Abstract
This study examined the sustained and moderating effects of a behavioural sleep intervention for autistic children in a randomised controlled trial. Autistic children (5–13 years) with sleep problems were randomised to the Sleeping Sound intervention or Treatment as Usual (TAU). At 12-month follow-up (n = 150), caregivers of children in the Sleeping Sound group reported greater reduction in child sleep problems compared to TAU (p < .001, effect size: − 0.4). The long-term benefits of the intervention were greater for children taking sleep medication, children of parents who were not experiencing psychological distress, and children with greater autism severity. The Sleeping Sound intervention demonstrated sustained improvements in child sleep. Identified moderators may inform treatment by indicating which subgroups may benefit from further support.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 50–80% of autistic children experience sleep disturbance (Singh & Zimmerman, 2015), involving a range of problems which often occur concurrently (Carnett et al., 2021). Disordered sleep has been associated with higher levels of autistic symptoms and daytime behaviour problems in autistic children (Veatch et al., 2017), and increased levels of stress and poorer mental health for parents (Martin et al., 2019). Empirical research evaluating the efficacy and durability of sleep interventions is therefore of great importance given the possible far-reaching benefits.
Evidence-based clinical guidelines recommend behavioural interventions as the first-line treatment for sleep problems in autism (National Institute for Health & Care Excellence, 2013; Williams Buckley et al., 2020). While there is promising meta-analytic evidence to support the short-term efficacy (ranging between 2 weeks and 2 months) of behavioural sleep interventions within this population (Keogh et al., 2019; Phillips et al., 2020), there is a dearth of literature examining whether benefits are sustained over time. As highlighted by Schreibman (2000), an intervention that fails to demonstrate sustainability of treatment effects should not be considered optimally effective. The maintenance of treatment gains following sleep interventions is of critical importance given the fundamental role that sleep plays in human functioning, particularly for autistic children whose sleep disturbances are persistent and less likely to resolve with age (Hodge et al., 2014; Humphreys et al., 2014).
Nonetheless, the few randomised controlled trials (RCTs) of behavioural sleep interventions in autism have conducted follow-ups over very short periods, ranging from 2 weeks to 2 months post-randomisation (Adkins et al., 2012; Johnson et al., 2013; Malow et al., 2014). Only three studies (Durand, 2002; van Deurs et al., 2019; Weiskop et al., 2001) have evaluated outcomes of behavioural sleep interventions for autistic children at 12-months or longer post-intervention. While each study reported sustained improvements in children’s sleep outcomes, findings are limited by the small sample sizes (n ≤ 3), single case designs, and lack of evaluation of secondary child/parent outcomes. The long-term benefits of behaviourally based sleep interventions therefore remain uncertain, particularly given evidence from adult studies suggesting that effects may diminish over time.
A recent meta-analysis of 29 RCTs examined the long-term effects of cognitive behavioural therapy for insomnia (CBT-I) in adults compared to non-active control groups (van der Zweerde et al., 2019). Findings indicated that although the CBT-I group outperformed the control groups at 12-month follow-up, effect sizes steadily decreased over time (g = 0.64 at 3-month, 0.40 at 6-month, 0.25 at 12-month follow-up). In the absence of these data within the autism field, clinicians managing sleep problems cannot make evidence-informed decisions regarding the level of further assessment and support required for autistic children. The paucity of follow-up sleep studies and subsequent clinical implications highlight the need for longer-term evaluations of behavioural sleep interventions for autism.
Sleeping Sound is a brief behavioural sleep intervention that provides tailored sleep strategies to families across two face-to-face consultations. The efficacy of Sleeping Sound was initially demonstrated in a large RCT of children with attention-deficit hyperactivity disorder (ADHD) (n = 244) (Hiscock et al., 2015), with benefits found up to 12 months later (Sciberras et al., 2020). Compared to TAU controls, children with ADHD who received the intervention showed greater improvements in sleep problems, ADHD severity, quality of life, daily functioning, and behaviour at 12 months post-randomisation. A recent RCT involving 245 autistic children aged 5–13 years has also confirmed the benefits of Sleeping Sound (Papadopoulos et al., 2022). Children receiving the intervention showed a reduction in sleep problems at 3 (moderate-to-large effect size; − 0.7) and 6 months (small-to-moderate effect size; − 0.4) post-randomisation compared with TAU controls. The intervention was also associated with small to moderate improvements in secondary child (emotional behavioural disturbances, internalising behaviour, and quality of life) and parent/caregiver (parenting stress, mental health, and quality of life) outcomes up to 6 months post-randomisation; however, these effects became non-significant when controlling for multiple comparisons. The present evaluation of the Sleeping Sound intervention at 12 months will determine whether the intervention is associated with long-term benefits for autistic children, or if continued support is needed to enable the maintenance of treatment gains beyond 6 months post-intervention.
In addition to establishing the short and long-term efficacy of an intervention, it is crucial to understand for whom, and under what conditions an intervention may be more or less effective (Farmer et al., 2012). Moderators of treatment outcomes can be defined as the characteristics that influence the relationship between intervention and outcome (Kraemer et al., 2006). Identifying moderators of treatment efficacy yields important clinical implications as it enables clinicians to prospectively recommend treatments to meet individual needs and maximise positive outcomes. The identification of treatment moderators is particularly pertinent in autism research, as the clinical heterogeneity of the disorder may result in different treatment responses from different subgroups of children (Hudry et al., 2018; Stahmer et al., 2016; Vivanti et al., 2014).
A number of child and family factors have been proposed as moderators of behavioural intervention outcomes for autistic children. Child factors include variables such as age, gender, autism symptomatology and severity, IQ, co-occurring psychiatric conditions, and medication use (see Crank et al., 2021; Gates et al., 2017 for reviews). Family characteristics include level of parent education, family socio-economic status, parent age, parental mental health, and parental stress levels (see Shalev et al., 2020; Trembath et al., 2019 for reviews). Despite this research, there is a dearth of literature examining moderators of treatment outcomes for behavioural sleep interventions in autistic or youth populations more broadly. A RCT evaluating moderators of a cognitive-behavioural and mindfulness-based group sleep intervention for typically developing adolescents found that improvements in sleep quality were greatest among those with higher levels of anxiety and depressive symptoms (Blake et al., 2018). Moderators of behavioural sleep interventions have also been explored in a RCT using a sample of children with ADHD (Sciberras et al., 2020). The results identified medication use and parental depression as moderators of treatment efficacy, indicating that the Sleeping Sound intervention was less efficacious for children who did not take ADHD medication and children of parents with depression. To date, no RCTs have explored moderators of treatment outcomes for autistic children receiving a behavioural sleep intervention.
In summary, behavioural sleep interventions are recognised as an efficacious, first-line approach for treating sleep problems in autism (Keogh et al., 2019). However, the degree to which these improvements are maintained over time is currently unknown. Moreover, little is known about the particular child or family characteristics that may be associated with varying degrees of treatment efficacy. The aims of the present study were threefold. First, we aimed to extend previous findings and examine whether the Sleeping Sound intervention for autistic children is associated with reduced sleep problems at 12 months post-randomisation. Second, we aimed to investigate the secondary longer-term impacts of the intervention on children’s quality of life, social, emotional, and behavioural functioning, and parent/caregivers’ stress levels, mental health, and quality of life. Third, we aimed to conduct an exploratory analysis to determine whether several putative child and family factors including child co-occurring conditions, age, sex, medication use, symptom severity, socioeconomic factors, and parent mental health are moderators of treatment outcomes over time. It was hypothesised that autistic children who received the Sleeping Sound intervention would show greater improvement in sleep problems and daytime functioning at 12 months post-randomisation, compared to a TAU control group.
