalization of baby schema sensitivity across species, it would be recommended to test the visual exploration of human babies versus human adults while presenting the stimuli simultaneously, as well as of cute animals versus non-cute animals by manipulating their physical traits (see Borgi et al., 2014). Further, responses to baby schema may depend on the stimuli characteristics (i.e., static, dynamic, real-world) (Chevallier et al., 2015; Klin et al., 2002; Mouga et al., 2021), rendering generalizations difficult. For example, a tactile interface allowing interaction with stimuli (Lio et al., 2019) might increase the ecological relevance of the findings and the robustness of eye-tracking measures. Using multi-method approaches to examine simultaneously attentional processes and the subjective experience towards cute stimuli may provide additional insight. Finally, an important methodological limitation refers to the Tobii Studio software which provides only a qualitative visual verification of the calibration accuracy and precision. Using software providing a quantitative procedure to evaluate the eye-tracking metrics quality for each participant would improve the validity of the findings and conclusions (Dalrymple et al., 2018).
Conclusion
The current study provides evidence for an altered attentional bias toward baby schema in children with high autism severity and suggests that the decreased attention to cute stimuli may be related to social difficulties. These findings might help better understand the appraised relevance, social reward processing, and emotional experience during the exploration of cute stimuli across the autism spectrum. Variations linked to the symptom severity observed in the cuteness responses may have important implications in considering individualized approaches in therapies or in the design of interactive agents used in interventions for autistic children. Future studies should examine the extent to which the emotional reward learning of baby schema (Kinard et al., 2020; Stussi et al., 2018) and the sensitivity to other cute features (Golonka et al., 2023) are affected in autism. Finally, it is worthy to examine how baby schema sensitivity concretely contributes to the unfolding of social interactions and prosocial behavior across development.
Data Availability
The datasets analyzed during the current study are available in the Open Science Framework repository, DOI https://doi.org/10.17605/OSF.IO/CA8VU.
References
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Association.
Antezana, L., Coffman, M. C., DiCriscio, A. S., & Richey, J. A. (2022). Effects of nonsocial and circumscribed interest images on neural mechanisms of emotion regulation in autistic adults. Frontiers in Behavioral Neuroscience, 16, 1–18. https://doi.org/10.3389/fnbeh.2022.1057736
Atherton, G., Morimoto, Y., Nakashima, S., & Cross, L. (2023). Does the study of Culture Enrich our understanding of Autism? A cross-cultural exploration of life on the Spectrum in Japan and the West. Journal of Cross-Cultural Psychology, 54(5), 610–634. https://doi.org/10.1177/00220221231169945
Bast, N., Mason, L., Freitag, C. M., Smith, T., Portugal, A. M., Poustka, L., Banaschewski, T., Johnson, M., Ahmad, J., Ambrosino, S., Auyeung, B., Baron-Cohen, S., Baumeister, S., Beckmann, C. F., Bolte, S., Bourgeron, T., Bours, C., Brammer, M., Brandeis, D., & Zwiers, M. P. (2021). Saccade dysmetria indicates attenuated visual exploration in autism spectrum disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines, 62(2), 149–159. https://doi.org/10.1111/jcpp.13267
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, 57(1), 289–300.
Borgi, M., & Cirulli, F. (2013). Children’s preferences for infantile features in dogs and cats. Human-Animal Interaction Bulletin, 1(2), 1–15. https://doi.org/10.1079/hai.2013.0010
Borgi, M., Cogliati-Dezza, I., Brelsford, V., Meints, K., & Cirulli, F. (2014). Baby schema in human and animal faces induces cuteness perception and gaze allocation in children. Frontiers in Psychology, 5, 1–12. https://doi.org/10.3389/fpsyg.2014.00411
Bottini, S. (2018). Social reward processing in individuals with autism spectrum disorder: A systematic review of the social motivation hypothesis. Research in Autism Spectrum Disorders, 45, 9–26. https://doi.org/10.1016/j.rasd.2017.10.001
Brosch, T., Sander, D., & Scherer, K. R. (2007). That Baby Caught My Eye… attention capture by infant faces. Emotion, 7(3), 685–689. https://doi.org/10.1037/1528-3542.7.3.685
Brosch, T., Sander, D., Pourtois, G., & Scherer, K. R. (2008). Beyond fear: Rapid spatial orienting toward positive emotional stimuli. Psychological Science, 19(4), 362–370.
