Abstract
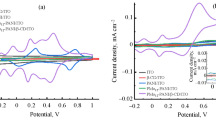
Monomers: gentian violet (GV), brilliant green (BG), aniline, and methyl violet (MV), as well as their copolymers, were electropolymerized and used to modify glassy carbon electrode (GCE) by enhancing the sensitivity of the electrode. This is due to their special characteristics of sensing ability of the electropolymerized polymers. The electropolymerization and characterization of these monomers and their copolymers were done using cyclic voltammetry (CV) on GCE as a working electrode against Ag/AgCl reference electrode and platinum wire as the counter electrode. The copolymers showed higher current peaks than their corresponding homopolymers. The electrochemical characterization of these polymers and copolymers was studied by scan rate effect each between 10 and 300 mV/s and pH effect between 1.56 and 5.30. After being modified by the copolymers: poly(BG-GV) and poly(BG-MV), the modified GCE showed good results in resolving the peaks of ascorbic acid and uric acid which otherwise showed an overlapped broad peak on bare GCE. The CV in bare GCE was scanned at different scan rates of 4 mM K3[Fe(CN)6] in 1 M KNO3 solution. The square root of scan rate dependence of peak current was plotted and the Randles–Sevcik equation was applied to determine the diffusion coefficient of Fe(CN)63− ion and which was 1.13 × 10–14 cm2/s. This was used to determine the area of the modified electrode after running the CV of 4 mM K3[Fe(CN)6] in 1 M KNO3 at different scan rates. Therefore, the electrochemical polymerization of organic dye polymers was able to increase the sensitivity of the electrode by increasing its area.
Graphical abstract










Similar content being viewed by others
References
Guo X, Facchetti A (2020) The journey of conducting polymers from discovery to application. Nat Mater 19:922–928. https://doi.org/10.1038/s41563-020-0778-5
Namsheer K, Rout CS (2021) Conducting polymers: a comprehensive review on recent advances in synthesis, properties and applications. RSC Adv 11(10):5659–5697. https://doi.org/10.1039/d0ra07800j
Janata J, Josowicz M (2003) Conducting polymers in electronic chemical sensors. Nat Mater 2(1):19–24. https://doi.org/10.1038/nmat768
Alışık F, Burç M, Titretir Duran S, Güngör Ö, Cengiz MA, Köytepe S (2021) Development of gum-Arabic-based polyurethane membrane-modified electrodes as voltammetric sensor for the detection of phenylalanine. Polym Bull 78(8):4699–4719. https://doi.org/10.1007/s00289-021-03605-0
Tkach VV, de Martins JIFP, Ivanushko YG, Yagodynets PI (2022) Dye electropolymerization for electrochemical analysis. A brief review. Biointerface Res Appl Chem 12(3):4028–4047. https://doi.org/10.33263/BRIAC123.40284047
Aksoy B, Güngör Ö, Köytepe S, Seckin T (2015) Preparation of novel sensors based on polyimide membrane for sensitive and selective determination of dopamine. Polym Plast Technol Eng 55:150827134246008. https://doi.org/10.1080/03602559.2015.1055503
Saidman S (2002) The effect of PH on the electrochemical polymerisation of pyrrole on aluminium. J Electroanal Chem J Electroanal Chem 534:39–45. https://doi.org/10.1016/S0022-0728(02)01102-6
Fomo G, Waryo T, Feleni U, Baker P, Iwuoha E (2019) Electrochemical polymerization. 105–131. https://doi.org/10.1007/978-3-319-95987-0_3
Chani MTS, Karimov KS, Khalid FA, Moiz SA (2013) Polyaniline based impedance humidity sensors. Solid State Sci 18:78–82. https://doi.org/10.1016/j.solidstatesciences.2013.01.005
Aqeel K, Mubarak HA, Amoako-Attah J, Abdul-Rahaim LA, Al Khaddar R, Abdellatif M, Al-Janabi A, Hashim KS (2020) Electrochemical removal of brilliant green dye from wastewater. IOP Conf Ser Mater Sci Eng. https://doi.org/10.1088/1757-899X/888/1/012036
