Abstract
The aim of this research was to study the effect of a horseback-riding programme on postural control in a group of autistic children (ASD). Nine children aged 9 to 12 years participated in this study through a multiple baseline across subjects design. The whole programme took place over nine months. Participants followed a previously developed specific horseback-riding programme, consisting of 45-minute sessions held twice a week for at least three months. To evaluate postural control, the average velocity of the centre of pressure displacement was measured by means of a posturographic platform. Results indicated that this intervention with horses had a positive effect on the postural control in children with ASDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterised by a significant deficit in social communication and repetitive behaviours, restricted interests, and/or atypical sensory behaviour. The spectrum of autism refers to the great variability of the symptoms presented by each affected individual. ASD is often associated with other disorders or medical conditions and may co-occur with intellectual disability and language impairment, ranging from mutism to articulatory and grammatical correction. In all cases adaptive skills are significantly impaired and can be classified into three levels of severity depending on the support required (APA, 2013).
In recent years, ASD has become one of the most prevalent neurodevelopmental disorders in children due, partly, to new diagnostic criteria, greater sensitivity in society, and increased specialist training (Hyman et al., 2020). It is currently estimated that one in 59 children under the age of 8 years has symptoms that meet ASD criteria (Maenner et al., 2020).
ASD Associated Disorders
ASD is a complex reality and has often been associated with other disorders such as stress (Ogawa et al., 2017; Corbett et al., 2016), anxiety (Ezell et al., 2019), sleep (Neumeyer et al., 2019), gastrointestinal (Madra et al., 2020), obsessive–compulsive (Martin et al., 2020), attention deficit hyperactivity (Muskens et al., 2017), sensory processing (Rydzewska et al., 2019), and motor disorders (Licari et al., 2020), among others. There is often a circular relationship between some of these disorders and the expression of ASD symptoms. For instance, greater sleep disturbances may induce higher severity of expression of ASD symptoms and vice versa, as do anxiety, sensory processing disorders, and motor disorders (Cohen et al., 2014; Mazurek & Petroski, 2015).
The relationship between some of these disorders and ASD is sometimes so close, as in the case of motor disorders, that some authors have even considered them to be typical of autism (Crespo-Eguílaz & Narbona-García, 2009; MacDonald et al., 2012, 2013).
Motor Disorders and Postural Control
Although not regarded as an essential symptom of ASD, motor disorders appear as an associated symptomatology in the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (APA, 2013), as these symptoms seem to show a peculiar and different functioning from other conditions such as developmental coordination disorders. (Caeyenberghs et al., 2016; Valverde-Esteve et al., 2020). Dziuk et al. (2007) have suggested that movement impairments in ASD have more similarities than differences with impairments found in developmental coordination disorder. Jansiewicz et al. (2006) found impaired balance and gait, slower speed of timed movements and greater “sprawl” movements in autistic children than in normotypical children. However, the breadth of the autistic spectrum makes it difficult to establish a common pattern.
Also, Fournier et al. (2010) argued that the deficit of postural control, which is basic for a good balance control, is an aspect that is repeated throughout the autism spectrum, presenting a less stable and more variable posture in comparison with neurotypical children of the same age. This was also endorsed by Lourenço et al. (2020) since, in his research, autistic children presented significantly lower scores in relation to motor skills, including balance. In several studies, such as that of Fernándes (2020), it was observed that autistic people scored significantly lower on the balance scales. Similarly, Larson and Mostofosky (2009) found in their work that autistic children have greater difficulty in balance and gait compared to normotypical children.
Moreover, motor disorders can help to establish the diagnosis and obtain prognostic indicators regarding the adaptive behaviour of these individuals (Stevenson et al., 2017). However, although very common, motor disorders are often not systematically assessed by diagnostic teams (Licari et al., 2020).
The typology of motor disorders in ASD is very diverse. Some of the most common problems are difficulties in general dynamic coordination, problems in flexibility and motor tone, gait disorders, difficulties in fine motor coordination, motor praxis, and difficulties in postural control and balance (Paquet et al., 2016; Wang et al., 2016; Molloy et al., 2003; Lum et al., 2020). Among these motor difficulties, balance disorders are of particular interest as they directly affect the success of carrying out everyday activities (Travers et al., 2017). Balance is the ability of our body to remain upright through compensatory movements when stationary or in motion (Mosston & Ashworth, 2008). Balance provides the body with a stable basis for locomotion and manual and facial actions, thus facilitating effective interaction with the environment, both physical and social (Adolph & Franchak, 2017). In fact, everyday tasks such as soaping and getting out of the shower, cooking, or dressing become more difficult if there is a lack of good stability in the upright position (Smith & Fisher, 2018). We use the term ‘postural control’ to refer to the complex ability that involves different sensory–motor processes and that aims to achieve a correct balance in both static and dynamic activities (Winter et al., 1990; Westcott et al., 1997). Guzmán-Muñoz et al. (2019) consider that good postural control is necessary for correct balance in both static and dynamic activities. Montes-Castillo et al. (2000) believe that postural control helps the body to be stable and stay balanced.
Balance is a complex behaviour in which three sensory systems act in a coordinated way: the vestibular system, which determines the position of the head with gravity and the movements of acceleration and deceleration of the head (Ayres, 2008); vision, which provides information on the position of the body in relation to space and objects (Aartolahti et al., 2013; Bronstein, 2019); and the proprioceptive system, which intervenes in the processes of discrimination and localisation of body parts, helping to modulate the force of contraction, temporalisation of movement, and straightening reactions, among others (Speers et al., 2002). When these three systems function correctly, balance issues are usually absent. However, when one of these systems does not function correctly, due to neurophysiological alterations (Maurer & Damasio, 2008; Nayate et al., 2005), compensatory mechanisms involving the remaining sensory systems may make postural control possible, though less effective (Lakie & Loram, 2006; Parreira et al., 2017).
Balance Improvement Interventions
Due to the important role of balance in a person’s adaptive capacities, it is essential to develop interventions to improve balance in children in whom this skill is impaired (Wuang et al., 2020; Stins & Emck, 2018; Peters & Wood, 2017).
Programmes that stimulate the sensory systems and aim to improve balance in people with acquired brain damage or cognitive impairment have been successful, but interventions aimed at improving balance in autistic children are very rare and have yet to yield definitive results (Hilton et al., 2014; Dobell et al., 2020). Although results are not conclusive, they have been encouraging with sensory stimulation interventions (Sam et al., 2017), visual feedback through video games (Travers et al., 2018; Hilton et al., 2014; Somogyi et al., 2016; Peña et al., 2020), gymnastic and neuromuscular training (Akyol & Pektas, 2018; Najafabadi et al., 2018; Shavikloo & Norasteh, 2018), therapeutic skating (Casey et al., 2015), Tai Chi Chuan (Sarabzadeh et al., 2019), and also with the help of elephants (Nuntanee & Daranee, 2019) and horses (Portela-Pino et al., 2019).