Cai, R. Y., Richdale, A. L., Dissanayake, C., Trollor, J., & Uljarević, M. (2019). Emotion regulation in autism: Reappraisal and suppression interactions. Autism, 23(3), 737–749. https://doi.org/10.1177/1362361318774558
Celani, G. (2002). Human beings, animals and inanimate objects. What do people with autism like? SAGE Social Science Collections, 6(1), 93–102.
Chawarska, K., Ye, S., Shic, F., & Chen, L. (2016). Multilevel Differences in ASD. 25(3), 289–313. https://doi.org/10.1111/cdev.12473.Multilevel.
Chaxiong, P., Burrows, C., Botteron, K. N., Dager, S. R., Estes, A. M., Hazlett, H. C., Schultz, R. T., Zwaigenbaum, L., Piven, J., Wolff, J., Piven, J., Hazlett, H. C., Chappell, C., Shen, M., Swanson, M., Shaw, D., John, T. S., Constantino, J., Schultz, R., & Shen, M. (2022). Relations of restricted and Repetitive Behaviors to Social Skills in toddlers with Autism. Journal of Autism and Developmental Disorders, 52(4), 1423–1434. https://doi.org/10.1007/s10803-021-05014-8
Chevallier, C., Parish-Morris, J., Mcvey, A., Rump, K. M., Sasson, N. J., Herrington, J. D., & Schultz, R. T. (2015). Measuring social attention and motivation in autism spectrum disorder using eye-tracking: Stimulus type matters. Autism Research, 8(5), 620–628. https://doi.org/10.1002/aur.1479
Chita-Tegmark, M. (2016). Social attention in ASD: A review and meta-analysis of eye-tracking studies. Research in Developmental Disabilities, 48, 79–93. https://doi.org/10.1016/j.ridd.2015.10.011
Dalrymple, K. A., Manner, M. D., Harmelink, K. A., Teska, E. P., & Elison, J. T. (2018). An examination of recording accuracy and precision from eye tracking data from toddlerhood to adulthood. Frontiers in Psychology, 9, 1–12. https://doi.org/10.3389/fpsyg.2018.00803
Damon, F., Quinn, P. C., & Pascalis, O. (2021). When novelty prevails on familiarity: Visual biases for child versus infant faces in 3.5- to 12-month-olds. Journal of Experimental Child Psychology, 210, 1–17. https://doi.org/10.1016/j.jecp.2021.105174
Wood de Wilde, H., Kojovic, N., Robertson, C., Karr, C., Akman, L., Caccia, F., Costes, A., Etienne, M., Franchini, M., Gentaz, E., & Schaer, M. (2023). Remote intensive intervention for young children on the autism spectrum during COVID-19: The experience of caregivers and service providers. Advances in Neurodevelopmental Disorders, 8, 338–354. https://doi.org/10.1007/s41252-023-00339-0
Doebel, S., Stucke, N. J., & Pang, S. (2022). Kindchenschema and cuteness elicit interest in caring for and playing with young children, but less so when children are masked. Scientific Reports, 12(1), 1–8. https://doi.org/10.1038/s41598-022-15922-z
Elbeltagi, R., Al-Beltagi, M., Saeed, N. K., & Alhawamdeh, R. (2023). Play therapy in children with autism: Its role, implications, and limitations. World Journal of Clinical Pediatrics, 12(1), 1–22. https://doi.org/10.5409/wjcp.v12.i1.1
Esler, A. N., Val, H., Guthrie, V., Wetherby, W., Ellis Weismer, A., S., & Lord, C. (2015). The Autism Diagnostic Observation schedule, Toddler Module: Standardized severity scores. Journal of Autism and Developmental Disorders, 45(9), 2704–2720. https://doi.org/10.1007/s10803-015-2432-7