Tong Z, Zheng P, Bai B, Wang H, Suo Y (2016) Adsorption performance of methyl violet via α-Fe2O3@porous hollow carbonaceous microspheres and its effective regeneration through a fenton-like reaction. Catalysts. https://doi.org/10.3390/catal6040058
Zahran M (2023) Conducting dyes as electro-active monomers and polymers for detecting analytes in biological and environmental samples. Heliyon 9(9):e19943. https://doi.org/10.1016/j.heliyon.2023.e19943
Jarjes ZA, Samian MR, Ab Ghani S (2015) Conductive polymers: their preparations and catalyses on NADH oxidation at carbon cloth electrodes. Arab J Chem 8(5):726–731. https://doi.org/10.1016/j.arabjc.2013.05.021
Güngör Ö, Özgül O, Aksoy B, Okuşluk F, Köytepe S (2022) Titanium dioxide-multiwalled carbon nanotube/polyimide composite film modified electrodes for simultaneous voltammetric detection of ascorbic acid, uric acid and dopamine as biomarker molecules. Polym Bull 79(12):11051–11077. https://doi.org/10.1007/s00289-022-04077-6
Climent V, Feliu JM (2018) Cyclic voltammetry. In: Wandelt KBT-E. of I. C., Ed. Elsevier, Oxford, pp 48–74. https://doi.org/10.1016/B978-0-12-409547-2.10764-4.
Xiao Z, Subbiah J, Sun K, Jones DJ, Holmes AB, Wong WWH (2014) Synthesis and photovoltaic properties of thieno[3,2-b]thiophenyl substituted benzo[1,2-b:4,5-B′]dithiophene copolymers. Polym Chem 5(23):6710–6717. https://doi.org/10.1039/c4py00827h
Ravichandran R, Sundarrajan S, Venugopal JR, Mukherjee S, Ramakrishna S (2010) Applications of conducting polymers and their issues in biomedical engineering. J R Soc Interface. https://doi.org/10.1098/rsif.2010.0120.focus
Mbacké MK, Kane C, Diallo NO, Diop CM, Chauvet F, Comtat M, Tzedakis T (2016) Electrocoagulation process applied on pollutants treatment- experimental optimization and fundamental investigation of the crystal violet dye removal. J Environ Chem Eng 4(4):4001–4011. https://doi.org/10.1016/j.jece.2016.09.002
Horáková E, Barek J, Vyskočil V (2016) Determination of methyl violet 2B using polarographic and voltammetric methods at mercury electrodes. Anal Lett 49:56–65. https://doi.org/10.1080/00032719.2015.1004576
Ganesh PS, Kumara Swamy BE, Fayemi OE, Sherif ESM, Ebenso EE (2018) Poly(crystal violet) modified pencil graphite electrode sensor for the electroanalysis of catechol in the presence of hydroquinone. Sens Bio-Sens Res 20(August):47–54. https://doi.org/10.1016/j.sbsr.2018.08.001
Porjazoska-Kujundziski A, Chamovska D, Grchev T (2016) Electroconducting materials based on polypyrrole. Zast Mater 57(2):282–295. https://doi.org/10.5937/zasmat1602282p
Cihaner A (2004) Electrochemical synthesis of crowned conducting polymers: nature of radical cations in polymerization and mechanism of conductivity. Thesis, No. June, 114
Pielichowski K (1997) Kinetic analysis of the thermal decomposition of polyaniline. Solid State Ionics 104(1):123–132. https://doi.org/10.1016/S0167-2738(97)00396-2
Song E, Choi J-W (2013) Conducting polyaniline nanowire and its applications in chemiresistive sensing. Nanomaterials 3:498–523. https://doi.org/10.3390/nano3030498
Korent A, Žagar Soderžnik K, Šturm S, Žužek Rožman K (2020) A correlative study of polyaniline electropolymerization and its electrochromic behavior. J Electrochem Soc 167(10):106504. https://doi.org/10.1149/1945-7111/ab9929
Pifferi V, Barsan MM, Ghica ME, Falciola L, Brett CMA (2013) Synthesis, characterization and influence of poly(brilliant green) on the performance of different electrode architectures based on carbon nanotubes and poly(3,4-ethylenedioxythiophene). Electrochim Acta 98:199–207. https://doi.org/10.1016/j.electacta.2013.03.048
