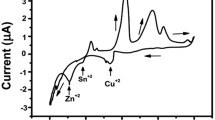
Abstract
The primary purpose of this article is to synthesize electrochemically a binary semiconductor material ZnS that is generally used for manufacturing solar cells. It has been shown that the properties and composition of the deposits are closely linked to the synthesis conditions, namely the applied potential, the electrolyte concentration and its composition. The electrodeposition was realized from an acidic medium with a pH ranging from 3.5 to 4.5 and containing respectively 0.02 M of zinc chloride (ZnCl2), 0.02 M of sodium thiosulphate (Na2S2O3), and 0.15 M of trisodium citrate (Na3C6H5O7) as complexing agent. The deposition mechanism of ZnS binary alloys was investigated using various electrochemical techniques. ZnS thin films were electrochemically deposited on a tin-doped indium oxide (ITO) substrate in one single step, at a potential of −1 V/SCE. The effects of the concentration ratio ([zinc]/[sulfur]) on the electrochemical, optical, and compositional properties of thin films as well as their surface morphology were also investigated.
Graphical abstract












Similar content being viewed by others
References
Kassim A, Nagalingam S, Min HS, Karrim N (2010) XRD and AFM studies of ZnS thin films produced by electrodeposition method. Arab J Chem 3:243–249. https://doi.org/10.1016/j.arabjc.2010.05.002
Choi YI, Lee S, Kim SK et al (2016) Fabrication of ZnO, ZnS, Ag-ZnS, and Au-ZnS microspheres for photocatalytic activities, CO oxidation and 2-hydroxyterephthalic acid synthesis. J Alloys Compd 675:46–56. https://doi.org/10.1016/j.jallcom.2016.03.070
ÜZAR N, ARIKAN MÇ (2011) Synthesis and investigation of optical properties of ZnS nanostructures. Bull Mater Sci 34:287–292. https://doi.org/10.1007/s12034-011-0085-5
Fang X, Zhai T, Gautam UK et al (2011) ZnS nanostructures: from synthesis to applications. Prog Mater Sci 56:175–287. https://doi.org/10.1016/j.pmatsci.2010.10.001
Popa M, Zakhidov A, Tiginyanu I (2018) Perovskite solar cells with ZnS as electron transport layer. Proc Romanian Acad Ser -Math Phys Tech Sci Inf Sci 19:559–566
Kumar V, Sharma M, Gaur J, Sharma T (2008) Polycrystalline ZnS thin films by screen printing method and its characterization. Chalcogenide Lett 5:289–295
Mach R, Müller GO (1982) Physical concepts of high-field, thin-film electroluminescence devices. Phys Status Solidi A 69:11–66
Ledger AM (1979) Inhomogeneous interface laser mirror coatings. Appl Opt 18:2979–2989
Jones PL, Moore DM, Smith SC (1976) A new method for melting and recrystallization of lanthanum ZnS nanoparticle. J Phys E 9:312–316
Jadhav UM, Shinde MS, Patel SN, Patil RS (2014) Structural, optical and electrical properties of nanocrystalline cadmium sulphide thin films deposited by novel chemical route. Indian J Pure Appl Phys 52:39–43
Kumar V, Manickam D, Venkatachalam M et al (2007) Structural and Optical Properties of Hydrothermally Grown Zns Thin Films. Int J Innov Res Sci Eng Technol 3297:2319–8753
Sartale SD, Sankapal BR, Lux-Steiner M, Ennaoui A (2005) Preparation of Nanocrystalline ZnS by a new chemical bath deposition route. Thin Solid Films 480–481:168–172. https://doi.org/10.1016/j.tsf.2004.11.054
Roy P, Ota JR, Srivastava SK (2006) Crystalline ZnS thin films by chemical bath deposition method and its characterization. Thin Solid Films 515:1912–1917. https://doi.org/10.1016/j.tsf.2006.07.035
Lădar M, Popovici E-J, Baldea I et al (2007) Studies on chemical bath deposited zinc sulphide thin films with special optical properties. J Alloys Compd 434–435:697–700. https://doi.org/10.1016/j.jallcom.2006.08.226
Gunasekaran M, Gopalakrishnan R, Ramasamy P (2004) Deposition of ZnS thin films by photochemical deposition technique. Mater Lett 58:67–70. https://doi.org/10.1016/S0167-577X(03)00416-6
Shao L-X, Chang K-H, Hwang H-L (2003) Zinc sulfide thin films deposited by RF reactive sputtering for photovoltaic applications. Appl Surf Sci 212–213:305–310. https://doi.org/10.1016/S0169-4332(03)00085-0
Durrani SMA, Al-Shukri AM, Iob A, Khawaja EE (2000) Optical constants of zinc sulfide films determined from transmittance measurements. Thin Solid Films 379:199–202. https://doi.org/10.1016/S0040-6090(00)01539-X
Dimitrova V, Tate J (2000) Synthesis and characterization of some ZnS-based thin film phosphors for electroluminescent device applications. Thin Solid Films 365:134–138. https://doi.org/10.1016/S0040-6090(99)01089-5