Hariri et al. (2022), in a systematic review of the literature, shed light on the available intervention strategies for improving postural control in people with ASD. This study has reported that most interventions affect motor function in ASD by improving cerebellar or balance function, integration and processing of sensory information, muscle strength and correction of body alignment. Nevertheless, all these studies have limitations associated with insufficient sample size and the lack of a control group which could potentially compromise the validity of the results of such studies. This situation was also observed in a systematic review by Trzmiel et al. (2019) where specifically equine-assisted activities and therapies in autistic children were studied. It was concluded that, although the different studies claim an effective impact of the therapies, as previously shown in the previous literature review, the available publications often present flaws in the methodology, mainly derived from the small sample size and the lack of a control group. The use of different therapeutic protocols, and especially different methods to measure efficacy, often reduce the quality of a study.
Horseback Riding Benefits
Horse-riding has a high potential to facilitate the postural control of autistic children and thus improve their balance, since it is an activity in which multiple stimuli from different sensory channels need to be coordinated (Hameury et al., 2010). It is also a highly specific intensive exercise for improving balance, since the horse’s gait induces continuous changes in the rider’s centre of gravity requiring immediate positional readjustments to avoid falling off (Bronson et al., 2010; Muñoz Lasa et al., 2015; Tseng et al., 2013). Moreover, horseback riding meets two other criteria related to the successful care of autistic people: it is a motivating and highly structured activity (Kern et al., 2011; García-Gómez et al., 2014).
In recent years, multiple studies have emerged on the benefits of horseback riding, especially for people with ASD. Peters and Wood (2017) conducted a systematic review of the scientific literature on the topic, focusing on studies with interventions. The authors concluded that most of the studies had methodological weaknesses, but collectively provided evidence that people with ASD who participated in these interventions experienced improvements in social interaction, positive emotions, stress, communication, and especially motor skills. An important finding of this study highlights some authors’ proposal that the sensory nature of riding, including gradual vestibular, proprioceptive, and tactile input, promotes self-regulation in children with ASD, which, according to Gabriels et al. (2012), contributes to a feeling of calm in a child with ASD. Such evidence is consistent with the scientific literature that suggests interventions that include graded sensory input for children with ASD can improve academic responsive behaviour, concentration behaviour, and stereotypical behaviour (Jenkins & Reed, 2013; Bass et al., 2009).
Objective
The aim of this research was to study whether a therapeutic programme based on horseback riding can improve postural control in autistic children, as measured by postural control data from standing on a platform.
Materials and Methods
Study Design
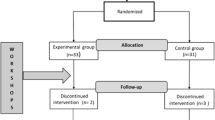
A mixed single-case design was chosen, with a multiple baseline design across subjects. Four groups responded to an AB scheme (A being the baseline and B the intervention), in which a new post-treatment baseline phase was added to one of the groups, which would respond to an ABA scheme (Fig. 1).
Following the current standards for these type of studies (Kratochwill et al., 2013), the design contemplates the existence of more than three participants and at least three measurement points in each phase. In addition, the allocation of each participant to each intervention group and the times at which each participant started the intervention were randomly decided by drawing lots.
In the present design the independent variable is the intervention programme, and the dependent variable is the postural control.
Participants
A sample of nine autistic children belonging to different organisations of ASD in Castellón (Spain) was selected. The sample was recruited from volunteer parents willing to collaborate throughout the development of the intervention programme. The criteria required for inclusion in the sample were as follows: having a clinical diagnosis of ASD with a degree of affectation 1 or 2 issued by a health centre; being between 9 and 12 years oldnever having ridden a horse; and being able to respond to simple orders (Table 1). Candidates with serious behavioural problems who were taking medication that could affect the nervous system or who were overweight were excluded, the latter for animal ethics and security reasons. Eight participants were male, and one was female.
Before the study began, the participants’ legal guardians signed an informed consent document for their children to participate in the study. Both the informed consent and the information collected in the study were in line with the recommendations of the 2013 Declaration of Helsinki. This research was approved by the Deontological Commission of the Universitat Jaume I of Castellón (Spain), with reference code CD/54/2019.
Instruments
To assess the objective of postural control, we used a Medicapteurs branded posturographic platform, model T-Plate®, approved by the French Posturology Association. Through the pressures exerted by the subjects’ bodies on the platform sensors, the T-Plate evaluated the area covered by the centre of pressure (COP).
T-Plate offers the possibility of studying alterations to the different sensory systems involved in postural control by analysing multiple parameters such as scanning area, angle of travel, average velocity, lateral velocity, antero-posterior velocity, maximum average lateral and antero-posterior forces, and others.
In the present study we have only considered the average velocity with which the centre of pressure (AVCOP) moves, which is regarded as a parameter that correlates with the efficiency of postural control and therefore with balance (Viguier, 2009). The AVCOP is expressed in millimetres per second (mm/s). Magnitude interpretation considers that the smaller the area of deviation of the COP and, therefore, the less time spent in covering this area, the better the postural stability (Leanderson et al., 1996).
Data Collection Procedure and Protocol
The nine participants were randomly assigned to four groups that started the intervention in consecutive months. Figure 1 shows a graph illustrating the grouping and the different moments of the evaluation.
Following the proposal of Hsu et al. (2009) postural control was evaluated under four experimental conditions: on a hard surface with participants’ eyes open; on a hard surface with participants’ eyes closed; on a padded surface with participants’ eyes open; and on a padded surface with participants’ eyes closed. This protocol enables analysis of the predominance of the sensory channel used in postural control. Closing the eyes sets in motion the proprioceptive and vestibular channels, and the padded surface inhibits proprioceptive stimuli by foregrounding the vestibular information.
For tests with closed eyes, we used a mask. For the padded surface we used a soft foam pad (50 × 50 × 6 centimetres and a density of 40 kg/m3).
In accordance with the work of Gagey and Weber (2004), the measurements were taken as follows: with the direct help of an adult, participants stood on the platform barefoot with their feet at an angle of 30º, their heels two centimetres apart, and their arms by their sides. Once these requirements had been met, each participant was given the following instruction: “Stand as still as possible, looking at the point in front of you.”
At the count of three the platform was activated to analyse the participant’s movements. Two measurements were taken each time, and the best of them was used.
Nine evaluations (one for every month) of each participant were performed throughout the study in order to have enough points to establish the baseline and the intervention line.
