Abstract
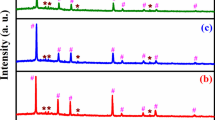
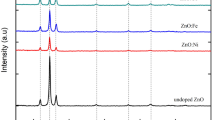
Copper-zinc-tin-sulfide (Cu2ZnSnS4 or CZTS) is a promising p-type semiconductor material as absorber layer in thin film solar cells. The sulfides of copper and tin as well as zinc and sulfur powders were dissolved in hydrazine. The effect of chemical reaction between precursor species, at room temperature, was assessed for 6 to 22 h. For 22 h reaction time, the effect of spin coated film thickness on the resulting composition, after annealing under N2 flow at 500 °C for 1 h, was investigated. The morphology, composition, and optical properties of the annealed films were determined by means of x-ray diffraction, scanning electron microscope, and spectrophotometer studies. It was found that, for less than optimal reaction time of 22 h or film thickness below 1.2 µm, other ternary phases namely Cu4SnS4, Cu5Sn2S7, and ZnS co-exist in different proportions besides CZTS. Formation of phase-pure CZTS films also exhibited a tendency to minimize film cracking during annealing. Depending on the processing conditions, the band gap (E g) values were determined to be in the range of 1.55 to 1.97 eV. For phase-pure annealed CZTS film, an increase in the E g value may be attributed to quantum confinement effect due to small crystallite size.








Similar content being viewed by others
References
PV Magazine, ZSW sets 21.7% thin film efficiency record, http://www.pv-magazine.com/news/details/beitrag/zsw-sets-217-thin-film-efficiency-record_100016505/#axzz3NAQ5uFEp, 22 Sep 2014.
PV Magazine, Thin-film competition hots up as TSMC Solar breaks day-old CIGS efficiency record, http://www.pv-tech.org/news/thin_film_competition_hots_up_as_tsmc_producer_breaks_day_old_cigs_efficien, 28 April, 2015
S. Siebentritt and A.S. Schorr, Kesterites—A Challenging Material for Solar Cells, Prog. Poovoltaics, 2012, 20, p 512–519
M. Jiang, Yan X, Cu2ZnSnS4 Thin Film Solar Cells: Present Status and Future Prospects. In: Arturo Morales-Acevedo (Ed.) Solar Cells—Research and Application Perspectives, InTech (2013)
I.V. Fisher, S.R. Cohen, A. Ruzin, and D. Cahen, How Polycrystalline Devices Can Outperform Single-Crystal Ones Thin Film CdTe/CdS Solar Cells, Adv. Mater., 2004, 16, p 879–883
L. Arora, V.N. Singh, G. Partheepan, T.D. Senguttuvan, and K. Jain, One-Step Synthesis of Size-Controlled CZTS Quantum Dots, Appl. Nanosci., 2015, doi:10.1007/s13204-015-0404-z (Feb 13)
D.-C. Nguyen, S. Ito, and D.V.A. Dung, Effects of Annealing Conditions on Crystallization of the CZTS Absorber and Photovoltaic Properties of Cu(Zn, Sn)(S, Se)2 Solar Cells, J. Alloys Compd., 2015, 632, p 676–680
R. Schurr, A. Hölzing, S. Jost, R. Hock, T. Voβ, J. Schulze, A. Kirbs, A. Ennaoui, M.L. Steiner, A. Weber et al., The Crystallisation of Cu2ZnSnS4 Thin Film Solar Cell Absorbers from Co-Electroplated Cu-Zn-Sn Precursors, Thin Solid Films, 2009, 517(7), p 2465–2468
T. Todorov, O. Gunawan, S.J. Chey, T.G. de Monsabert, A. Prabhakar, and D.B. Mitzi, Progress Towards Marketable Earth-Abundant Chalcogenide Solar Cells, Thin Solid Films, 2011, 519(21), p 7378–7381
K. Hironori, J. Kazuo, Y. Satoru, K.T.M.W. Shwe, F. Tatsuo, I. Tadashi, and M. Tomoyoshi, Enhanced Conversion Efficiencies of Cu2ZnSnS4-Based Thin Film Solar Cells by Using Preferential Etching Technique, Appl. Phys. Express, 2008, 1(4), p 041201
A. Shavel, J. Arbiol, and A. Cabot, Synthesis of Quaternary Chalcogenide Nanocrystals: Stannite Cu2ZnxSnySe1+x+2y, J. Am. Chem. Soc., 2010, 132(1), p 4414–4415
H. Wang, Progress in Thin Film Solar Cells Based on Cu2ZnSnS4, Int. J. Photoenergy, 2011, 2011, p 1–10
G. Ma, T. Minegishi, D. Yokoyama, J. Kubota, and K. Domen, Photoelectrochemical Hydrogen Production on Cu2ZnSnS4/Mo-Mesh Thin-Film Electrodes prEpared by Electroplating, Chem. Phys. Lett., 2011, 501(4–6), p 619–622
S.C. Riha, B.A. Parkinson, and A.L. Prieto, Solution-Based Synthesis and Characterization of Cu2ZnSnS4 Nanocrystals, J. Am. Chem. Soc., 2009, 131(34), p 12054–12055