Franchini, M., Glaser, B., Wood De Wilde, H. W., Gentaz, E., Eliez, S., & Schaer, M. (2017). Social orienting and joint attention in preschoolers with autism spectrum disorders. Plos One, 12(6). https://doi.org/10.1371/journal.pone.0178859
Frazier, T. W., Strauss, M., Klingemier, E. W., Zetzer, E. E., Hardan, A. Y., Eng, C., & Youngstrom, E. A. (2017). A Meta-analysis of Gaze differences to Social and Nonsocial Information between individuals with and without Autism. Journal of the American Academy of Child and Adolescent Psychiatry, 56(7), 546–555. https://doi.org/10.1016/j.jaac.2017.05.005
Glocker, M. L., Langleben, D. D., Ruparel, K., Loughead, J. W., Gur, R. C., & Sachser, N. (2009). Baby schema in infant faces induces cuteness perception and motivation for caretaking in adults. Ethology, 115(3), 257–263. https://doi.org/10.1111/j.1439-0310.2008.01603.x
Golonka, E. M., Jones, K. M., Sheehan, P., Pandža, N. B., Paletz, S. B. F., Rytting, C. A., & Johns, M. A. (2023). The construct of cuteness: A validity study for measuring content and evoked emotions on social media. Frontiers in Psychology, 14, 1068373. https://doi.org/10.3389/fpsyg.2023.1068373
Gotham, K., Risi, S., Pickles, A., & Lord, C. (2007). The autism diagnostic observation schedule: Revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders, 37(4), 613–627. https://doi.org/10.1007/s10803-006-0280-1
Gotham, K., Pickles, A., & Lord, C. (2009). Standardizing ADOS scores for a measure of severity in Autism Spectrum disorders. Journal of Autism and Developmental Disorders, 39(5), 693–705. https://doi.org/10.1007/s10803-008-0674-3.Standardizing
Grandgeorge, M., Degrez, C., Alavi, Z., & Lemonnier, E. (2016). Face processing of animal and human static stimuli by children with autism spectrum disorder: A pilot study. Human-Animal Interaction Bulletin, 4(2), 39–53. https://doi.org/10.1079/hai.2016.0005
Groppe, D. (2015). fdr_bh. Retrieved October 25, 2022, from https://www.mathworks.com/matlabcentral/fileexchange/27418-fdr_bh
Hadjikhani, N., Zürcher, N. R., Rogier, O., Hippolyte, L., Lemonnier, E., Ruest, T., Ward, N., Lassalle, A., Gillberg, N., Billstedt, E., Helles, A., Gillberg, C., Solomon, P., Prkachin, K. M., & Gillberg, C. (2014). Emotional contagion for pain is intact in autism spectrum disorders. Translational Psychiatry, 4, e343. https://doi.org/10.1038/tp.2013.113
Hus, V., Katherine, G., & Lord, C. (2014). Standardizing ADOS Domain scores: Separating severity of Social Affect and Restricted and repetitive behaviors. Journal of Autism and Developmental Disorders, 44(10), 2400–2412. https://doi.org/10.1007/s10803-012-1719-1
Jacques, C., Courchesne, V., Mineau, S., Dawson, M., & Mottron, L. (2022). Positive, negative, neutral—or unknown? The perceived valence of emotions expressed by young autistic children in a novel context suited to autism. Autism, 26(7), 1833–1848. https://doi.org/10.1177/13623613211068221
Johnson, M. H. (2014). Autism: Demise of the innate social orienting hypothesis. Current Biology, 24(1), R30–R31. https://doi.org/10.1016/j.cub.2013.11.021