Method
Design
A RCT design was used to examine the efficacy of a brief behavioural sleep intervention in treating sleep problems in children on the autism spectrum, compared with a TAU control group. This paper will focus on the 12-month follow-up data. This study was approved by the ethics committees from the Royal Children’s Hospital Melbourne (36154), Deakin University (2017-130), the Catholic Education Office Melbourne (0501), and the Victorian Department of Education and Early Childhood Development (2016_003134). The RCT is registered with the International Trial Registry (ISRCTN14077107).
Recruitment and Eligibility
A total of 247 autistic children aged between 5 and 13 years was recruited through Victorian paediatric clinics and study advertisements in research, clinical, and community networks. Children were eligible if they had a multidisciplinary diagnosis of ASD, were aged 5–13 years, and scored ≥ 11 on the Social Communication Lifetime form (Rutter et al., 2003). Children also needed to have moderate to severe caregiver-reported sleep problem(s) and meet diagnostic criteria for at least one caregiver-reported sleep problem as defined by the International Classification of Sleep Disorders (American Academy of Sleep Medicine, 2014). Children were excluded if they had an intellectual disability, suspected sleep disordered breathing or Obstructive Sleep Apnoea, or co-occurring medical conditions known to impact sleep. Children of parents with insufficient English proficiency were also excluded. A comprehensive summary of the inclusion and exclusion criteria can be found in Appendix B. Two participants were excluded post-randomisation as they did not have formal diagnoses of ASD confirmed by a multidisciplinary team.
Procedure
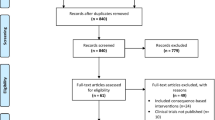
Parents/caregivers who registered interest in the study were contacted by a member of the research team via telephone to assess eligibility. The study was described and verbal informed consent obtained at the start of the screening call with parents. Eligible and interested families were provided with an information sheet, consent form, and baseline survey by email or post. Parents/caregivers who received the information and consent form by post provided consent by signing the hard copy document and returning it to researchers in a reply paid envelope. Parents/caregivers who received the information and consent form by email provided consent by typing their name and clicking ‘Yes’ to consent statements on a secure research database. Upon completion of consent form and baseline survey, participants were randomised to either the intervention group or the TAU control group by an independent researcher. Parents were sent surveys via email or post to assess outcomes at 12 months post-randomisation. Surveys were also sent to children’s school teachers if optional consent was provided by parents. The flow of participants from recruitment to follow-up assessments is displayed in Fig. 1.
Randomisation and Blinding
Upon completion of the consent form and baseline survey, participants were randomised to the intervention group or the TAU control group. Families allocated to the TAU control group continued with their standard service of clinical care available in the community. With the exception of web-based psychoeducational resources, there are currently no services and supports tailored to Australian families of autistic children who have sleep difficulties. Sleep problems in autism are often addressed among a variety of other behavioural issues and advice is often generic. Treatment allocation was determined by an independent researcher using a computer-generated block randomisation sequence and was stratified by gender to ensure equal representation. Families with siblings enrolled in the study were assigned to the same group. Knowledge of group allocation was limited to the project coordinator and study clinicians. Chief investigators, members of the research team, and the statistician were blinded to participant group status to minimise potential bias. A detailed summary of the randomisation and blinding is outlined in our protocol paper (Papadopoulos et al., 2019) (Fig. 2).
Intervention
Families allocated to the intervention group received the Sleeping Sound behavioural sleep intervention by a study-employed clinician (paediatrician or psychologist) with experience working with autistic children. The intervention comprised two consecutive 50-min face-to-face consultations and one follow-up phone call at 2-week intervals consisting of assessment, goal-setting, psychoeducation, and a tailored sleep management plan. An overview of some of the key behavioural sleep strategies recommended to families are provided in Appendix A. To ensure treatment fidelity, study clinicians adhered to a standardised intervention manual and met fortnightly to discuss any clinical questions or issues. A detailed description of the Sleeping Sound intervention content is outlined in our protocol paper (Papadopoulos et al., 2019).
Outcome Measures
The primary outcome was child sleep problems measured by the Children’s Sleep Habit Questionnaire (CSHQ; Owens et al., 2000) at 12 months post-randomisation. Secondary outcomes included child social, emotional, and behavioural functioning, child quality of life, child sleep hygiene and daytime sleepiness, child school attendance, parent stress and mental health, parent quality of life, and parent work attendance. Study measures and relevant psychometrics are summarised in Appendix C. Additional information collected at baseline included family and child demographics (e.g., gender, age, household income), and medical information (e.g., co-occurring conditions, medication use).
Statistical Analyses
Analyses were undertaken using Stata version 15 and conducted on an intention-to-treat basis, with participant data included as per initial treatment group allocation. To determine whether the Sleeping Sound intervention was associated with longer-term benefits, comparisons of continuous outcomes between the intervention and TAU groups at 12 months were made using linear mixed models. This approach involved fitting a single mixed model comparing baseline scores on an outcome measure to 3-month, 6-month, and 12-month scores for the same outcome, enabling a separate treatment effect at each follow-up time point. Count-based outcomes (days missed from school, days off work) were modelled using mixed-effects negative binomial models to control for overdispersion. This paper focuses on the 12-month treatment effects to extend our previously published work (Papadopoulos et al., 2022). Our study protocol (Papadopoulos et al., 2019) proposed clustering by individual (Level 2) and paediatrician (Level 3). However, for most variables, the clustering effect for paediatrician did not significantly deviate from zero based on comparison of log likelihood values for models with and without random intercepts at Level 3. Consequently, with the exception of outcomes that had a significant random intercept for paediatrician (school attendance, AQoL4d dimension: mental health), outcomes were tested with two-level mixed models, with clustering for within-participant effects.
Models are reported in unadjusted form, as well as adjusting for covariates identified a priori. Covariates used (child sex, age, ASD symptom severity, medication use, and socioeconomic status) were consistent with our published 3- and 6-month trial outcomes to ensure comparability (Papadopoulos et al., 2022). Effect sizes are reported as standardised mean differences, with values of ~ 0.20 considered small, ~ 0.50 moderate, and ~ 0.80 as large. Primary outcomes were tested with unadjusted p values, whereas p values reported for secondary outcomes were corrected for multiple comparisons using the Benjamini–Hochberg approach (Benjamini & Hochberg, 1995).
Missing data were dealt with using conditional maximum likelihood estimation. Sensitivity analyses were undertaken to evaluate the robustness of attained results to the possible presence of non-ignorable missingness patterns (i.e., missing not at random; MNAR). For the sensitivity analyses, pattern mixture models were used via the mimix package (Cro et al., 2016). Several multiple imputation options were tested with the mimix package, including last mean carried forward (LMCF; which imputes the mean at the previous timepoint from one’s assigned group), jump to reference (J2R; in which an individual’s missing data is imputed with the mean value from the control group at that timepoint) and copy increments in reference (CIR; in which an individual’s missing data is imputed with the mean increment from the previous timepoint for the control group regardless of treatment assignment at baseline). Interim missingness (i.e., when a participant has missing data at one timepoint but returns for a later wave) was treated as missing at random (MAR), which is a reasonable assumption for an intermittent response rather than complete dropout (Cro et al., 2016). A total of 50 imputations were undertaken per model.