Intervention Programme Design
The work was carried out over a nine-month period. Participants attended regularly at a rate of two 45-minute sessions per week for at least three months. Table 2 details the number of sessions that each participant received. A design comprising different length of intervention was made to observe whether the time of intervention influenced the results. Adherence to programme scheduled was 100%.
The intervention programme for improving balance and postural control is based on the performance of several different exercises on a horse. The exercises are divided into three large blocks of increasing difficulty: with the horse standing, walking, and trotting. Within each block the exercises also increase in difficulty: holding the cinch with the eyes open and closed, and not holding the cinch with the eyes open or closed. A mask is used for the exercises with eyes closed. All these exercises have been standardised and measured with a rubric that is both a complementary evaluation system and a guide to the exercises to be followed (Vives-Vilarroig et al., 2021).
The participant mounts the horse barefoot (wearing socks) and is led by a guide and two safety monitors who remain by his/her side. No saddle or stirrups are used. The horse’s back is protected by two padded saddlebags held in place with a girth strap so that the participant can hold on.
Data Analysis
Following the recommendations of Parker et al. (2011) and Kratochwill et al. (2013), a visual analysis of the changes in level, trend, and latency of the intervention with respect to the baseline was carried out in the first instance. To complete this analysis, two numerical indicators were provided since the visual interpretation of changes was not always evident.
For the analysis of individual differences, the NAP statistic (percentage of non-overlap between all pairs) is shown. This method is an adaptation made for single-case studies by Parker et al. (2011) of Mann Whitney’s test and shows the magnitude of the effect between adjacent phases. For their interpretation, Parker and Vannest (2009, p. 4) examine the size of the effect between phases by considering the following figures: large effect between 93% and 100%; medium effect between 66% and 92%; and finally, weak effect between 0% and 65% of the sample. In addition, we calculated Cohen’s d (1988). For its interpretation as an indicator of the magnitude of effect between adjacent phases of a design n = 1, the restrictive criteria of Harrington and Velicer (2015) should be used: small effect 0–0.99; medium effect 1–2.49 and large effect > 2.50.
For analysis of the combined effect of all participants between adjacent phases, the Between-case standardised mean difference (BC–SMD) effect magnitude indicator, known as The HPS d statistic (Hedges et al., 2013), is provided. The value of BC–SMD is interpreted as Cohen’s d (1988) since it must be understood as if it were a group study: small effect > 0.2, medium > 0.5 and grade > 0.8. The calculations were made with the web-based calculator scdhlm (Valentine et al., 2016; Pustejovsky, 2016).
Results
The results obtained for the average velocity of the centre of pressure (AVCOP) are presented according to the four different experimental conditions studied: bipodal position on a hard surface with eyes open; bipodal position on a hard surface with eyes closed; bipodal position on a padded surface with eyes open; and bipodal position on a padded surface with eyes closed.
Bipodal Position on a Hard Surface with Eyes Open and Closed
Visual analysis of the graphs (Fig. 2) reveals no evident pattern of immediate level change in most participants, although a change in the trend of the intervention line is intuited, the effect being appreciated from the second and third month of intervention.
Baseline and intervention line graphs of AVCOP results (mm/s) on hard surface with participants’ eyes open (left) and closed (right). Numbers on the right side of the graph indicate the subject to which they correspond. The horizontal axis indicates the temporal scale, each point representing a consecutive month. The scale in the vertical axis corresponds to the AVCOP expressed in mm/s. Vertical dotted lines separate the baseline before the intervention (A left), the intervention (B), and the post-treatment baseline (A right, when measured). Grouping in the left side correspond to those indicated in Fig. 1
Moreover, analysis of the reversal lines of the first three cases shows that the effect of the intervention was maintained three months after the end of the intervention programme (Tables 3 and 4).
Bipodal Position on a Padded Surface with Participants’ Eyes Open and Closed
Also, visual analysis of the graphs (Fig. 3) reveals no evident pattern of immediate level change in most participants. However, a change in the trend of the intervention line is observed, the effect being seen from the second and third month of intervention. Moreover, analysis of the reversal lines of the first three participants shows that the effect of the intervention programme is maintained after three months.
Baseline and intervention line graphs of AVCOP results (mm/s) on a padded surface with participants’ eyes open (left) and closed (right). Numbers on the right side of the graph indicate the subject to which they correspond. The horizontal axis indicates the temporal scale, each point representing a consecutive month. The scale in the vertical axis corresponds to the AVCOP expressed in mm/s. Vertical dotted lines separate the baseline before the intervention (A left), the intervention (B), and the post-treatment baseline (A right, when measured). Grouping in the left side correspond to those indicated in Fig. 1
The quantitative data indicate that in eight of the nine participants the effect of the intervention programme was large (Table 5). In addition, analysis of the aggregate data (Table 6) indicates that the magnitude of the effect of the intervention programme is large for both open-eye (BC–SMD = 1.033) and closed-eye (BC–SMD = 0.838) conditions.
Discussion
The objective of this paper was to study whether a programme of therapeutic horse-assisted activities can improve postural control in autistic children. For this purpose, an experimental design of a multiple base group between subjects was carried out, with four groups responding to an AB scheme (A being the baseline and B the intervention). A new reversal phase (ABA) was added to one of the groups to obtain data about maintaining the treatment effect in the absence of intervention.
The baseline data on the two surfaces studied show that the starting point is far removed from the regulatory data for the corresponding age in neurotypical children. Our group of participants’ AVCOP on a hard surface was 20.3 mm/s for open eyes and 27.9 mm/s for closed eyes, the normative scores for both conditions being around 3.5 mm/s. Our group’s AVCOP on a padded surface was 93.6 mm/s (open eyes) and 64.7 mm/s (closed eyes), the standard scores also being around 3.5 mm/s (Shams et al., 2020).
The fact that the worst scores were obtained on a padded surface (inhibition of proprioceptive stimuli) and with the participants’ eyes open (93.7 mm/s) seems to indicate that vestibular input and poor use of visual information confer a peculiar style of postural control to autistic children (Doumas et al., 2016). Regarding this issue, some characteristics have been observed in this research corroborating the scientific literature, such as the specific deficit in the use of vision for action. Some authors have highlighted this fact, trying to explain that, besides limitations in the reception and processing of vestibular stimuli, there is a specific deficit in the use of vision for action in autistic children, which can explain in part the social and motor difficulties associated to this syndrome (Morris et al., 2015).
Another feature observed is postural impairment, which in the case of people with ASD may be related to abnormal neurophysiology based on atypical connectivity between brain regions during sensory stimulation (Lim et al., 2017), as they have structural or functional impairments (Allen & Courchesne, 2003; Nayate et al., 2005). These impairments are exacerbated in situations where children with ASD experience a disruption of some of the channels that support sensory integration, as corroborated by previous studies indicating that postural flopping (lack of postural control) is in part due to poor integration of vestibular, somatosensory and visual inputs (Kohen-Raz et al., 1992; Molloy et al., 2003). This atypical connectivity would determine the poor processing of sensory signals received by some sensory channels and the multiple sensory processing of these signals (Lim et al., 2017).