Baba A, Tian S, Stefani F, Xia C, Wang Z, Advincula RC, Johannsmann D, Knoll W (2004) Electropolymerization and doping/dedoping properties of polyaniline thin films as studied by electrochemical-surface plasmon spectroscopy and by the quartz crystal microbalance. J Electroanal Chem 562(1):95–103. https://doi.org/10.1016/j.jelechem.2003.08.012
Elugoke SE, Fayemi OE, Adekunle AS, Sherif ESM, Ebenso EE (2022) Electrochemical sensor for the detection of adrenaline at poly(crystal violet) modified electrode: optimization and voltammetric studies. Heliyon 8(10):e10835. https://doi.org/10.1016/j.heliyon.2022.e10835
Yoon S-B, Yoon E-H, Kim K-B (2011) Electrochemical properties of leucoemeraldine, emeraldine, and pernigraniline forms of polyaniline/multi-wall carbon nanotube nanocomposites for supercapacitor applications. J Power Sources 196(24):10791–10797. https://doi.org/10.1016/j.jpowsour.2011.08.107
Zaulkiflee ND, Ahmad AL, Che Lah NF, Low SC, Norikazu N (2023) Collation of PANI as electrode material for supercapacitor: effect of different substrate and its performances. J Mater Sci Mater Electron. https://doi.org/10.1007/s10854-023-10326-9
El Aggadi S, Loudiyi N, Chadil A, El Abbassi Z, El Hourch A (2020) Electropolymerization of aniline monomer and effects of synthesis conditions on the characteristics of synthesized polyaniline thin films. Mediterr J Chem 10(2):138–145. https://doi.org/10.13171/mjc102020021114sea
Sezgin S, Ates M, Parlak EA, Sarac AS (2012) Scan rate effect of 1-(4-methoxyphenyl)-1H-pyrrole electro-coated on carbon fiber: characterization via cyclic voltammetry, FTIR-ATR and electrochemical impedance spectroscopy. Int J Electrochem Sci 7(2):1093–1106. https://doi.org/10.1016/s1452-3981(23)13397-9
Paredes-Salazar EA, Herrero E, Varela H (2023) Mass transfer phenomena induced by surface gas flow rate in the hanging meniscus configuration: a case study of the methanol electro-oxidation reaction on Pt(100). Electrochim Acta 464:142917. https://doi.org/10.1016/j.electacta.2023.142917
King AR (1995) Mass transfer cycles in CV BT—cataclysmic variables. In: Bianchini A, Della Valle M, Orio M (eds) Springer Netherlands, Dordrecht, pp 523–532
Billet R, Schultes M (1993) Predicting mass transfer in packed columns. Chem Eng Technol 16(1):1–9. https://doi.org/10.1002/ceat.270160102
Liapis AI (2005) Expression for the film mass-transfer coefficient of charged solutes in a liquid stream flowing in packed beds of charged particles and charged porous monoliths. Ind Eng Chem Res 44(14):5380–5387. https://doi.org/10.1021/ie049120w
Iqbal MZ, Haider SS, Siddique S, Karim MRA, Zakar S, Tayyab M, Faisal MM, Sulman M, Khan A, Baghayeri M, Kamran MA, Alherbi T, Iqbal MJ, Hussain T (2020) Capacitive and diffusion-controlled mechanism of strontium oxide based symmetric and asymmetric devices. J Energy Storage 27:101056. https://doi.org/10.1016/j.est.2019.101056
García-Salinas M, de Las Nieves FJ (2003) Influence of counterion type and diffusion on the primary electroviscous effect. Colloids Surfaces A Physicochem Eng Asp 222:65–77. https://doi.org/10.1016/S0927-7757(03)00235-8
Jang HJ, Shin BJ, Jung EY, Bae GT, Kim JY, Tae H-S (2023) Polypyrrole film synthesis via solution plasma polymerization of liquid pyrrole. Appl Surf Sci 608:155129. https://doi.org/10.1016/j.apsusc.2022.155129
Spencer M, Holzapfel N, You K-E, Mpourmpakis G, Augustyn V (2024) Participation of electrochemically inserted protons in the hydrogen evolution reaction on tungsten oxides. Chem Sci. https://doi.org/10.1039/d4sc00102h