To identify child and family moderators of the primary treatment outcome (child sleep problems as measured by the CSHQ), moderation analyses were conducted. Subgroups were defined via 13 dichotomous variables measured at baseline based on parent report: psychotropic medication use (yes/no), sleep medication use (yes/no), internalising comorbidity (anxiety and/or depression diagnosed or treated by a health professional: yes/ no), externalising comorbidity (conduct disorder and/or oppositional defiant disorder diagnosed or treated by a health professional: yes/no), combined internalising and externalising comorbidity (yes/no), ADHD comorbidity diagnosed or treated by a health professional (yes/no), ASD symptom severity (< 15/ ≥ 15 on the SCQ total score), child age (5–9/10–13 years), child biological sex (male/female), parent level of education (has/not completed high school), parent mental health (< 20/ ≥ 20 on the K10 total score), clinically significant parent stress (< 114/ ≥ 114 on the PSI total score), and weekly household income categorised as below or above the national average reported in the most recent Australian Bureau of Statistics (ABS) Household Income and Wealth report (< $2000/ ≥ $2000 a week) (ABS, 2019). This allowed us to examine the effect of each subgroup on outcomes over the 12-month study period. Number of participants in moderator subgroups can be found in Appendix D. Once subgroups were defined, we repeated the linear mixed models with the inclusion of a 3-way interaction (subgroup x trial arm x timepoint) term to test whether the treatment effects differed for each of the subgroups over time. A significant 3-way interaction would indicate that the efficacy of the intervention varied as a function of the moderator. Sensitivity analyses (pattern mixture models) and follow-up simple slope analyses were undertaken on any significant 3-way interaction effects.
Results
COVID-19 Disruptions to Follow-Up
Data collection ceased early due to the impact of the coronavirus (COVID-19) pandemic. In response to the pandemic, the Victorian Government declared a State of Emergency and implemented restrictions between March and May of 2020. During these times, there were only four reasons for Victorians to leave their home: food and supplies, medical care and care giving, exercise, and work or education if necessary (Victoria State Government Department of Health, 2020). Victorian schools were subject to remote learning throughout Term 2 and Term 3 and all research projects were paused, with students learning from home unless they were unable to be supervised. Following discussion with the research team, it was decided that sending follow-up surveys and reminders could place additional burden on participating families. The impact of the pandemic on children’s daily routines and consequent changes in parent-reported sleep problems were also considered. Accordingly, several parent (n = 24) and teacher (n = 24) final surveys and reminders that were due to be sent during the Victorian state of emergency (March – May 2020) were not sent. The last surveys were collected in February 2020, prior to the first lockdown being implemented in Victoria.
Descriptive Statistics
At 12 months post-randomisation, 150 children completed the follow-up survey (61.22% of the original sample). There were no significant correlations between 12-month survey completion and CSHQ change scores at 3-month (r = − 0.03, p = 0.628) or 6-month (r = − 0.01, p = 0.876) follow-up. Moreover, there were no significant differences in CSHQ total sleep scores between 12-month survey completers and non-completers at baseline (t = 0.84, p = 0.402), 3-month (t = 0.30, p = 0.764) or 6-month (t = 0.29, p = 0.770) follow-up. Table 1 summarises the baseline characteristics of the 12-month survey completers and non-completers. Between group differences were non-significant, with the exception of parent age and parent-reported internalising disorders. Parents who completed the 12-month survey were significantly older (42.01 years vs. 40.55 years, t = − 2.16, p = 0.031) and reported significantly more child internalising disorders (82 vs. 34, t = − 2.92, p = 0.004).
Primary Outcome: Child Sleep Problems
Table 2 summarises the sustained effects of the intervention on the primary outcome. The unadjusted mean group difference in severity of child sleep problems was significant for the majority of CSHQ subscales by 12-month follow-up, with effect sizes ranging from small to moderate. Children randomised to the intervention group reported a greater reduction in total sleep problems (mean difference = -3.62, 95% confidence interval (CI) = -− 5.61 to -1.63, p < 0.001, effect size = -− 0.40), and fared better in the following CSHQ subscales: bedtime resistance (mean difference = − 0.77, 95% CI − 1.49 to − 0.05, p = 0.037, effect size = − 0.24), sleep onset delay (mean difference = − 0.45, 95% CI − 0.69 to − 0.21, p < 0.001, effect size = − 0.65), sleep duration (mean difference = − 0.76, 95% CI − 1.26 to − 0.25, p = 0.003, effect size = − 0.46), and parasomnias (mean difference = − 0.80, 95% CI − 1.32 to − 0.27, p = 0.003, effect size = − 0.34). There were no significant differences between the two groups with regard to sleep anxiety (p = 0.160) or night waking (p = 0.085) at 12-month follow-up.
These differences all remained significant after adjusting for covariates. Additionally, results that were significant in the adjusted analyses tended to remain significant in sensitivity analyses, with a few exceptions: (1) the significant effect of sleep duration at 12-months was non-significant in one of the three MNAR analyses (J2R; p = 0.053), (2) the significant effect of parasomnias at 12-months was non-significant in one of the three MNAR analyses (J2R; p = 0.714), and (3) the significant effect of total score at 12-months was non-significant in one of the three MNAR analyses (J2R; p = 0.296).
A series of one-way repeated measures ANOVA were run on the intervention group to determine if there were significant differences in their sleep problems following the Sleeping Sound program. The results showed that the Sleeping Sound program elicited statistically significant differences in mean CSHQ total scores over 12 months, F(3, 258) = 68.36, p < 0.001. With the exception of the CSHQ Sleep Disordered Breathing subscale, children in the intervention group reported significant reductions across all CSHQ subscales over the 12-month follow-up period.
Descriptive statistics were used to determine the proportion of children who went from the clinical (i.e. total CSHQ score ≥ 41) to the non-clinical range (i.e., total CSHQ score < 41) from baseline to 12-month follow-up. On average, children in the intervention group remained in the clinical range with a Mean CSHQ total score of 48.85 (SD: 8.63) at 12-month follow-up. A total of 16.22% of children in the intervention group moved from the clinical range to the non-clinical range at 12-month follow-up. Children in the TAU group also remained in the clinical range with a Mean CSHQ total score of 54.76 (SD: 9.47) at 12-month follow-up. A total of 4.26% of children in the TAU group moved from the clinical range to the non-clinical range at 12-month follow-up.
Secondary Outcomes
Child Outcomes
There was one significant effect of intervention on secondary child outcomes found in unadjusted models at 12-month follow-up. Children randomised into the intervention group had lower scores on the SCQ Reciprocal Social Interaction subscale (mean difference = 0.83, 95% confidence interval = 0.17 to 1.49, p = 0.014, effect size = 0.29) compared with TAU children. This effect remained significant after adjusting for covariates and controlling for multiple comparisons; however, it become non-significant in two of the three MNAR analyses (LMCF; p = 0.068, CIR, p = 0.110). There were no other significant group differences with regard to sleep hygiene (p = 0.088), daytime sleepiness (p = 0.539), parent- (p = 0.563) and teacher- (p = 0.487) reported behavioural and social functioning, or quality of life (p = 0.144). For the count-based outcome school attendance, incidence rate ratios (IRR) were used. There were no significant differences in days off school between the intervention and TAU group (IRR = 0.83, p = 0.422).
Parent/Caregiver Outcomes
At 12-month follow-up, one significant effect of intervention on parent/caregiver outcomes was found in the unadjusted model for mental health. Parents/caregivers of children in the intervention group had lower total K10 scores at 12-month follow-up (mean difference = -2.40, 95% CI − 4.33 to − 0.46, p = 0.015, effect size = -0.34) compared to parents/caregivers in the TAU control group. This effect remained significant after adjusting for covariates and controlling multiple comparisons; however, it became non-significant in two of the three MNAR analyses (J2R; p = 0.177, CIR, p = 0.051). Parents/caregivers of children in the intervention group also had lower parental distress relating to their role as a parent (PSI Parental Distress subscale) in the adjusted model (mean difference = -3.57, 95% CI − 6.99 to − 0.14, p = 0.041, effect size = -0.35); however, this difference was not significant in the unadjusted model and became non-significant in all three MNAR analyses. There were no significant differences between groups with regard to parenting stress (p = 0.115) or quality of life (p = 0.278). Results of the mixed-effects negative binomial regression revealed no significant difference in work attendance between the intervention and TAU group (IRR = 1.11, p = 0.422).