Regarding the effects of the intervention programme, the results indicate that the intervention with horses had a positive effect on the postural control of participants with autism. This effect was moderate when participants were evaluated on hard surfaces, but large when they were evaluated on padded surfaces, even when their eyes were closed.
It should be noted that the greatest effect occurred when data were obtained on padded surfaces with participants’ eyes open, which seems to indicate that working with horses produces sensory stimulation in all channels, but especially in vestibular and visual stimuli. This fact is related to the observations made by some authors (Bronson et al., 2010; Muñoz Lasa et al., 2015; Tseng et al., 2013; Olivier et al., 2017) who consider that riding a horse involves intensive exercise which is highly specific for improving balance, since the horse’s gait induces continuous changes in the rider’s centre of gravity requiring positional readjustments as the horse moves.
This continuous repositioning due to the movements of horse-riding has been described as being facilitated by the vestibular system (Campbell & Garrett, 1996). It is perhaps the greatest success of this intervention, since the vestibular stimulation that involves riding a horse is difficult to simulate elsewhere. Obviously, this vestibular stimulation requires of a special and specific training.
However, despite the statistically significant effect of the intervention programme on participants’ postural control, the results at the end of the intervention programme were not within the normotypical values for the corresponding age. This fact may be related to the duration and frequency of the intervention programme, but also to the persistence of motor symptoms in autism, as they are usually still present even in the adult population (Lim et al., 2017). Nevertheless, the persistence of symptoms encourages us to think about planning longer intervention programmes than those followed in our work. In this study, no significant differences were observed between the number of sessions assigned to each group in the range studied, due to the limited size number and the high dispersion of data associated to ASD individuals.
Although there is very little information about the therapeutic and re-educational alternatives to improve the postural control of people with autism, some experimental programmes have offered encouraging results (Cheldavi et al., 2014).
These programmes have been successful to a greater or lesser extent. However, future research should provide information about which programme is most effective for different conditions and age groups, and about the relevance, feasibility, and cost effectiveness of the different techniques. In addition, the intensity and duration of the sessions and the permanence in time of the achievements in the medium and long term should be investigated.
Finally, we would like to point out that care of postural control in autistic children in whom this ability is affected is an indispensable task since this deficit directly influences personal autonomy (Fears et al., 2022) and the successful performance of routine activities (Travers et al., 2017). On the other hand, the fact that deficits in postural control can be observed before the appearance of social and communicative symptoms (Radonovich et al., 2013) allows us to venture that appropriate early intervention may decisively influence the severity of the evolution of the disorder (Nayate et al., 2005). In this regard we support the suggestions of Li et al. (2021) who emphasise that early intervention in children with ASD is very important for improving postural control through the development of postural stability and motor function. These authors suggest that interventions could include opportunities to experience different body movements or positions that require different postural control strategies. In addition, several authors (Muñoz Lasa et al., 2015) argue that much of the ability to learn comes from the ability to integrate sensory information in the first years of life. However, they conclude that more research is needed on deficits in postural control complexity for young autistic children, along with evaluation of the efficacy of early interventions in this aspect.
Conclusively, the data here presented reveal that the horseback-riding intervention programme significantly improved participants’ postural control. Some factors that might explain this improvement could be that they were developed in a highly structured and motivating context providing intense and specific multisensory stimulation adjusted to the specific needs of the participants.
Limitations
Once the effect of the intervention programme on the postural control of autistic children has been demonstrated with multiple baseline across subjects design, efforts should be made to carry out group, randomised, and double-blind experimental designs with adequate statistical power.
The limited sample used does not allow investigation of the effect of the treatment programme on some differential characteristics such as age, gender, or the level of severity of the disorder.
Regarding our design in particular, we understand that there is appreciable variability in the baseline of some of the participants, although participants with baselines with more than three evaluations have tended towards stabilisation. Presumably, a longer baseline period would have enabled a clearer visual analysis of the effect of the intervention.
On the other hand, although a reversionary design (ABA) with three measurements after the intervention programme was carried out in one of the groups, we have no information about the long-term effects of the intervention programme. It was not possible to obtain comparative results between the groups to which different intervention times were applied, in part due to the heterogeneity of each subject with ASD and their own evolutionary development.
Finally, it should be noted that, although the participants were selected on the basis of their ability to follow orders, their difficulties in attention and concentration, combined with the high sensitivity of the posturography sensors, meant that the measurements were not free of difficulty, which also resulted in higher data dispersion.
These limitations are the basis for further research.
References
Aartolahti, E., Häkkinen, A., Lönnroos, E., Kautiainen, H., Sulkava, R., & Hartikainen, S. (2013). Relationship between functional vision and balance and mobility performance in community-dwelling older adults. Aging Clinical and Experimental Research, 25(5), 545–552. https://doi.org/10.1007/s40520-013-0120-z.
Adolph, K. E., & Franchak, J. M. (2017). The development of motor behavior. Wiley Interdisciplinary Reviews: Cognitive Science, 8(1–2), e1430. https://doi.org/10.1002/wcs.1430.
Akyol, B., & Pektas, S. (2018). The effects of Gymnastics Training combined with music in children with Autism Spectrum Disorder and Down Syndrome. International Education Studies, 11(11), 45–51. https://eric.ed.gov /?q=music+and+autism&ft=on&id=EJ1194665.
Allen, G., & Courchesne, E. (2003). Differential effects of developmental cerebellar abnormality on cognitive and motor functions in the cerebellum: An fMRI study of autism. American Journal of Psychiatry, 160(2), 262–273.
APA (2013). Diagnostic and Statistical Manual of Mental Disorders Fifth Edition. DSM-5 (2013.a ed.). American Psychiatric Association.
Ayres, A. (2008). La integración sensorial en Los niños: Desafíos sensoriales ocultos (25 ed.). TEA Ediciones, S.A.
Bass, M. M., Duchowny, C. A., & Llabre, M. M. (2009). The effect of therapeutic horseback riding on social functioning in children with autism. Journal of Autism and Developmental Disorders, 39(9), 1261–1267.
Bronson, C., Brewerton, K., Ong, J., Palanca, C., & Sullivan, S. J. (2010). Does hippotherapy improve balance in persons with multiple sclerosis: A systematic review. European Journal of Physical and Rehabilitation Medicine, 46, 347–353.