Aleksić M, Lijeskic N, Pantic J, Kapetanovic V (2013) Electrochemical behavior and differential pulse voltammetric determination of ceftazidime, cefuroxime-axetil and ceftriaxone. Facta Univ Ser Phys Chem Technol 11:55–66. https://doi.org/10.2298/FUPCT1301055A
Cesarino V, Cesarino I, Moraes F, Machado SA, Mascaro L (2014) Carbon nanotubes modified with SnO2 rods for levofloxacin detection. J Braz Chem Soc. https://doi.org/10.5935/0103-5053.20140017
Van Slyke DD, Zacharias G (1914) The effect of hydrogen ion concentration and of inhibiting substances on urease. J Biol Chem 19(2):181–210. https://doi.org/10.1016/s0021-9258(18)88301-6
Turbale M, Moges A, Dawit M, Amare M (2020) Adsorptive stripping voltammetric determination of tetracycline in pharmaceutical capsule formulation using poly(malachite green) modified glassy carbon electrode. Heliyon 6:e05782. https://doi.org/10.1016/j.heliyon.2020.e05782
Alhemiary N, Rizk M (2017) Sensitive and validated voltammetric methods for determination of zileuton in serum, urine and pharmaceutical dosage forms at activated glassy carbon electrode. Asian J Chem 29:2627–2633. https://doi.org/10.14233/ajchem.2017.20737
Elsayed EM, Rashad MM, Khalil HFY, Ibrahim IA, Hussein MR, El-Sabbah MMB (2016) The effect of solution ph on the electrochemical performance of nanocrystalline metal ferrites MFe2O4 (M=Cu, Zn, and Ni) thin films. Appl Nanosci 6(4):485–494. https://doi.org/10.1007/s13204-015-0453-3
He Q, Zhang R, Hu Y, Li J, Yu H, Zheng Y, Qian J (2024) The effect of feeding sequence on the structure and properties of the ethylene/1-octene copolymer in the semi-continuous polymerization reaction system. Polymers (Basel). https://doi.org/10.3390/polym16040526
Anbarasan R, Ponprapakaran K, Subramani HR, Rajendran B, Tung K-L (2019) Synthesis, characterization and catalytic activity of copolymer/metal oxide nanocomposites. Polym Bull. https://doi.org/10.1007/s00289-018-2591-8
Majeed AH, Mohammed LA, Hammoodi OG, Sehgal S, Alheety MA, Saxena KK, Dadoosh SA, Mohammed IK, Jasim MM, Salmaan NU (2022) A review on polyaniline: synthesis, properties, nanocomposites, and electrochemical applications. Int J Polym Sci 2022:9047554. https://doi.org/10.1155/2022/9047554
Govindarajan N, Xu A, Chan K (2022) How PH affects electrochemical processes. Science 375(6579):379–380. https://doi.org/10.1126/science.abj2421
Ghica ME, Brett CMA (2014) Poly(brilliant green) and poly(thionine) modified carbon nanotube coated carbon film electrodes for glucose and uric acid biosensors. Talanta 130:198–206. https://doi.org/10.1016/j.talanta.2014.06.068
Guimard NK, Gomez N, Schmidt CE (2007) Conducting polymers in biomedical engineering. Prog Polym Sci 32(8–9):876–921. https://doi.org/10.1016/j.progpolymsci.2007.05.012
Park Y, Jung J, Chang M (2019) Research progress on conducting polymer-based biomedical applications. Appl Sci. https://doi.org/10.3390/app9061070
Acknowledgements
This work was financially supported by the Ministry of Education of Ethiopia (MoE) and was done at Hawassa University.
Author information
Authors and Affiliations
Contributions
J. N. G., T. T. B., and S. T. A. Prepared the conception, S. T. A. secured the research fund, T. T. B. Organized the data, J. N. G has undergone the experimental work and wrote the draft manuscript, All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guyasa, J.N., Beyene, T.T. & Anshebo, S.T. Electrochemical syntheses and characterization of some polydyes and their application for the simultaneous determination of ascorbic acid and uric acid. J Appl Electrochem (2024). https://doi.org/10.1007/s10800-024-02126-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10800-024-02126-8