Kinard, J. L., Mosner, M. G., Greene, R. K., Addicott, M., Bizzell, J., Petty, C., Cernasov, P., Walsh, E., Eisenlohr-Moul, T., Carter, R. M. K., McLamb, M., Hopper, A., Sukhu, R., & Dichter, G. S. (2020). Neural mechanisms of social and nonsocial reward prediction errors in adolescents with Autism Spectrum Disorder. Autism Research, 13(5), 715–728. https://doi.org/10.1002/aur.2273
Klin, A., Jones, W., Schultz, R., Volkmar, F., & Cohen, D. (2002). Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry, 59(9), 809–816. https://doi.org/10.1001/archpsyc.59.9.809
Komeda, H., Kosaka, H., Fujioka, T., Jung, M., & Okazawa, H. (2019). Do individuals with autism spectrum disorders help other people with autism spectrum disorders? An investigation of empathy and helping motivation in adults with autism spectrum disorder. Frontiers in Psychiatry, 10, 1–9. https://doi.org/10.3389/fpsyt.2019.00376
Kringelbach, M. L., Stark, E. A., Alexander, C., Bornstein, M. H., & Stein, A. (2016). On cuteness: Unlocking the parental brain and beyond. Trends in Cognitive Sciences, 20(7), 545–558. https://doi.org/10.1016/j.tics.2016.05.003
Kumazaki, H., Warren, Z., Muramatsu, T., Yoshikawa, Y., Matsumoto, Y., Miyao, M., Nakano, M., Mizushima, S., Wakita, Y., Ishiguro, H., Mimura, M., Minabe, Y., & Kikuchi, M. (2017). A pilot study for robot appearance preferences among high-functioning individuals with autism spectrum disorder: Implications for therapeutic use. Plos One, 12(10), 1–13. https://doi.org/10.1371/journal.pone.0186581
Lefort-Besnard, J., Vogeley, K., Schilbach, L., Varoquaux, G., Thirion, B., Dumas, G., & Bzdok, D. (2020). Patterns of autism symptoms: Hidden structure in the ADOS and ADI-R instruments. Translational Psychiatry, 10(1), 1–12. https://doi.org/10.1038/s41398-020-00946-8
Lei, Y., Xia, Q., Mo, Z., & Li, H. (2020). The attention bias effect of infant face: The mechanism of cuteness and familiarity. Acta Psychologica Sinica, 52(7), 811–822. https://doi.org/10.3724/SP.J.1041.2020.00811
Levinson, S. C. (2022). The interaction engine: Cuteness selection and the evolution of the interactional base for language. Philosophical Transactions of the Royal Society B: Biological Sciences, 377, 1–7. https://doi.org/10.1098/rstb.2021.0108
Lio, G., Fadda, R., Doneddu, G., Duhamel, J. R., & Sirigu, A. (2019). Digit-tracking as a new tactile interface for visual perception analysis. Nature Communications, 10(1), 1–13. https://doi.org/10.1038/s41467-019-13285-0
LoBue, V. (2014). The Child Affective Facial Expression (CAFE) set. Databrary. https://doi.org/10.17910/B7301K
LoBue, V., & Thrasher, C. (2015). The child affective facial expression (CAFE) set: Validity and reliability from untrained adults. Frontiers in Psychology, 5, 1–8. https://doi.org/10.3389/fpsyg.2014.01532
Lord, C., Risi, S., Lambrecht, L., Cook, H. C. J., Leventhal, B. L., DiLavore, P. C., Pickles, A., & Rutter, M. (2000). The Autism Diagnostic Observation Schedule–Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. Journal of Autism and Developmental Disorders, 30(3), 205–223.
Lord, C., Rutter, M., DiLavore, P., Risi, S., Gotham, K., & Bishop, S. (2012). The Autism Diagnostic Observation Schedule, Second Edition (Western Ps).
Lorenz, K. (1943). Die angeborenen Formen Moeglicher Erfahrung. Z Tierpsychologie, 5, 235–409.