Bronstein, A. M. (2019). A conceptual model of the visual control of posture. En Progress in Brain Research (Vol. 248, pp. 285–302). Elsevier. https://doi.org/10.1016/bs.pbr.2019.04.023.
Caeyenberghs, K., Taymans, T., Wilson, P. H., Vanderstraeten, G., Hosseini, H., & van Waelvelde, H. (2016). Neural signature of developmental coordination disorder in the structural connectome Independent of comorbid autism. Developmental Science, 19(4), 599–612. https://doi.org/10.1111/desc.12424.
Campbell, J., & Garrett, S. (1996). Therapeutic riding as a treatment modality for clients with a sensory integrative dysfunction. Riding Tall, 6, 5–6.
Casey, A. F., Quenneville-Himbeault, G., Normore, A., Davis, H., & Martell, S. G. (2015). A therapeutic skating intervention for children with Autism Spectrum Disorder. Pediatric Physical Therapy, 27(2), 170–177. https://doi.org/10.1097/PEP.0000000000000139.
Cheldavi, H., Shakerian, S., Boshehri, S. N. S., & Zarghami, M. (2014). The effects of balance training intervention on postural control of children with autism spectrum disorder: Role of sensory information. Research in Autism Spectrum Disorders, 8, 8–14. https://doi.org/10.1016/j.rasd.2013.09.016.
Cohen. (1988). Statistical power analysis for the behavioral sciences. L. Erlbaum Associates.
Cohen, S., Conduit, R., Lockley, S. W., Rajaratnam, S. M., & Cornish, K. M. (2014). The relationship between sleep and behavior in autism spectrum disorder (ASD): A review. Journal of Neurodevelopmental Disorders, 6(1), https://doi.org/10.1186/1866-1955-6-44.
Corbett, B. A., Muscatello, R. A., & Blain, S. D. (2016). Impact of sensory sensitivity on physiological stress response and novel peer interaction in children with and without Autism Spectrum Disorder. Frontiers in Neuroscience, 10. https://doi.org/10.3389/fnins.2016.00278.
Crespo-Eguílaz, N., & Narbona-García, J. (2009). Procedural learning disorder: Neuropsychological characteristics. Revista De Neurología, 49(08), 409. https://doi.org/10.33588/rn.4908.2009079.
Dobell, A., Pringle, A., Faghy, M. A., & Roscoe, C. M. P. (2020). Fundamental Movement skills and Accelerometer-measured physical activity levels during early childhood: A systematic review. Children, 7(11), 224. https://doi.org/10.3390/children7110224.
Doumas, M., McKenna, R., & Murphy, B. (2016). Postural control deficits in Autism Spectrum Disorder: The role of sensory integration. Journal of Autism and Developmental Disorders, 46, 853–861. https://doi.org/10.1007/s10803-015-2621-4.
Dziuk, M. A., Gidley Larson, J. C., Apostu, A., Mahone, E. M., Denckla, M. B., & Mostofsky, S. H. (2007). Dyspraxia in autism: Association with motor, social, and communication deficits. Dev Med Child Neurol, 49, 734–739.
Ezell, J., Hogan, A., Fairchild, A., Hills, K., Klusek, J., Abbeduto, L., & Roberts, J. (2019). Prevalence and predictors of anxiety disorders in adolescent and adult males with Autism Spectrum Disorder and Fragile X Syndrome. Journal of Autism and Developmental Disorders, 49(3), 1131–1141. https://doi.org/10.1007/s10803-018-3804-6.
Fears, N. E., Templin, T. N., Sherrod, G. M., Bugnariu, N. L., Patterson, R. M., & Miller, H. L. (2022). Autistic children use less efficient goal-Directed whole body movements compared to Neurotypical Development. Journal of Autism and Developmental Disorders, 1–12.
Fernándes, C. R. (2020). Influencia da fisioterapia no acompanhamento de crianças portadoras do TEA (Trasntorno do espectro autista).TCC (Graduação).Unifasb, Barreiras.
Fournier, K. A., Kimberg, C. I., Radonovich, K. J., Tillman, M. D., Chow, J. W., Lewis, M. H., Bodfish, J. W., & Hass, C. J. (2010). Decreased static and dynamic postural control in children with autism spectrum disorders. Gait & Posture, 32(1), 6–9. https://doi.org/10.1016/j.gaitpost.2010.02.007.
Gabriels, R. L., Agnew, J. A., Holt, K. D., Shoffner, A., Zhaoxing, P., Ruzzano, S., & Mesibov, G. (2012). Pilot study measuring the effects of therapeutic horseback riding on school-age children and adolescents with autism spectrum disorders. Research in Autism Spectrum Disorders, 6(2), 578–588.
Gagey, P. M., & Weber, B. (2004). Posturologie: Régulation et dérèglements de la station debout. Elsevier Masson.
García-Gómez, A., Risco, M. L., Rubio, J. C., Guerrero, E., & García-Peña, I. M. (2014). Effects of a program of adapted therapeutic Horse-Riding in a Group of Autism Spectrum Disorder Children. Electronic Journal of Research in Educational Psychology, 12(1), 107–128.
Guzmán-Muñoz, E., Méndez-Rebolledo, G., Villouta-Gutiérrez, O., Concha-Cisternas, Y., & Valdés-Badilla, P. (2019). Análisis De Los Sistemas sensoriales que contribuyen al control postural en personas con síndrome de Down. Neurología, 11(2), 75–80. https://doi.org/10.1016/j.neuarg.2019.02.004.
Hameury, L., Delavous, P., Teste, B., Leroy, C., Gaboriau, J. C., & Berthier, A. (2010). Équithérapie et autisme. Annales Médico-psychologiques Revue Psychiatrique, 168(9), 655–659. https://doi.org/10.1016/j.amp.2009.12.019.
Hariri, R., Nakhostin-Ansari, A., Mohammadi, F., Memari, A. H., Oskouie, I. M., & Haghparast, A. (2022). An Overview of the Available Intervention Strategies for Postural Balance Control in Individuals with Autism Spectrum Disorder. Autism Research and Treatment, 2022.
Harrington, M., & Velicer, W. F. (2015). Comparing visual and statistical analysis in single-case studies using published studies. Multivariate Behavioral Research, 50(2), 162–183. https://doi.org/10.1080/00273171.2014.973989.
Hedges, L. V., Pustejovsky, J. E., & Shadish, W. R. (2013). A standardized mean difference effect size for multiple baseline designs across individuals. Research Synthesis Methods, 4(4), 324–341. https://doi.org/10.1002/jrsm.1086.
Hilton, C. L., Cumpata, K., Klohr, C., Gaetke, S., Artner, A., Johnson, H., & Dobbs, S. (2014). Effects of exergaming on executive function and motor skills in Children with Autism Spectrum disorder: A pilot study. American Journal of Occupational Therapy, 68(1), 57–65. https://doi.org/10.5014/ajot.2014.008664.