Moderators of Treatment Outcomes (Child Sleep Problems)
At 12-month follow-up, baseline ASD severity moderated sleep onset delay (mean difference = − 0.53, 95% CI − 1.02 to − 0.04, p = 0.033), parent mental health moderated night waking (mean difference = 1.07, 95% CI = 0.02 to 2.13, p = 0.046) and sleep duration (mean difference = 1.10, 95% CI = 0.04 to 2.16, p = 0.041), and sleep medication moderated sleep duration (mean difference = − 1.26, 95% CI − 2.31 to − 0.21, p = 0.019). Simple slope analyses conducted on the significant 3-way interactions indicated that the benefits of the intervention were more likely to be sustained among children who had greater baseline ASD severity, children whose parents were not experiencing psychological distress, and children who were taking sleep medication. These effects remained significant after adjusting for covariates, with the exception of parent mental health on sleep duration (p = 0.067). However, they became non-significant in a number of sensitivity analyses: (1) the significant moderating effect of ASD severity on onset delay was non-significant in one of three MNAR analyses (J2R: p = 0.079), (2) the significant moderating effect of parent mental health on night waking was non-significant in all three MNAR analyses (CIR; p = 0.136, J2R, p = 0.135, LMCF, p = 0.127), and (3) the significant moderating effect of sleep medication on sleep duration was non-significant in two of three MNAR analyses (CIR; p = 0.128, J2R, p = 0.203).
Discussion
Sleeping Sound, a brief behavioural sleep intervention that provides individually tailored strategies to children and their families, was associated with small to moderate sustained benefits in sleep at 12-month follow-up. Autistic children who were randomised to the intervention group fared better in terms of bedtime resistance, sleep onset delay, sleep duration, parasomnias, and overall sleep problems compared to the TAU control group. While sustained benefits in child and parent daytime functioning were limited, there were small improvements in child reciprocal social interaction and parent mental health at 12-month follow-up. However, these effects became non-significant in two of three sensitivity analyses. Exploratory moderation analyses identified ASD severity, parent mental health, and sleep medication at baseline as moderators of long-term treatment outcomes.
Our findings are consistent with previous research indicating sustained improvements in sleep for autistic children who received a parent-mediated behavioural sleep intervention (Durand, 2002; van Deurs et al., 2019; Weiskop et al., 2001). While these previous studies are limited by their small sample size and lack of control group, the current findings contribute to a limited body of evidence highlighting the durability of behavioural sleep interventions for autistic children. Moreover, the small to moderate effect sizes related to improved sleep in this study are consistent with the 6-month treatment outcomes of the Sleeping Sound with ASD trial (Papadopoulos et al., 2022) and the 12-month treatment outcomes of the Sleeping Sound with ADHD trial (Sciberras et al., 2020). Our results provide further support for the long-term efficacy of Sleeping Sound in the treatment of sleep problems in children with neurodevelopmental disorders. It is noteworthy that improvements in sleep onset delay were maintained with a moderate effect size. As highlighted in a recent systematic review, difficulty falling asleep is prioritised by parents of autistic children as an outcome that is an important indicator of their child’s progress over time (McConachie et al., 2018). It is also one of the most common sleep concerns reported by parents of autistic children (Cortesi et al., 2010), therefore a sustained reduction in sleep latency as demonstrated in the present study is likely highly valuable to parents.
In contrast to our findings at 3- and 6-month follow-up (Papadopoulos et al., 2022), there were no significant differences in sleep anxiety and night waking between the intervention and TAU groups at 12-month follow-up. This may indicate that a booster session reinforcing strategies relating to the management of sleep anxiety and night waking might benefit parents/caregivers, though further research is required to identify the optimal period for this to be offered. The main behavioural strategy to address night wakings is bedtime fading, which has been shown to have lower regularity of use among parents of children with neurodevelopmental disorders compared to strategies related to healthy sleep practices (Pattison et al., 2022; Sciberras et al., 2022). The Sleeping Sound intervention does not address all behaviours captured on the CSHQ parasomnias subscale; however, autistic children who received the intervention reported lower parasomnia scores than children in the TAU group at 12-month follow-up. Some parasomnias such as night terrors are more likely to occur if a child is not getting enough sleep, thus it is possible that the significant improvements observed in sleep duration among the intervention group produced a flow-on effect.
Outcomes such as ability to manage relationships and parent wellbeing have been shown to be highly valued outcome measures for parents of autistic children (McConachie et al., 2018). We found significant, albeit small improvements at 12-month follow-up in child reciprocal social interaction and parent/caregiver mental health for families who received the Sleeping Sound intervention. However, improvements in secondary outcomes became non-significant in two of three sensitivity analyses. In an ADHD sample, Sleeping Sound was associated with small to moderate improvements in secondary child (ADHD severity, quality of life, daily functioning, behaviour) outcomes at 12-months post-randomisation (Sciberras et al., 2020). The lack of sustained improvements across various parent- or teacher-reported secondary outcomes in the present study may be indicative of a decline in efficacy of treatment effects over time. This aligns with meta-analytic findings demonstrating a weakening of long-term effectiveness of CBT-I for sleep problems in non-autistic adults across a 12-month period (van der Zweerde et al., 2019).
We found evidence that sleep medication, ASD severity, and parent mental health were moderators of various sleep outcomes at 12-month follow-up. It should be noted that these effects also became non-significant in one or more sensitivity analyses with the most extreme assumptions and the following findings should be interpreted with caution. Children who were taking sleep medication at baseline showed greater long-term improvements in sleep duration than those not taking sleep medication. This finding is consistent with research that has demonstrated that melatonin can improve overall sleep time among autistic children (Abdelgadir et al., 2018; Beresford et al., 2018; Cuomo et al., 2017; Gringras et al., 2017). The present study was not designed to evaluate the interaction between medication and behavioural therapy for the treatment of sleep disorders. Notwithstanding, the current finding suggests that sleep medication may serve as a ‘primer’ for behavioural sleep strategies, possibly through an additive physiological effect or by decreasing the stress of the bedtime routine. A RCT conducted by Cortesi et al. (2012) provides some preliminary evidence that combining melatonin and CBT for insomnia in autistic children may be more efficacious than melatonin or CBT alone; however, more research on the relationship between medication and behavioural intervention is needed.
Our findings also indicated that children whose parents were not experiencing psychological distress at baseline fared better at 12-months post-randomisation with regard to night waking and sleep duration. This result is perhaps not surprising, as parent mental health has been found significantly to influence response to parent-based interventions (Reyno & McGrath, 2006) and symptoms of anxiety and depression may make it challenging for parents/caregivers to consistently implement intervention strategies over an extended period of time. For parents/caregivers in the present study, poorer mental health at baseline may have hindered their capacity to understand and apply the strategies discussed during the intervention sessions. Consequently, the provision of additional support may be required to reinforce strategies and optimise outcomes for children of parents experiencing psychological distress.