Luo, L., Ma, X., Zheng, X., Zhao, W., Xu, L., Becker, B., & Kendrick, K. M. (2015). Neural systems and hormones mediating attraction to infant and child faces. Frontiers in Psychology, 6, 1–22. https://doi.org/10.3389/fpsyg.2015.00970
Ma, D. S., Correll, J., & Wittenbrink, B. (2015). The Chicago face database: A free stimulus set of faces and norming data. Behavior Research Methods, 47(4), 1122–1135. https://doi.org/10.3758/s13428-014-0532-5
Mastergeorge, A. M., Kahathuduwa, C., & Blume, J. (2021). Eye-Tracking in infants and Young children at risk for Autism Spectrum disorder: A systematic review of visual stimuli in experimental paradigms. Journal of Autism and Developmental Disorders, 51(8), 2578–2599. https://doi.org/10.1007/s10803-020-04731-w
Mouga, S., Castelhano, J., Café, C., Sousa, D., Duque, F., Oliveira, G., & Castelo-Branco, M. (2021). Social attention deficits in children with autism spectrum disorder: Task dependence of objects vs. faces observation bias. Frontiers in Psychiatry, 12, 1–13. https://doi.org/10.3389/fpsyt.2021.640599
Mullen, E. M. (1995). Mullen scales of early learning. American Guidance Service Inc.
O’Connor, R. A. G., Stockmann, L., & Rieffe, C. (2019). Spontaneous helping behavior of autistic and non-autistic (Pre-)adolescents: A matter of motivation? Autism Research, 12(12), 1796–1804. https://doi.org/10.1002/aur.2182
Olsen, A. (2012). The Tobii I-VT fixation filter: Algorithm description. Tobii Technology, 21. https://www.tobiipro.com/siteassets/tobii-pro/learn-and-support/analyze/how-do-we-classify-eye-movements/tobii-pro-i-vt-fixation-filter.pdf
Pierce, K., Conant, D., Hazin, R., Stoner, R., & Desmond, J. (2011). Preference for geometric patterns early in life as a risk factor for autism. Archives of General Psychiatry, 68(1), 101–109. https://doi.org/10.1001/archgenpsychiatry.2010.113
Pierce, K., Marinero, S., Hazin, R., McKenna, B., Barnes, C., C., & Malige, A. (2016). Eye-tracking reveals abnormal visual preference for geometric images as an early biomarker of an ASD Subtype Associated with increased symptom severity. Biological Psychiatry, 79(8), 657–666. https://doi.org/10.1016/j.biopsych.2015.03.032
Pool, E., Brosch, T., Delplanque, S., & Sander, D. (2016). Attentional bias for positive emotional stimuli: A meta-analytic investigation. Psychological Bulletin, 142(1), 79–106. https://doi.org/10.1037/bul0000026
Prothmann, A., Christine Ettrich, & Sascha Prothmann. (2009). Preference for, and responsiveness to, people, dogs and objects in children with autism. Anthrozoos, 22(2), 161–171. https://doi.org/10.2752/175303709X434185
Robain, F., Franchini, M., Kojovic, N., Wood de Wilde, W., H., & Schaer, M. (2020). Predictors of treatment outcome in preschoolers with autism spectrum disorder: An observational study in the Greater Geneva Area, Switzerland. Journal of Autism and Developmental Disorders, 50(11), 3815–3830. https://doi.org/10.1007/s10803-020-04430-6
Roberts, J. E., Ezell, J. E., Fairchild, A. J., Klusek, J., Thurman, A. J., McDuffie, A., & Abbeduto, L. (2018). Biobehavioral composite of social aspects of anxiety in young adults with fragile X syndrome contrasted to autism spectrum disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 177(7), 665–675. https://doi.org/10.1002/ajmg.b.32674
Sanefuji, W., Ohgami, H., & Hashiya, K. (2007). Development of preference for baby faces across species in humans (Homo sapiens). Journal of Ethology, 25(3), 249–254. https://doi.org/10.1007/s10164-006-0018-8