Hsu, Y. S., Kuan, C. C., & Young, Y. H. (2009). Assessing the development of balance function in children using stabilometry. International Journal of Pediatric Otorhinolaryngology, 73(5), 737–740. https://doi.org/10.1016/j.ijporl.2009.01.016.
Hyman, S. L., Levy, S. E., Myers, S. M., & Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. (2020). Identification, evaluation, and management of children with Autism Spectrum Disorder. Pediatrics, 145(1), e20193447. https://doi.org/10.1542/peds.2019-3447.
Jansiewicz, E. M., Goldberg, M. C., Newschaffer, C. J., Denckla, M. B., Landa, R., & Mostofsky, S. H. (2006). Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. Journal of Autism and Developmental Disorders, 36, 613–621.
Jenkins, S. R., & Reed, F. D. D. (2013). An experimental analysis of the effects of therapeutic horseback riding on the behavior of children with autism. Research in Autism Spectrum Disorders, 7(6), 721–740.
Kern, J. K., Fletcher, C. L., Garver, C. R., Mehta, J. A., Grannemann, B. D., Knox, K. R., Richardson, T. A., & Trivedi, M. H. (2011). Prospective trial of equine-assisted activities in autism spectrum disorder. Alternative Therapies in Health and Medicine, 17(3), 14–20. https://utsouthwestern.pure.elsevier.com/en/publications/prospective-trial-of-equine-assisted-activities-in-autism-spectru.
Kohen-Raz, R., Volkman, F. R., & Cohen, D. J. (1992). Postural control in children with autism. Journal of Autism and Developmental Disorders, 22(3), 419–432.
Kratochwill, T. R., Hitchcock, J. H., Horner, R. H., Levin, J. R., Odom, S. L., Rindskopf, D. M., & Shadish, W. R. (2013). Single-case intervention Research Design standards. Remedial and Special Education, 34(1), 26–38. https://doi.org/10.1177/0741932512452794.
Lakie, M., & Loram, I. D. (2006). Manually controlled human balancing using visual, vestibular and proprioceptive senses involves a common, low frequency neural process: Balancing with altered sensory modalities. The Journal of Physiology, 577(1), 403–416. https://doi.org/10.1113/jphysiol.2006.116772.
Larson, J. C. G., & e Mostofosky, S. H. (2009). Déficits motores no autismo. In R. Tuchman E I. Rapin (Orgs). Autismo: Abordagem neurobiológica (pp. 249–266). Artmed.
Leanderson, J., Eriksson, E., Nilsson, C., & Wykman, A. (1996). Proprioception in Classical Ballet dancers: A prospective study of the influence of an ankle sprain on Proprioception in the Ankle Joint. The American Journal of Sports Medicine, 24(3), 370–374. https://doi.org/10.1177/036354659602400320.
Li, Y., Liu, T., & Venuti, C. E. (2021). Development of postural stability in children with autism spectrum disorder: A cross-sectional study. International Biomechanics, 8(1), 54–62.
Licari, M. K., Alvares, G. A., Varcin, K., Evans, K. L., Cleary, D., Reid, S. L., Glasson, E. J., Bebbington, K., Reynolds, J. E., Wray, J., & Whitehouse, A. J. O. (2020). Prevalence of Motor difficulties in Autism Spectrum Disorder: Analysis of a Population-based cohort. Autism Research, 13(2), 298–306. https://doi.org/10.1002/aur.2230.
Lim, Y. H., Partridge, K., Girdler, S., & Morris, S. L. (2017). Standing Postural Control in individuals with Autism Spectrum Disorder: Systematic review and Meta-analysis. Journal of Autism and Developmental Disorders, 47(7), 2238–2253. https://doi.org/10.1007/s10803-017-3144-y.
Lourenço, C., Esteves, D., Nunes, C., & Liu, T. (2020). Motor proficiency of children with autism spectrum disorder and typically developing children in Portugal. Journal of Physical Education and Sport, 20(3), 1491–1496.
Lum, J. A. G., Shandley, K., Albein-Urios, N., Kirkovski, M., Papadopoulos, N., Wilson, R. B., Enticott, P. G., & Rinehart, N. J. (2020). Meta-Analysis Reveals Gait Anomalies in Autism. Autism Research, n/a(n/a). https://doi.org/10.1002/aur.2443.
MacDonald, M., Esposito, P., Hauck, J., Jeong, I., Hornyak, J., Argento, A., & Ulrich, D. A. (2012). Bicycle training for youth with Down syndrome and autism spectrum disorders. Focus on Autism and Other Developmental Disabilities, 27(1), 12–21.
MacDonald, M., Lord, C., & Ulrich, D. A. (2013). The relationship of Motor skills and Social communicative skills in school-aged children with Autism Spectrum Disorder. Adapted Physical Activity Quarterly, 30(3), 271–282. https://doi.org/10.1123/apaq.30.3.271.
Madra, M., Ringel, R., & Margolis, K. G. (2020). Gastrointestinal issues and Autism Spectrum Disorder. Child and Adolescent Psychiatric Clinics, 29(3), 501–513. https://doi.org/10.1016/j.chc.2020.02.005.
Maenner, M. J., Shaw, K. A., Baio, J., EdS1, Washington, A., Patrick, M., DiRienzo, M., Christensen, D. L., Wiggins, L. D., Pettygrove, S., Andrews, J. G., Lopez, M., Hudson, A., Baroud, T., Schwenk, Y., White, T., Rosenberg, C. R., Lee, L. C., Harrington, R. A., & Dietz, P. M. (2020). Prevalence of Autism Spectrum Disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2016. MMWR Surveillance Summaries, 69(4), 1–12. https://doi.org/10.15585/mmwr.ss6904a1.
Martin, A. F., Jassi, A., Cullen, A. E., Broadbent, M., Downs, J., & Krebs, G. (2020). Co-occurring obsessive-compulsive disorder and autism spectrum disorder in young people: Prevalence, clinical characteristics and outcomes. European Child & Adolescent Psychiatry, 29(11), 1603–1611. https://doi.org/10.1007/s00787-020-01478-8.
Maurer, R. G., & Damasio, A. R. (2008). Vestibular dysfunction in autistic children. Developmental Medicine & Child Neurology, 21(5), 656–659. https://doi.org/10.1111/j.1469-8749.1979.tb01682.x.
Mazurek, M. O., & Petroski, G. F. (2015). Sleep problems in children with autism spectrum disorder: Examining the contributions of sensory over-responsivity and anxiety. Sleep Medicine, 16(2), 270–279. https://doi.org/10.1016/j.sleep.2014.11.006.