We also found evidence that children with greater initial ASD severity fared better longer-term with regard to falling asleep compared to children with lower baseline ASD severity. One possible explanation for the current finding is that children with more severe autism are more likely to form rigid and persistent behavioural routines than children with milder forms of autism. Indeed, children with greater initial ASD severity in the present study had significantly higher scores on the Restricted, Repetitive, and Stereotyped Patterns of Behaviour subscale of the SCQ (M = 4.41, SD = 2.00) compared to children with lower baseline ASD severity (M = 3.14, SD = 2.27), t = -3.56, p < 0.001. As such, strategies targeted at managing sleep onset delay (e.g., setting regular wake up time) may have been adhered to more strictly by children with greater ASD severity. While the developers of the SCQ state that the measure can be used as an index of autism severity (Rutter et al., 2003), it should be noted that this is an approximate score and there is a dearth of research evaluating its use in efficacy trials. Future research exploring the relationship between autism severity and the maintenance of behaviour is warranted. Overall, the identification of very few moderators suggests that the Sleeping Sound intervention is likely suitable for most families of autistic children.
The present study has a number of strengths. It is the first 12-month follow-up of a RCT examining the efficacy of a brief behavioural sleep intervention for primary school-aged autistic children with sleep problems. Moreover, it is the first RCT to explore moderators of behavioural sleep treatment outcomes. Finally, our study is strengthened by its large, community sample.
Our study also has some limitations. We did not include an objective measure of sleep (i.e., actigraphy) and therefore relied on unblinded, parent-reported outcomes. While studies have shown high concordance between objective measures and parent reports of sleep problems (Souders et al., 2009; Wiggs & Stores, 2004), subjective measures pose potential limitations such as reporter bias and an unawareness of some sleep difficulties that may be unobservable or not communicated by the child (see Hodge et al., 2012 for review). The dichotomisation of continuous variables in the moderation analysis also poses limitations, including loss of statistical power, increased risk of false positive results, and underestimates of variability within groups (see Altman & Royston, 2006 for review). The generalisability of the findings is limited by the exclusion of autistic children with an intellectual disability and autistic children from non-English speaking backgrounds, thus the efficacy of the intervention in additional populations is unknown. Additional study limitations include the loss to follow-up at 12 months and the small teacher sample. We observed an attrition rate of 39% at the 12-month follow-up for parent/caregiver surveys, and 45% for teacher surveys. These limitations were further compounded by the impact of the COVID-19 pandemic on data collection and changes in children’s teachers and schools over the 12-month period. There was no evidence to suggest that participants who did not complete the 12-month follow-up survey were those who observed less benefit at 3- or 6-month follow-up. As such, the larger attrition rate is unlikely to have led to inflated estimates of efficacy at 12-month follow-up.
Sleep problems are prevalent, burdensome, and persistent among autistic children. Sleeping Sound, a tailored behavioural sleep intervention, can provide families with an accessible and brief treatment option that yields sustained benefits. The intervention appears to be more beneficial in the longer-term for children taking sleep medication, children of parents who are not experiencing psychological distress, and children with greater ASD severity. As highlighted in the recent Lancet Commission on the future of care and clinical research in autism (Lord et al., 2022), clinical practice needs to respect the heterogeneity of the condition and recognise the unique strengths, needs, abilities, preferences, and circumstances of autistic individuals and their families. Personalised assessment and intervention should be accessible and equitable to everyone, and should encourage participation and empowerment of autistic individuals and their families (Lord et al., 2022). The Sleeping Sound intervention that was administered in our study is strengthened by its tailored nature, brevity, and applicability to everyday clinical practice. Our findings are important for clinicians seeking time efficient interventions for sleep problems in autism that can help to maximise positive and sustainable outcomes for children and their families. Future research investigating parental perceptions of participating in Sleeping Sound will inform future iterations of the intervention and provide a greater understanding of how the intervention can be optimised and translated into community healthcare settings.
Appendix A: Overview of Sleeping Sound Intervention
Sleep problem | Example of behavioural sleep strategies |
|---|---|
Delayed sleep phase Child falls asleep late and wakes up late | Bedtime fading: Temporarily set later bedtime to when child is feeling sleepy, gradually bring it forward when child is falling asleep within 30 min, continue until desired bedtime is reached. Wake child at regular pre-set time each morning and encourage light exposure Healthy sleep habits: Set consistent bedtime and wake-up time every day, reduce caffeine and stimulating activities, remove electronic devices from bedroom |
Sleep onset association disorder Child needs an object or person to fall asleep | Checking method: Parent visits child at regular intervals in the night to check on them and reassure them. Gradually stretch interval times Camping out: Parent sits in chair or camp bed next to child’s bed until child falls asleep and then gradually moves further away over next few nights. Process is repeated until child can fall asleep alone |
Bedtime resistance Child is non-compliant at bedtime and delays sleep onset | Bedtime pass: Child is instructed they can only leave their bedroom one time before sleep to promote compliant behaviour Ignore child complaints/protests: Parent ignores protests/ complaints about bedtime, calmly tells child it is time for bed, and gently guides child back to bed. Consistency is important |
Insomnia Child has difficulty falling or staying asleep | Relaxation training: Parent teaches child ways to relax to help them fall asleep. This might include controlled breathing, progressive muscle relaxation (PMR), or visual imagery Restricting time in bed: Parent encourages child to get out of bed and leave their bedroom to do something quiet (e.g., reading) if they cannot fall asleep |
Appendix B: Study inclusion and exclusion criteria
Inclusion Criteria | Exclusion Criteria |
|---|---|
∙ Clinically confirmed, multidisciplinary diagnosis of ASD a ∙ Aged 5–12 years or 13 years and attending primary school at the time of recruitment ∙ Clinical cut-off score ≥ 11 for ASD symptom severity on the Social Communication ∙ Questionnaire – Lifetime formb Parent/caregiver-reported sleep problem(s) are moderate to severe and persisting for ≥ 4 weeks ∙ Meet diagnostic criteria for ≥ 1 of the following parent/caregiver-reported child sleep problem as defined by the International ∙ ∙ ∙ Classification of Sleep Disorders – Third Edition diagnostic criteriac: chronic insomnia and/or delayed sleep–wake phase | ∙ Parent/caregiver-reported child intellectual disabilityd ∙ Co-occurring medical conditions known to disturb regular sleep patterns (e.g., blindness), or genetic conditions associated with intellectual impairment (e.g., Fragile X disorder) e ∙ Suspected Sleep Disordered Breathing or ∙ Obstructive Sleep Apnoea as indicated by parent responses on the CSHQ and followed up by phone review with study paediatrician ∙ Parents/caregivers with insufficient English proficiency to provide informed consent, complete study measures, and/or participate in the intervention treatment programf |