Sari, N. P., Luijk, M. P. C. M., Prinzie, P., van IJzendoorn, M. H., & Jansen, P. W. (2021). Children’s autistic traits and peer relationships: Do non-verbal IQ and externalizing problems play a role? Child and Adolescent Psychiatry and Mental Health, 15(1), 1–12. https://doi.org/10.1186/s13034-021-00421-2
Sasson, N. J., & Touchstone, E. W. (2014). Visual attention to competing social and object images by preschool children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(3), 584–592. https://doi.org/10.1007/s10803-013-1910-z
Sasson, N. J., Turner-Brown, L. M., Holtzclaw, T. N., Lam, K. S. L., & Bodfish, J. W. (2008). Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Research, 1(1), 31–42. https://doi.org/10.1002/aur.4
Sasson, N. J., Dichter, G. S., & Bodfish, J. W. (2012). Affective responses by adults with autism are reduced to social images but elevated to images related to circumscribed interests. Plos One, 7(8), e42457. https://doi.org/10.1371/journal.pone.0042457
Sasson, N. J., Faso, D. J., Langleben, D. D., & Gur, R. C. (2012b). Individuals with Autism Exhibit Reduced Sensitivity to Infant Cuteness. International Meeting for Autism Research. https://www.researchgate.net/publication/268128844_Individuals_with_Autism_Exhibit_Reduced_Sensitivity_to_Infant_Cuteness
Saxton, T. K., Pollet, T. V., Panagakis, J., Round, E. K., Brown, S. E., & Lobmaier, J. S. (2020). Children aged 7–9 prefer cuteness in baby faces, and femininity in women’s faces. Ethology, 126(11), 1048–1060. https://doi.org/10.1111/eth.13081
Sharma, S., Woolfson, L. M., & Hunter, S. C. (2014). Maladaptive cognitive appraisals in children with high-functioning autism: Associations with fear, anxiety and theory of mind. Autism, 18(3), 244–254. https://doi.org/10.1177/1362361312472556
Sparrow, S. S., Cicchetti, D., & Balla, D. A. (2005). Vineland adaptive behavior scales (2nd ed., Vineland-II). APA PsycTests. https://doi.org/10.1037/t15164-000
Stallmann, L., Dukes, D., Tran, M., Durand de Gevigney, V., Rudrauf, D., & Samson, A. C. (2022). Socially supported by an embodied agent: The development of a virtual-reality paradigm to study social emotion regulation. Frontiers in Virtual Reality, 3, 1–14. https://doi.org/10.3389/frvir.2022.826241
Stussi, Y., Pourtois, G., & Sander, D. (2018). Supplemental Material for enhanced pavlovian aversive conditioning to positive emotional stimuli. Journal of Experimental Psychology: General, 147(6), 905–923. https://doi.org/10.1037/xge0000424.supp
Takamatsu, R. (2023). Responses to infantile cuteness explain the link between autistic traits and reduced maternal attachment. Journal of Genetic Psychology, 184(1), 1–8. https://doi.org/10.1080/00221325.2022.2110854
Tang, S., Sun, N., Floris, D. L., Zhang, X., Di Martino, A., & Yeo, B. T. T. (2020). Reconciling dimensional and categorical models of Autism Heterogeneity: A brain connectomics and behavioral study. Biological Psychiatry, 87(12), 1071–1082. https://doi.org/10.1016/j.biopsych.2019.11.009
Toutain, M., Dollion, N., Henry, L., & Grandgeorge, M. (2024). How do children and adolescents with ASD look at animals? A scoping review. Children, 11, 1–41. https://doi.org/10.3390/children11020211
Walle, E. A., & Özden, Z. B. (2024). How appraisal development shapes the development of emotion. In A. C. Samson, D. Sander, & U. Kramer (Eds.), Change in emotion and mental health (pp. 141–157). Academic Press.