Molloy, C. A., Dietrich, K. N., & Bhattacharya, A. (2003). Postural Stability in Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 33(6), 643–652. https://doi.org/10.1023/B:JADD.0000006001.00667.4c.
Montes-Castillo, M. L., Benítez, M. P., Díaz-Barriga, A. S., & y Jasso, A. V. (2000). El balance y las caídas en la tercera edad: Consecuencias, evaluación y tratamiento. Revista Mexicana De Medicina física Y rehabilitación, 12(1), 16–20. https://www.medigraphic.com/pdfs/fisica/mf-2000/mf001c.pdf.
Morris, S. L., Foster, C. J., Parsons, R., Falkmer, M., Falkmer, T., & Rosalie, S. M. (2015). Differences in the use of vision and proprioception for postural control in autism spectrum disorder. Neuroscience, 307, 273–280. https://doi.org/10.1016/j.neuroscience.2015.08.040.
Mosston, M., & Ashworth, S. (2008). Teaching Physical Education. First Online Edition). Pearson Education.
Muñoz Lasa, S., Máximo Bocanegra, N., Alcaide, V., Atín, R., Arratibel, M. A., Donoso, V., E., & Ferriero, G. (2015). Animal assisted interventions in neurorehabilitation: A review of the most recent literature. Neurología (English Edition), 30(1), 1–7. https://doi.org/10.1016/j.nrleng.2013.01.010.
Muskens, J. B., Velders, F. P., & Staal, W. G. (2017). Medical comorbidities in children and adolescents with autism spectrum disorders and attention deficit hyperactivity disorders: A systematic review. European Child & Adolescent Psychiatry, 26(9), 1093–1103. https://doi.org/10.1007/s00787-017-1020-0.
Najafabadi, M. G., Sheikh, M., Hemayattalab, R., Memari, A. H., Aderyani, M. R., & Hafizi, S. (2018). The effect of SPARK on social and motor skills of children with autism. Pediatrics & Neonatology, 59(5), 481–487. https://doi.org/10.1016/j.pedneo.2017.12.005.
Nayate, A., Bradshaw, J. L., & Rinehart, N. J. (2005). Autism and Asperger’s disorder: Are they movement disorders involving the cerebellum and/or basal ganglia? Brain Research Bulletin, 67(4), 327–334. https://doi.org/10.1016/j.brainresbull.2005.07.011.
Neumeyer, A. M., Anixt, J., Chan, J., Perrin, J. M., Murray, D., Coury, D. L., Bennett, A., Farmer, J., & Parker, R. A. (2019). Identifying associations among Co-occurring Medical conditions in children with Autism Spectrum disorders. Academic Pediatrics, 19(3), 300–306. https://doi.org/10.1016/j.acap.2018.06.014.
Nuntanee, S., & Daranee, S. (2019). Effect of Motorized Elephant-Assisted Therapy Program on Balance Control of Children with Autism Spectrum Disorder. Occupational Therapy International, 2019, 1–10. https://doi.org/10.1155/2019/5914807.
Ogawa, S., Lee, Y. A., Yamaguchi, Y., Shibata, Y., & Goto, Y. (2017). Associations of acute and chronic stress hormones with cognitive functions in autism spectrum disorder. Neuroscience, 343, 229–239.
Olivier, A., Faugloire, E., Lejeune, L., Biau, S., & Isableu, B. (2017). Head Stability and Head-Trunk Coordination in Horseback Riders: The Contribution of Visual Information According to Expertise. Frontiers in Human Neuroscience, 11. https://doi.org/10.3389/fnhum.2017.00011.
Paquet, A., Olliac, B., Bouvard, M. P., Golse, B., & Vaivre-Douret, L. (2016). The Semiology of Motor Disorders in Autism Spectrum Disorders as Highlighted from a Standardized Neuro-Psychomotor Assessment. Frontiers in Psychology, 7. https://doi.org/10.3389/fpsyg.2016.01292.
Parker, R. I., & Vannest, K. (2009). An improved effect size for single-case research: Nonoverlap of all pairs. Behavior Therapy, 40(4), 357–367. https://doi.org/10.1016/j.beth.2008.10.006.
Parker, R. I., Vannest, K. J., & Davis, J. L. (2011). Effect size in Single-Case Research: A review of nine nonoverlap techniques. Behavior Modification, 35(4), 303–322.
Parreira, R. B., Grecco, L. A. C., & Oliveira, C. S. (2017). Postural control in blind individuals: A systematic review. Gait & Posture, 57, 161–167. https://doi.org/10.1016/j.gaitpost.2017.06.008.
Peña, O., Cibrian, F. L., & Tentori, M. (2020). Circus in Motion: A multimodal exergame supporting vestibular therapy for children with autism. Journal on Multimodal User Interfaces. https://doi.org/10.1007/s12193-020-00345-9.
Peters, B. C. M., & Wood, W. (2017). Autism and equine-assisted interventions: A systematic mapping review. Journal of Autism and Developmental Disorders, 47(10), 3220–3242.
Portela-Pino, I., Bouzo-Gónzalez, S., & Pino-Juste, M. (2019). Evaluation of an equine therapy program in students with autism spectrum disorder. Journal of Human Sport and Exercise, 15(4), https://doi.org/10.14198/jhse.2020.154.06.
Pustejovsky, J. E. (2016). scdhlm. A web-based calculator for between-case standardized mean differences (Version 0.3.1) [Web application]. https://jepusto.shinyapps.io/scdhlm.
Radonovich, K. J., Fournier, K. A., & Hass, C. J. (2013). Relationship between postural control and restricted, repetitive behaviors in autism spectrum disorders. Frontiers in Integrative Neuroscience, 7. https://doi.org/10.3389/fnint.2013.00028.
Rydzewska, E., Hughes-McCormack, L. A., Gillberg, C., Henderson, A., MacIntyre, C., Rintoul, J., & Cooper, S. A. (2019). Prevalence of sensory impairments, physical and intellectual disabilities, and mental health in children and young people with self/proxy-reported autism: Observational study of a whole country population. Autism, 23(5), 1201–1209. https://doi.org/10.1177/1362361318791279.
Sam, K. L. S., Smith, A. W., & Lo, S. K. (2017). Visual cognition and dynamic balance in persons with autism spectrum disorder. International Journal of Social Science and Humanity, 7(5), 274–277. https://doi.org/10.18178/ijssh.2017.7.5.833.
Sarabzadeh, M., Azari, B. B., & Helalizadeh, M. (2019). The effect of six weeks of Tai Chi Chuan training on the motor skills of children with Autism Spectrum Disorder. Journal of Bodywork and Movement Therapies, 23(2), 284–290. https://doi.org/10.1016/j.jbmt.2019.01.007.