Appendix C: Summary of Outcome Measures Included in the Study
Outcomes | Measure | Source | B | 3 | 6 | 12 |
|---|---|---|---|---|---|---|
Primary outcome: child | ||||||
Overall child sleep problems | Children’s Sleep Habits Questionnaire (CSHQ)a. 33-item validated measure of sleep that can distinguish clinical from community samples. Provides a measure of total sleep problems and eight subscale scores reflecting major behavioural sleep disorders: Bedtime Resistance, Sleep-Onset Delay, Sleep Duration, Sleep Anxiety, Night Waking, Parasomnias, Sleep-Disordered breathing, Daytime Sleepiness. This measure was shown to have good internal consistency in the present study, α = 0.86 | Parent | • | • | • | • |
Secondary outcomes: child | ||||||
Sleep hygiene | Sleep Hygiene Scale. 7-item study developed measure adapted from the Bedroom Routines Scale. This measure was shown to have acceptable internal consistency in the present study, α = 0.74 | Parent | • | • | • | • |
Daytime sleepiness | Teacher Daytime Sleepiness Questionnaireb. 10-item validated report scale of daytime sleepiness at school. This measure was shown to have good internal consistency in the present study, α = 0.80 | Teacher | • | • | • | • |
Behavioural and social functioning | Strengths and Difficulties Questionnaire (SDQ)c. 25-item measure assessing the following subscales: Hyperactivity/Inattention, Conduct Problems, Emotional Symptoms, Peer Relationship Problems, and Prosocial Behaviour. This measure was shown to have good internal consistency in the present study (parent: α = 0.80, teacher: α = 0.87) | Parent and teacher | • | • | • | • |
Social communication functioning | Social Communication Questionnaire (SCQ) Currentd. 40-item measure of ASD symptoms in past 3 months to measure change in ASD social-communication symptoms over time. Provides a total score and three subscale scores: Reciprocal Social Interaction, Language and Communication, Stereotyped Patterns of Behaviour. This measure was shown to have good internal consistency in the present study, α = 0.81 | Parent | • | • | • | • |
School attendance | School attendance over the preceding three months, measured by the number of days missed from school during that period | Parent | • | • | • | • |
Quality of life | Child Health Utility 9D (CHU9D)e. 9-item measure of child quality of life. This measure was shown to have acceptable internal consistency in the present study, α = 0.78 | Parent | • | • | • | • |
Secondary outcomes: parent | ||||||
Stress | Parenting Stress Index 4SF (PSI-4SF)f. 36-item measure of parenting stress. Provides a measure of total parenting stress and three subscales reflecting the major sources of parenting stress: Parental Distress, Difficult Child, Parent–Child Dysfunctional Interaction. This measure was shown to have excellent internal consistency in the present study, α = 0.93 | Parent | • | • | • | • |
Mental health | Kessler 10 (K10)g. A 10-item validated measure of adult psychological distress. This measure was shown to have excellent internal consistency in the present study, α = 0.90 | Parent | • | • | • | • |
Quality of life | Assessment of Quality of Life (AQoL4D)h. 12-item measure of parent quality of life. This measure was shown to have acceptable internal consistency in the present study, α = 0.78 | Parent | • | • | • | • |
Work attendance | Paid work attendance over the preceding three months, measured by the number of hours of missed from paid work during that period to care for their child | Parent | • | • | • | • |
Appendix D: Number of Participants in Moderator Subgroups
Moderator subgroup | n | % |
|---|---|---|
Psychotropic medication use | ||
Yes | 73 | 31.06% |
No | 163 | 68.94% |
Sleep medication use | ||
Yesa | 117 | 52.23% |
No | 107 | 47.77% |
Internalising comorbidity | ||
Yes | 116 | 47.35% |
No | 129 | 52.65% |
Externalising comorbidity | ||
Yes | 24 | 9.80% |
No | 221 | 90.20% |
Combined internalising and externalising comorbidity | ||
Yes | 116 | 47.35% |
No | 129 | 52.65% |
ADHD comorbidity b | ||
Yes | 96 | 39.18 |
No | 149 | 60.82% |
ASD symptom severity | ||
< 15 SCQ total score | 136 | 55.51% |
≥ 15 SCQ total score | 109 | 44.49% |
Child age | ||
5–9 years | 170 | 69.39% |
10–13 years | 75 | 30.61% |
Child biological sex | ||
Male | 161 | 65.71% |
Female | 84 | 34.29% |
Parent level of education | ||
Not completed high school | 26 | 10.61% |
Completed high school | 219 | 89.39% |
Parent mental health | ||
< 20 K10 total score | 165 | 67.35% |
≥ 20 K10 total score | 80 | 32.65% |
Parent stress | ||
< 114 PSI total score | 138 | 56.33% |
≥ 114 PSI total score | 107 | 43.67 |
Weekly household income | ||
< $2000 a week | 143 | 59.09% |
≥ $2000 a week | 99 | 40.91% |
References
Abdelgadir, I. S., Gordon, M. A., & Akobeng, A. K. (2018). Melatonin for the management of sleep problems in children with neurodevelopmental disorders: A systematic review and meta-analysis. Archives of Disease in Childhood, 103(12), 1155–1162. https://doi.org/10.1136/archdischild-2017-314181
Adkins, K. W., Molloy, C., Weiss, S. K., Reynolds, A., Goldman, S. E., Burnette, C., Clemons, T., Fawkes, D., & Malow, B. A. (2012). Effects of a standardized pamphlet on insomnia in children with Autism Spectrum Disorders. Pediatrics, 130(Supplement 2), S139–S144. https://doi.org/10.1542/peds.2012-0900K
Altman, D. G., & Royston, P. (2006). The cost of dichotomising continuous variables. BMJ, 332(7549), 1080.
American Academy of Sleep Medicine. (2014). International Classification of Sleep Disorders (ISCD-3) (3rd ed.). American Academy of Sleep Medicine.
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (methodological), 57(1), 289–300.
Beresford, B., McDaid, C., Parker, A., Scantlebury, A., Spiers, G., Fairhurst, C., Hewitt, C., Wright, K., Dawson, V., Elphick, H., & Thomas, M. (2018). Pharmacological and non-pharmacological interventions for non-respiratory sleep disturbance in children with neurodisabilities: A systematic review. Health Technology Assessment, 22(60), 1–296. https://doi.org/10.3310/hta22600
Blake, M. J., Blake, L. M., Schwartz, O., Raniti, M., Waloszek, J. M., Murray, G., Simmons, J. G., Landau, E., Dahl, R. E., McMakin, D. L., Dudgeon, P., Trinder, J., & Allen, N. B. (2018). Who benefits from adolescent sleep interventions? Moderators of treatment efficacy in a randomized controlled trial of a cognitive-behavioral and mindfulness-based group sleep intervention for at-risk adolescents. Journal of Child Psychology and Psychiatry, 59(6), 637–649. https://doi.org/10.1111/jcpp.12842
Carnett, A., McLay, L., Hansen, S., France, K., & Blampied, N. (2021). Sleep problems in children and adolescents with autism: Type, severity and impact. Journal of Developmental and Physical Disabilities, 33(6), 977–991. https://doi.org/10.1007/s10882-020-09783-5
Cortesi, F., Giannotti, F., Ivanenko, A., & Johnson, K. (2010). Sleep in children with autistic spectrum disorder. Sleep Medicine, 11(7), 659–664. https://doi.org/10.1016/j.sleep.2010.01.010
Cortesi, F., Giannotti, F., Sebastiani, T., Panunzi, S., & Valente, D. (2012). Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: A randomized placebo-controlled trial. Journal of Sleep Research, 21(6), 700–709. https://doi.org/10.1111/j.1365-2869.2012.01021.x
Crank, J. E., Sandbank, M., Dunham, K., Crowley, S., Bottema-Beutel, K., Feldman, J., & Woynaroski, T. G. (2021). Understanding the effects of naturalistic developmental behavioral interventions: A project AIM meta-analysis. Autism Research, 14(4), 817–834. https://doi.org/10.1002/aur.2471
Cro, S., Morris, T. P., Kenward, M. G., & Carpenter, J. R. (2016). Reference-based sensitivity analysis via multiple imputation for longitudinal trials with protocol deviation. Stata Journal, 2, 443.