Wang, X., Chen, L., Liu, P., Polk, J. R., & Feng, T. (2020). Orientation to and processing of social stimuli under normal and competitive conditions in children with autism spectrum disorder. Research in Autism Spectrum Disorders, 78, 1–13. https://doi.org/10.1016/j.rasd.2020.101614
Wilson, C. E., Brock, J., & Palermo, R. (2010). Attention to social stimuli and facial identity recognition skills in autism spectrum disorder. Journal of Intellectual Disability Research, 54(12), 1104–1115. https://doi.org/10.1111/j.1365-2788.2010.01340.x
Yao, L., Dai, Q., Wu, Q., Liu, Y., Yu, Y., Guo, T., Zhou, M., Yang, J., Takahashi, S., Ejima, Y., & Wu, J. (2022). Eye size affects cuteness in different facial expressions and ages. Frontiers in Psychology, 12, 1–13. https://doi.org/10.3389/fpsyg.2021.674456
Zagury-Orly, I., Kroeck, M. R., Soussand, L., & Cohen, A. L. (2022). Face-Processing performance is an independent predictor of Social affect as measured by the Autism Diagnostic Observation schedule across large-scale datasets. Journal of Autism and Developmental Disorders, 52(2), 674–688. https://doi.org/10.1007/s10803-021-04971-4
Zaharia, A., Noir-Kahlo, K., Bressoud, N., Sander, D., Dukes, D., & Samson, A. C. (2021). Proof of concept: A brief psycho-educational training program to increase the use of positive emotion regulation strategies in individuals with autism spectrum disorder. Frontiers in Psychology, 12, 705937. https://doi.org/10.3389/fpsyg.2021.705937
Zaharia, A., Dell’Angela, L., Sander, D., & Samson, A. C. (2022). Play and games: Means to support emotional development. In D. Dukes, A. C. Samson, & E. A. Walle (eds), The Oxford handbook of emotional development (online edn, pp. 354–370). Oxford Academic. https://doi.org/10.1093/oxfordhb/9780198855903.013.9
Acknowledgements
We thank all study participants and their families. We thank Ben Meuleman for statistical advice and help with graphs, Giona Di Poi for help with Fig. 1, and Autism Brain and Behavior lab team members for assisting in data collection.
Funding
Open access funding provided by University of Fribourg. This work was supported by the Swiss National Science Foundation (Grants 163859, 190084 & 212653 for M.S., PP00P1_176722 for A.C.S., as well as funding from the National Centre of Competence in Research Synapsy (Grant 51NF40–185897) and by the Fondation Pôle Autisme (https://www.pole-autisme.ch).
Open access funding provided by University of Fribourg
Author information
Authors and Affiliations
Contributions
A.Z. conducted the statistical analyses, co-led the data curation, wrote the first manuscript draft, interpreted the results, and revised the manuscript. N.K. supervised participants’ recruitment, the clinical assessments, and the data collection and curation, interpreted the results, and revised the manuscript. T.R. designed the experimental task, conducted preliminary analyses, and revised the manuscript. D.S. contributed to the conceptualization of the study, provided resources, and revised the manuscript. M.S. conceptualized the study, provided funding for the data collection in the context of the Geneva Autism Cohort, and revised the manuscript. A.C.S. conceptualized the study, provided personnel funding and resources, and co-wrote and revised the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The study was approved by the Ethics commission of the canton, Commission cantonale d’éthique de la recherche - CCER, Geneva, Switzerland, Protocole 12–163/Psy 12–014, referred under the number PB_2016 − 01880, accepted on September 25th, 2012.
Consent to Publication
Informed consent was obtained from legal guardians for all the participants included in the study.
Conflict of Interest
The authors have no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaharia, A., Kojovic, N., Rojanawisut, T. et al. Examining the Link Between Social Affect and Visual Exploration of Cute Stimuli in Autistic Children. J Autism Dev Disord (2024). https://doi.org/10.1007/s10803-024-06504-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s10803-024-06504-1