Shams, A., Vameghi, R., Shamsipour Dehkordi, P., Allafan, N., & Bayati, M. (2020). The development of postural control among children: Repeatability and normative data for computerized dynamic posturography system. Gait & Posture, 78, 40–47. https://doi.org/10.1016/j.gaitpost.2020.03.002.
Shavikloo, J., & Norasteh, A. (2018). The effect of integrative neuromuscular training on postural control of children with autism spectrum. Neurology and Neurosurgery, 1(2), https://doi.org/10.15761/NNS.1000107.
Smith, J. A., & Fisher, B. E. (2018). Anticipatory postural adjustments and spatial organization of motor cortex: Evidence of adaptive compensations in healthy older adults. Journal of Neurophysiology, 120(6), 2796–2805. https://doi.org/10.1152/jn.00428.2018.
Somogyi, E., Kapitány, E., Kenyeres, K., Donauer, N., Fagard, J., & Kónya, A. (2016). Visual feedback increases postural stability in children with autism spectrum disorder. Research in Autism Spectrum Disorders, 29–30, 48–56. https://doi.org/10.1016/j.rasd.2016.06.001.
Speers, R. A., Kuo, A. D., & Horak, F. B. (2002). Contributions of altered sensation and feedback responses to changes in coordination of postural control due to aging. Gait & Posture, 16(1), 20–30. https://doi.org/10.1016/S0966-6362(02)00003-6.
Stevenson, J. L., Lindley, C. E., & Murlo, N. (2017). Retrospectively assessed Early Motor and Current Pragmatic Language skills in autistic and Neurotypical Children. Perceptual and Motor Skills, 124(4), 777–794. https://doi.org/10.1177/0031512517710379.
Stins, J. F., & Emck, C. (2018). Balance performance in autism: A brief overview. Frontiers in Psychology, 9, 901.
Travers, B. G., Bigler, E. D., Duffield, T. C., Prigge, M. D. B., Froehlich, A. L., Lange, N., Alexander, A. L., & Lainhart, J. E. (2017). Longitudinal development of manual motor ability in autism spectrum disorder from childhood to mid-adulthood relates to adaptive daily living skills. Developmental Science, 20(4), e12401. https://doi.org/10.1111/desc.12401.
Travers, B. G., Mason, A., Mrotek, L. A., Ellertson, A., Dean, D. C., Engel, C., Gomez, A., Dadalko, O. I., & McLaughlin, K. (2018). Biofeedback-Based, Videogame Balance Training in Autism. Journal of Autism and Developmental Disorders, 48(1), 163–175. https://doi.org/10.1007/s10803-017-3310-2.
Trzmiel, T., Purandare, B., Michalak, M., Zasadzka, E., & Pawlaczyk, M. (2019). Equine assisted activities and therapies in children with autism spectrum disorder: A systematic review and a meta-analysis. Complementary Therapies in Medicine, 42, 104–113. https://www.sciencedirect.com/science/article/pii/S0965229918308331#sec0045.
Tseng, S. H., Chen, H. C., & Tam, K. W. (2013). Systematic review and meta-analysis of the effect of equine assisted activities and therapies on gross motor outcome in children with cerebral palsy. Disability and Rehabilitation, 35(2), 89–99. https://doi.org/10.3109/09638288.2012.687033.
Valentine, J. C., Tanner-Smith, E. E., Pustejovsky, J. E., & Lau, T. S. (2016). Between‐case standardized mean difference effect sizes for single‐case designs: A primer and tutorial using the scdhlm web application. Campbell Systematic Reviews, 12(1), 1–31. https://doi.org/10.4073/cmdp.2016.1.
Valverde-Esteve, T., Chiva-Bartoll, O., Salvador-García, C., & Maravé-Vivas, M. (2020). Effect of a service-learning program on the active lifestyle of children with Autism Spectrum disorder: A pilot study. Sustainability, 12(11), 4354.
Viguier, M. (2009). Influence du changement de l’angle d’incidence entre le vecteur gravitationnel terrestre et l’axe sagittal du rachis sur les performances posturales statiques et dynamiques d’un individu [Phd, Université de Toulouse, Université Toulouse III - Paul Sabatier]. http://thesesups.ups-tlse.fr/2747/.
Vives-Vilarroig, J., Ruiz-Bernardo, M. P., & García-Gómez, A. (2021). Rubric for evaluating balance on the horse in children with autism. Retos, 41, 887–896. https://doi.org/10.47197/retos.v41i0.86378.
Wang, Z., Hallac, R. R., Conroy, K. C., White, S. P., Kane, A. A., Collinsworth, A. L., Sweeney, J. A., & Mosconi, M. W. (2016). Postural orientation and equilibrium processes associated with increased postural sway in autism spectrum disorder (ASD). Journal of Neurodevelopmental Disorders, 8(1), 43. https://doi.org/10.1186/s11689-016-9178-1.
Westcott, S. L., Lowes, L. P., & Richardson, P. K. (1997). Evaluation of Postural Stability in Children: Current theories and Assessment Tools. Physical Therapy, 77(6), 629–645. https://doi.org/10.1093/ptj/77.6.629.
Winter, D. A., Patla, A. E., & Frank, J. S. (1990). Assessment of balance control in humans. Medical Progress through Technology, 16(1–2), 31–51.
Wuang, Y. P., Huang, C. L., & Tsai, H. Y. (2020). Sensory integration and perceptual-motor profiles in school-aged children with autistic spectrum disorder. Neuropsychiatric Disease and Treatment, 16, 1661–1673. https://doi.org/10.2147/NDT.S253337.
Acknowledgements
This job was possible thanks to the volunteers, the involved children and the patience of those wonderful creatures that are the horses. J Vives also thanks his “Sensei” for wise counsel.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. No funds, grants, or other support was received.
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Paola Ruiz-Bernardo, Juan Vives-Vilarroig and Andrés García-Gómez. The first draft of the manuscript was written by Juan Vives-Vilarroig and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors report no declarations of interest.
Ethics Approval
The journal’s instructions for authors have been followed during the research and the writing of the manuscript, considering that the work has been done according to the ethical rules of the responsible human experimentation committee (Deontological Commission of the Universitat Jaume I of Castellón, Spain, with the reference code CD/54/2019) and in accordance with the World Medical Association and the Declaration of Helsinki.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vives-Vilarroig, J., Ruiz-Bernardo, P. & García-Gómez, A. Effects of Horseback Riding on the Postural Control of Autistic Children: A Multiple Baseline Across-subjects Design. J Autism Dev Disord (2024). https://doi.org/10.1007/s10803-023-06174-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s10803-023-06174-5