Cuomo, B. M., Vaz, S., Lee, E. A. L., Thompson, C., Rogerson, J. M., & Falkmer, T. (2017). Effectiveness of sleep-based interventions for children with Autism Spectrum Disorder: A meta-synthesis. Pharmacotherapy The Journal of Human Pharmacology and Drug Therapy, 37(5), 555–578. https://doi.org/10.1002/phar.1920
Durand, V. M. (2002). Treating sleep terrors in children with autism. Journal of Positive Behavior Interventions, 4(2), 66–72. https://doi.org/10.1177/109830070200400201
Farmer, C., Lecavalier, L., Yu, S., Arnold, L. E., McDougle, C. J., Scahill, L., Handen, B., Johnson, C. R., Stigler, K. A., Bearss, K., Swiezy, N. B., & Aman, M. G. (2012). Predictors and moderators of parent training efficacy in a sample of children with Autism Spectrum Disorders and serious behavioral problems. Journal of Autism and Developmental Disorders, 42(6), 1037–1044. https://doi.org/10.1007/s10803-011-1338-2
Gates, J. A., Kang, E., & Lerner, M. D. (2017). Efficacy of group social skills interventions for youth with autism spectrum disorder: A systematic review and meta-analysis. Clinical Psychology Review, 52, 164–181. https://doi.org/10.1016/j.cpr.2017.01.006
Gringras, P., Nir, T., Breddy, J., Frydman-Marom, A., & Findling, R. L. (2017). Efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with Autism Spectrum Disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 56(11), 948-957.e4. https://doi.org/10.1016/j.jaac.2017.09.414
Hiscock, H., Sciberras, E., Mensah, F., Gerner, B., Efron, D., Khano, S., & Oberklaid, F. (2015). Impact of a behavioural sleep intervention on symptoms and sleep in children with attention deficit hyperactivity disorder, and parental mental health: Randomised controlled trial. BMJ, 350, h68. https://doi.org/10.1136/bmj.h68
Hodge, D., Parnell, A. M. N., Hoffman, C. D., & Sweeney, D. P. (2012). Methods for assessing sleep in children with autism spectrum disorders: A review. Research in Autism Spectrum Disorders, 6(4), 1337–1344. https://doi.org/10.1016/j.rasd.2012.05.009
Hodge, D., Carollo, T. M., Lewin, M., Hoffman, C. D., & Sweeney, D. P. (2014). Sleep patterns in children with and without autism spectrum disorders: Developmental comparisons. Research in Developmental Disabilities, 35(7), 1631–1638. https://doi.org/10.1016/j.ridd.2014.03.037
Household Income and Wealth, Australia, 2017–18 financial year | Australian Bureau of Statistics. (2019, December 7). https://www.abs.gov.au/statistics/economy/finance/household-income-and-wealth-australia/latest-release
Hudry, K., McConachie, H., Le Couteur, A., Howlin, P., Barrett, B., & Slonims, V. (2018). Predictors of reliable symptom change: Secondary analysis of the Preschool Autism Communication Trial. Autism & Developmental Language Impairments, 3, 2396941518764760. https://doi.org/10.1177/2396941518764760
Humphreys, J. S., Gringras, P., Blair, P. S., Scott, N., Henderson, J., Fleming, P. J., & Emond, A. M. (2014). Sleep patterns in children with autistic spectrum disorders: A prospective cohort study. Archives of Disease in Childhood, 99(2), 114–118. https://doi.org/10.1136/archdischild-2013-304083
Johnson, C. R., Turner, K. S., Foldes, E., Brooks, M. M., Kronk, R., & Wiggs, L. (2013). Behavioral parent training to address sleep disturbances in young children with autism spectrum disorder: A pilot trial. Sleep Medicine, 14(10), 995–1004. https://doi.org/10.1016/j.sleep.2013.05.013
Keogh, S., Bridle, C., Siriwardena, N. A., Nadkarni, A., Laparidou, D., Durrant, S. J., Kargas, N., Law, G. R., & Curtis, F. (2019). Effectiveness of non-pharmacological interventions for insomnia in children with Autism Spectrum Disorder: A systematic review and meta-analysis. PLoS ONE, 14(8), e0221428. https://doi.org/10.1371/journal.pone.0221428
Kraemer, H. C., Frank, E., & Kupfer, D. J. (2006). Moderators of treatment outcomes: Clinical, research, and policy importance. JAMA, 296(10), 1286–1289. https://doi.org/10.1001/jama.296.10.1286
Lord, C., Charman, T., Havdahl, A., Carbone, P., Anagnostou, E., Boyd, B., Carr, T., de Vries, P. J., Dissanayake, C., Divan, G., Freitag, C. M., Gotelli, M. M., Kasari, C., Knapp, M., Mundy, P., Plank, A., Scahill, L., Servili, C., Shattuck, P., & McCauley, J. B. (2022). The Lancet Commission on the future of care and clinical research in autism. The Lancet, 399(10321), 271–334. https://doi.org/10.1016/S0140-6736(21)01541-5
Malow, B. A., Adkins, K. W., Reynolds, A., Weiss, S. K., Loh, A., Fawkes, D., Katz, T., Goldman, S. E., Madduri, N., Hundley, R., & Clemons, T. (2014). Parent-based sleep education for children with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 44(1), 216–228. https://doi.org/10.1007/s10803-013-1866-z
Martin, C. A., Papadopoulos, N., Chellew, T., Rinehart, N. J., & Sciberras, E. (2019). Associations between parenting stress, parent mental health and child sleep problems for children with ADHD and ASD: Systematic review. Research in Developmental Disabilities, 93, 103463. https://doi.org/10.1016/j.ridd.2019.103463
McConachie, H., Livingstone, N., Morris, C., Beresford, B., Le Couteur, A., Gringras, P., Garland, D., Jones, G., Macdonald, G., Williams, K., & Parr, J. R. (2018). Parents suggest which indicators of progress and outcomes should be measured in young children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 48(4), 1041–1051. https://doi.org/10.1007/s10803-017-3282-2
National Institute for Health and Care Excellence. (2013). Autism spectrum disorder in under 19s: Support and management, Clinical guideline [CG170]. https://www.nice.org.uk/guidance/CG170
Owens, J. A., Spirito, A., & McGuinn, M. (2000). The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep, 23(8), 1–9. https://doi.org/10.1093/sleep/23.8.1d
Papadopoulos, N., Sciberras, E., Hiscock, H., Williams, K., McGillivray, J., Mihalopoulos, C., Engel, L., Fuller-Tyszkiewicz, M., Bellows, S. T., Marks, D., Howlin, P., & Rinehart, N. (2019). Sleeping sound with autism spectrum disorder (ASD): Study protocol for an efficacy randomised controlled trial of a tailored brief behavioural sleep intervention for ASD. BMJ Open, 9(11), e029767.
Papadopoulos, N., Sciberras, E., Hiscock, H., Williams, K., McGillivray, J., Mihalopoulos, C., Engel, L., Fuller-Tyszkiewicz, M., Bellows, S. T., Marks, D., Howlin, P., & Rinehart, N. (2022). Sleeping Sound Autism Spectrum Disorder (ASD): A randomised controlled trial of a brief behavioural sleep intervention in primary school‐aged autistic children. Journal of Child Psychology and Psychiatry. https://doi.org/10.1111/jcpp.13590
Pattison, E., Mantilla, A., Fuller-Tyszkiewicz, M., Marks, D., Sciberras, E., McGillivray, J., Papadopoulos, N., & Rinehart, N. (2022). Acceptability of a behavioural sleep intervention for autistic children: A qualitative evaluation of Sleeping Sound. Sleep Medicine, 100, 378–389.
Phillips, N. L., Moore, T., Teng, A., Brookes, N., Palermo, T. M., & Lah, S. (2020). Behavioral interventions for sleep disturbances in children with neurological and neurodevelopmental disorders: A systematic review and meta-analysis of randomized controlled trials. Sleep. https://doi.org/10.1093/sleep/zsaa040
Reyno, S. M., & McGrath, P. J. (2006). Predictors of parent training efficacy for child externalizing behavior problems – a meta-analytic review. Journal of Child Psychology and Psychiatry, 47(1), 99–111. https://doi.org/10.1111/j.1469-7610.2005.01544.x
Rutter, M., Bailey, A., & Lord, C. (2003). The social communication questionnaire: Manual. Western Psychological Services.