Afifi HH, Mahmoud SA, Ashour A (1995) Structural study of ZnS thin films prepared by spray pyrolysis. Thin Solid Films 263:248–251. https://doi.org/10.1016/0040-6090(95)06565-2
Elidrissi B, Addou M, Regragui M et al (2001) Structure, composition and optical properties of ZnS thin films prepared by spray pyrolysis. Mater Chem Phys 68:175–179. https://doi.org/10.1016/S0254-0584(00)00351-5
López MC, Espinos JP, Martín F et al (2005) Growth of ZnS thin films obtained by chemical spray pyrolysis: the influence of precursors. J Cryst Growth 285:66–75. https://doi.org/10.1016/j.jcrysgro.2005.07.050
Hernández-Fenollosa MA, López MC, Donderis V et al (2008) Role of precursors on morphology and optical properties of ZnS thin films prepared by chemical spray pyrolysis. Thin Solid Films 516:1622–1625. https://doi.org/10.1016/j.tsf.2007.05.031
Zhu H, Huang JF, Wang Y et al (2011) Synthesis and characterisation of ZnS optical thin films through cathodic electrodeposition technique. Surf Eng 27:42–45. https://doi.org/10.1179/026708409X12490360426089
Kassim A, Ho SM, Abdul HA et al (2010) Influence of the depositio n time on the structure and morphology of the ZnS thin films electrodeposited on indium tin oxid e substrates. Dig J Nanomater Biostructures 5:975–980
Kim H, Moon JY, Lee HS (2012) Effect of ZnCl2 concentration on the growth of ZnO by electrochemical deposition. Curr Appl Phys 12:S35–S38. https://doi.org/10.1016/j.cap.2012.05.036
Moon JY, Kim H, Lee JH et al (2012) Synthesis of MgZnO nanostructures by electrochemical method. Curr Appl Phys 12:S52–S55. https://doi.org/10.1016/j.cap.2012.05.028
Benyahia K, Djeffal F, Ferhati H et al (2021) Microstructured ZnO-ZnS composite for earth-abundant photovoltaics: Elaboration, surface analysis and enhanced optical performances. Sol Energy 218:312–319. https://doi.org/10.1016/j.solener.2021.02.057
Azmand A, Kafashan H (2019) Al-doped ZnS thin films: physical and electrochemical characterizations. J Alloys Compd 779:301–313. https://doi.org/10.1016/j.jallcom.2018.11.268
Mosavi SM, Kafashan H (2019) Physical properties of Cd-doped ZnS thin films. Superlattices Microstruct 126:139–149. https://doi.org/10.1016/j.spmi.2018.12.002
Boosagulla D, Mandati S, Misra P et al (2021) Pulse electrodeposited zinc sulfide as an eco-friendly buffer layer for the cadmium-free thin-film solar cells. Superlattices Microstruct 160:107060. https://doi.org/10.1016/j.spmi.2021.107060
Bard AJ, Faulkner LR, White HS (2022) Electrochemical methods: fundamentals and applications. Wiley, Hoboken
Greef R, Peat R, Peter LM (1985) Electrocatalysis. Instrumental Methods in Electrochemistry. Oxford UK Albion Horwood Publication Ltd, Oxford, p 111
Djelloul A, Adnane M, Lerbah Y et al (2016) Élaboration et Caractérisation des Couches Minces de Sulfure de Zinc (ZnS) Préparés par la Technique SILAR (Successive Ionic Layer Adsorption and Reaction). Algerian J Res Technol 1:1–9
Hennayaka HMMN, Lee HS (2013) Structural and optical properties of ZnS thin film grown by pulsed electrodeposition. Thin Solid Films 548:86–90. https://doi.org/10.1016/j.tsf.2013.09.011
Acknowledgements
The authors wish to acknowledge the financial support from the Algerian Ministry of Higher Education and the Faculty of Science and Technology at Mohamed El Bachir El Ibrahimi University in the city of Bordj Bou Arréridj - Algeria.
Author information
Authors and Affiliations
Contributions
MH is presently working as a research at the Department of Matter Sciences at the Faculty of Science and Technology, University of Bordj Bou Arréridj, Algeria. She played role in data curation, formal analysis, investigation and writing—original draft preparation. MB contributed to supervision, validation, review and editing. OD contributed to experimentation and investigation. YCH contributed to experimentation and investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest related the present work. They have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hamla, M., Benaicha, M., Chetbani, Y. et al. Electrochemical synthesis and characterization of zinc sulfide (ZnS) semiconducting thin films from citrate-based plating bath. J Appl Electrochem 54, 1299–1307 (2024). https://doi.org/10.1007/s10800-023-02028-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-02028-1