K. Tanaka, Y. Fukui, N. Moritake, and H. Uchiki, Chemical Composition Dependence of Morphological and Optical Properties of Cu2ZnSnS4 Thin Films Deposited By Sol-Gel Sulfurization and Cu2ZnSnS4 Thin Film Solar Cell Efficiency, Solar Energy Mater. Solar Cells, 2011, 95(3), p 838–842
N. Nakayama and K. Ito, Sprayed Films of Stannite Cu2ZnSnS4, Appl. Surf. Sci., 1996, 92, p 5
M. Cao and Y. Shen, A Mild Solvothermal Route to Kesterite Quaternary Cu2ZnSnS4 Nanoparticles, J. Cryst. Growth, 2011, 318, p 4
T.K. Todorov, K.B. Reuter, and D.B. Mitzi, High-Efficiency Solar Cell with Earth-Abundant Liquid-Processed Absorber, Adv. Mater., 2010, 22(20), p E156–159
D.B. Mitzi, M. Yuan, W. Liu, A.J. Kellock, S.J. Chey, L. Gignac, and A.G. Schrott, Hydrazine-Based Deposition Route for Device-Quality CIGS Films, Thin Solid Films, 2009, 517(7), p 2158–2162
W.-C. Hsu, B. Bob, W. Yang, C.-H. Chung, and Y. Yang, Reaction Pathways for the Formation of Cu2ZnSn(Se, S)4 Absorber Materials from Liquid-Phase Hydrazine-Based Precursor Inks, Energy Environ. Sci., 2012, 5(9), p 8564
N. Moritake, Y. Fukui, M. Oonuki, K. Tanaka, and H. Uchiki, Preparation of Cu2ZnSnS4 Thin Film Solar Cells Under Non-vacuum Condition, Phys. Status Solidi C, 2009, 6, p 1233–1236
A. Ennaoui, M. Lux-Steiner, A. Weber, D. Abou-Ras, I. Kotschau, H.-W. Schock, R. Schurr, A. Holzing, S. Jost, R. Hock, T. Voß, J. Schulze, and A. Kirbs, Cu2ZnSnS4 thin Film Solar Cells from Electroplated Precursors: Novel Low-Cost Perspective, Thin Solid Films, 2009, 517, p 2511–2514
K. Timmo, M. Altosaar, J. Raudoja, K. Muska, M. Pilvet, M. Kauk, T. Varema, M. Danilson, O. Volobujeva, and E. Mellikov, Sulfur-Containing Cu2ZnSnSe4 Monograin Powders for Solar Cells, Sol. Energy Mater. Sol. Cells., 2010, 94, p 1889–1892
W. Wang, M.T. Winkler, O. Gunawan, T. Gokmen, T.K. Todorov, Y. Zhu, and D.B. Mitzi, Device Characteristics of CZTSSe Thin-Film Solar Cells with 12.6% Efficiency, Adv. Energy Mater., 2014, 4, p 1301465
J.J. Scragg, T. Ericson, T. Kubart, M. Edoff, and C. Platzer-Björkman, Chemical Insights into the Instability of Cu2ZnSnS4 Films during Annealing, Chem. Mater., 2011, 23, p 4625–4633
A. Weber, R. Mainz, and H.W. Schock, On the Sn Loss from Thin Films of the Material System Cu-Zn-Sn-S in High Vacuum, J. Appl. Phys., 2010, 107, p 013516
E.J. Silvester, F. Grieser, B.A. Sexton, and T.W. Healy, Spectroscopic Studies on Copper Sulfide Sols, Langmuir, 1991, 7, p 2917–2922
S.K. Haram, A.R. Mahadeshwar, and S.G. Dixit, Synthesis and Characterization of Copper Sulfide Nanoparticles in Triton-X 100 Water-in-Oil Microemulsions, J. Phys. Chem., 1996, 100, p 5868–5873
T. Kameyama, T. Osaki, K. Okazaki, T. Shibayama, A. Kudo, S. Kuwabatade, and T. Torimoto, Preparation and Photoelectrochemical Properties of Densely Immobilized Cu2ZnSnS4 Nanoparticle Films, J. Mater. Chem., 2010, 20, p 5319–5324
F.-J. Fan, L. Wu, M. Gong, G. Liu, Y.-X. Wang, S.-H. Yu, S. Chen, L.-W. Wang, and X.-G. Gong, Composition- and Band-Gap-Tunable Synthesis of Wurtzite-Derived Cu2ZnSn(S1–x Se x )4 Nanocrystals: Theoretical and Experimental Insights, ACS Nano, 2013, 7(2), p 1454–1463
A. Khare, A.W. Wills, L.M. Ammerman, D.J. Norrisz, and E.S. Aydil, Size Control and Quantum Confinement in Cu2ZnSnS4 Nanocrystals, Chem. Commun., 2011, 47, p 11721–11723
L. Arora, V.N. Singh, G. Partheepan, T.D. Senguttuvan and K. Jain, One-Step Synthesis of Size-Controlled CZTS Quantum Dots. Appl. Nanosci. doi:10.1007/s13204-015-0404-z
Acknowledgments
The work has been funded by the Higher Education Commission (HEC), Pakistan through the National Research Program for Universities (Grant No. 20-1603). The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no. RGP-VPP-283.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Safdar, A., Islam, M., Akram, M.A. et al. Reaction Time and Film Thickness Effects on Phase Formation and Optical Properties of Solution Processed Cu2ZnSnS4 Thin Films. J. of Materi Eng and Perform 25, 457–465 (2016). https://doi.org/10.1007/s11665-015-1874-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-015-1874-6