Schreibman, L. (2000). Intensive behavioral/psychoeducational treatments for autism: Research needs and future directions. Journal of Autism and Developmental Disorders, 30(5), 373–378. https://doi.org/10.1023/A:1005535120023
Sciberras, E., Mulraney, M., Hayes, N., Rinehart, N., Schuster, T., Mudiyanselage, S. B., & Hiscock, H. (2022). A brief clinician training program to manage sleep problems in ADHD: What works and what do clinicians and parents think? Sleep Medicine, 89, 185–192.
Sciberras, E., Mulraney, M., Mensah, F., Oberklaid, F., Efron, D., & Hiscock, H. (2020). Sustained impact of a sleep intervention and moderators of treatment outcome for children with ADHD: A randomised controlled trial. Psychological Medicine, 50(2), 210–219.
Shalev, R. A., Lavine, C., & Di Martino, A. (2020). A systematic review of the role of parent characteristics in parent-mediated interventions for children with Autism Spectrum Disorder. Journal of Developmental and Physical Disabilities, 32(1), 1–21. https://doi.org/10.1007/s10882-018-9641-x
Singh, K., & Zimmerman, A. W. (2015). Sleep in autism spectrum disorder and attention deficit hyperactivity disorder. Seminars in Pediatric Neurology, 22(2), 113–125. https://doi.org/10.1016/j.spen.2015.03.006
Souders, M. C., Mason, T. B. A., Valladares, O., Bucan, M., Levy, S. E., Mandell, D. S., Weaver, T. E., & Pinto-Martin, J. (2009). Sleep behaviors and sleep quality in children with Autism Spectrum Disorders. Sleep, 32(12), 13.
Stahmer, A. C., Suhrheinrich, J., & Mandell, D. S. (2016). The importance of characterizing intervention for individuals with autism. Autism, 20(4), 386–387. https://doi.org/10.1177/1362361316637503
Trembath, D., Gurm, M., Scheerer, N. E., Trevisan, D. A., Paynter, J., Bohadana, G., Roberts, J., & Iarocci, G. (2019). Systematic review of factors that may influence the outcomes and generalizability of parent-mediated interventions for young children with autism spectrum disorder. Autism Research, 12(9), 1304–1321. https://doi.org/10.1002/aur.2168
van der Zweerde, T., Bisdounis, L., Kyle, S. D., Lancee, J., & van Straten, A. (2019). Cognitive behavioral therapy for insomnia: A meta-analysis of long-term effects in controlled studies. Sleep Medicine Reviews, 48, 101208. https://doi.org/10.1016/j.smrv.2019.08.002
van Deurs, J. R., McLay, L. K., France, K. G., Blampied, N. M., Lang, R. B., & Hunter, J. E. (2019). Behavioral sleep intervention for adolescents with Autism Spectrum Disorder: A pilot study. Advances in Neurodevelopmental Disorders, 3(4), 397–410. https://doi.org/10.1007/s41252-019-00123-z
Veatch, O. J., Sutcliffe, J. S., Warren, Z. E., Keenan, B. T., Potter, M. H., & Malow, B. A. (2017). Shorter sleep duration is associated with social impairment and comorbidities in ASD. Autism Research, 10(7), 1221–1238. https://doi.org/10.1002/aur.1765
Vivanti, G., Prior, M., Williams, K., & Dissanayake, C. (2014). Predictors of outcomes in autism early intervention: Why don’t we know more? Frontiers in Pediatrics. https://doi.org/10.3389/fped.2014.00058
Weiskop, S., Matthews, J., & Richdale, A. (2001). Treatment of sleep problems in a 5-Year-old boy with autism using behavioural principles. Autism, 5(2), 209–221. https://doi.org/10.1177/1362361301005002009
Wiggs, L., & Stores, G. (2004). Sleep patterns and sleep disorders in children with autistic spectrum disorders: Insights using parent report and actigraphy. Developmental Medicine and Child Neurology, 46(6), 372–380. https://doi.org/10.1017/S0012162204000611
Williams Buckley, A., Hirtz, D., Oskoui, M., Armstrong, M. J., Batra, A., Bridgemohan, C., Coury, D., Dawson, G., Donley, D., Findling, R. L., Gaughan, T., Gloss, D., Gronseth, G., Kessler, R., Merillat, S., Michelson, D., Owens, J., Pringsheim, T., Sikich, L., & Ashwal, S. (2020). Practice guideline: Treatment for insomnia and disrupted sleep behavior in children and adolescents with autism spectrum disorder: Report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology, 94(9), 392–404. https://doi.org/10.1212/WNL.0000000000009033
Acknowledgments
We wish to thank the families, teachers, and paediatricians for giving their time to participate in this research study. We also thank the Sleeping Sound project staff and students who assisted with participant recruitment, delivery of the intervention, and data collection. This paper will form part of Emily Pattison’s dissertation for her Doctor of Psychology (Clinical) course. Emily Pattison is supported by a Deakin University Faculty of Health Postgraduate Research (DUPR) scholarship.
Funding
The data for this study was collected as part of the Sleeping Sound with Autism Spectrum Disorder project at Deakin University, which received funding from the Australian National Health and Medical Research Council (NHMRC; APP1101989).
Author information
Authors and Affiliations
Contributions
NR, NP, ES, HH, KW, JM, PH, and CM conceived the Sleeping Sound Autism Spectrum Disorder research trial. NR, NP, and EP were involved in the conception of this paper. DM and SB delivered the intervention program. EP and SB were involved in data collection. EP and MF-T were responsible for data analyses. EP drafted the manuscript under the supervision of NR, NP, MF-T, and JM. All authors contributed to the interpretation of results. All authors reviewed, contributed to, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Outside of the submitted work, Professor Nicole Rinehart, Professor Jane McGillivray, and Dr Nicole Papadopoulos receive philanthropic funding from Moose Toys, Ferrero Group Australia as part of its ‘Kinder Joy of Moving’ pillar of Corporate Social Responsibility initiatives, MECCA Brands, Wenig Family, Grace and Emilio Foundation, Geelong Community Foundation, and partnership with the Australian Football League. Professor Rinehart and Professor McGillivray also report industry partner funding from Victorian Department of Education outside of the submitted work. Professor Rinehart is a Director of AMAZE Board (Autism Victoria) and reports previous donations from VicHealth and the Bus Association Victoria; and Professor McGillivray reports a grant from Victorian Department of Justice. None of the companies, industry partners or organisational bodies listed above had a role in this research including the collection, analysis, and interpretation of data; in writing of the manuscript; and /or in the decision to submit the article for publication. The remaining authors have no competing interests to declare that are relevant to the content of this article.
Ethical Approval
This study was approved by the Human Research Ethics Committees from the Royal Children’s Hospital Melbourne (36154), Deakin University (2017–130), the Catholic Education Office Melbourne (0501), and the Victorian Department of Education and Early Childhood Development (2016_003134). All procedures performed in the study were done so in accordance with all applicable ethical standards, including the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pattison, E., Papadopoulos, N., Fuller-Tyszkiewicz, M. et al. Randomised Controlled Trial of a Behavioural Sleep Intervention, ‘Sleeping Sound’, for Autistic Children: 12-Month Outcomes and Moderators of Treatment. J Autism Dev Disord 54, 442–457 (2024). https://doi.org/10.1007/s10803-022-05809-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-022-05809-3