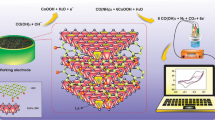
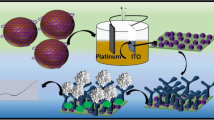
Abstract
Wheat gliadin is thought to be the main cause of wheat allergy leading to celiac disease. In this study, we reported an electrochemical immunosensor based on AuNPs/Zn/Ni-ZIF-8-800@graphene for simple, efficient, and sensitive detection of gliadin. A monoclonal antibody against gliadin were prepared by hybridoma technique. Zn/Ni-ZIF-8-800 with high stability was prepared by using zinc–nickel bimetallic organic framework MOFs, and graphene and gold nanoparticles with large surface area and excellent conductivity were introduced. Chitosan was used as a binder to fixed the composite on the surface of glassy carbon electrode. The fabricated materials and the corresponding sensors were comprehensively characterized by X-ray diffractometer, scanning electron microscope, transmission electron microscopy, Fourier transform infrared spectroscopy, Brunauer–Emmett–Teller, and cyclic voltammetry analyses. Under the optimal conditions, the linear range of the electrochemical immunosensor was 0.1–100 μg mL−1, and the detection limit was 0.950 μg mL−1. In addition, the fabricated sensor exhibited high selectivity and long-term stability. The recovery test showed that the prepared sensor was suitable for the detection of gliadin in real food and feed, exhibiting great promising of the developed sensor in practical application.
Graphical abstract


















Similar content being viewed by others
References
Steffolani ME, Ribotta PD, Pérez GT, León AE (2010) Effect of glucose oxidase, transglutaminase, and pentosanase on wheat proteins: relationship with dough properties and bread-making quality. J Cereal Sci 51:366–373. https://doi.org/10.1016/j.jcs.2010.01.010
Ekezie F-GC, Cheng J-H, Sun D-W (2018) Effects of nonthermal food processing technologies on food allergens: a review of recent research advances. Trends Food Sci Technol 74:12–25. https://doi.org/10.1016/j.tifs.2018.01.007
Biagi F, Zimmer K, Thomas P, Ellis H, Ciclitira PJ (1999) Is gliadin mispresented to the immune system in coeliac disease? A hypothesis. QJM Int J Med 92:119–122. https://doi.org/10.1093/qjmed/92.2.119
Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A et al (2013) The Oslo definitions for coeliac disease and related terms. Gut 62:43–52. https://doi.org/10.1136/gutjnl-2011-301346
Walker MM, Ludvigsson JF, Sanders DS (2017) Coeliac disease: review of diagnosis and management. Med J Aust 207:173–178. https://doi.org/10.5694/mja16.00788
Dieckman T, Koning F, Bouma G (2022) Celiac disease: new therapies on the horizon. Curr Opin Pharmacol 66:102268. https://doi.org/10.1016/j.coph.2022.102268
Lammers KM, Herrera MG, Dodero VI (2018) Translational chemistry meets gluten-related disorders. ChemistryOpen 7:217–232. https://doi.org/10.1002/open.201700197
Lowrie M, Garden O, Hadjivassiliou M, Harvey RJ, Sanders D, Powell R, Garosi L (2015) The clinical and serological effect of a gluten-free diet in border terriers with epileptoid cramping syndrome. J Vet Intern Med 29:1564–1568. https://doi.org/10.1111/jvim.13643
Daminet SC (1996) Gluten-sensitive enteropathy in a family of Irish setters. Can Vet J 37:745
Schleicher M, Cash SB, Freeman LM (2019) Determinants of pet food purchasing decisions. Can Vet J 60:644
Schubert-Ullrich P, Rudolf J, Ansari P, Galler B, Führer M, Molinelli A, Baumgartner S (2009) Commercialized rapid immunoanalytical tests for determination of allergenic food proteins: an overview. Anal Bioanal Chem 395:69–81. https://doi.org/10.1007/s00216-009-2715-y
Skerritt JH, Hill AS (1990) Monoclonal antibody sandwich enzyme immunoassays for determination of gluten in foods. J Agric Food Chem 38:1771–1778. https://doi.org/10.1021/jf00098a029
Valdés I, García E, Llorente M, Méndez E (2003) Innovative approach to low-level gluten determination in foods using a novel sandwich enzyme-linked immunosorbent assay protocol. Eur J Gastroenterol Hepatol 15:465–747. https://doi.org/10.1097/01.meg.0000059119.41030.df
van den Broeck H, Hongbing C, Lacaze X, Dusautoir J-C, Gilissen L, Smulders M, van der Meer I (2010) In search of tetraploid wheat accessions reduced in celiac disease-related gluten epitopes. Mol Biosyst 6:2206–2213. https://doi.org/10.1039/C0MB00046A
Camafeita E, Alfonso P, Mothes T, Méndez E (1997) Matrix-assisted laser desorption/ionization time-of-flight mass spectrometric micro-analysis: the first non-immunological alternative attempt to quantify gluten gliadins in food samples. J Mass Spectrom 32:940–947. https://doi.org/10.1002/(SICI)1096-9888(199709)32:9%3c940::AID-JMS550%3e3.0.CO;2-2
Mujico JR, Lombardía M, Mena MC, Méndez E, Albar JP (2011) A highly sensitive real-time PCR system for quantification of wheat contamination in gluten-free food for celiac patients. Food Chem 128:795–801. https://doi.org/10.1016/j.foodchem.2011.03.061
Talib NAA, Abidin SNJSZ, Salam F, Sulaiman Y (2016) Clenbuterol immunosensors based poly (3, 4-ethylenedioxythiophene)/multiwall carbon nanotube (PEDOT/MWCNT) hybrid composite. Procedia Chem 20:29–32. https://doi.org/10.1016/j.proche.2016.07.004
Wang C, Hu L, Zhao K, Deng A, Li J (2018) Multiple signal amplification electrochemiluminescent immunoassay for Sudan I using gold nanorods functionalized graphene oxide and palladium/aurum core-shell nanocrystallines as labels. Electrochim Acta 278:352–362. https://doi.org/10.1016/j.electacta.2018.05.061
Abdallah ZB, Grauby-Heywang C, Beven L, Cassagnere S, Moroté F, Maillard E, Sghaier H, Bouhacina TC (2019) Development of an ultrasensitive label-free immunosensor for fungal aflatoxin B1 detection. Biochem Eng J 150:107262. https://doi.org/10.1016/j.bej.2019.107262
Chen HA, Zhang JL, Huang R, Wang DJ, Deng DM, Zhang QX, Luo LQ (2023) The applications of electrochemical immunosensors in the detection of disease biomarkers: a review. Molecules 28:20. https://doi.org/10.3390/molecules28083605
Wang H-F, Chen L, Pang H, Kaskel S, Xu Q (2020) MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem Soc Rev 49:1414–1448. https://doi.org/10.1039/C9CS00906J
Li B, Wen HM, Cui Y, Zhou W, Qian G, Chen B (2016) Emerging multifunctional metal–organic framework materials. Adv Mater 28:8819–8860. https://doi.org/10.1002/adma.201601133
Shen K, Zhang L, Chen X, Liu L, Zhang D, Han Y, Chen J, Long J, Luque R, Li Y, Chen B (2018) Ordered macro–microporous metal–organic framework single crystals. Science 359:206–210. https://doi.org/10.1126/science.aao3403
Park KS, Ni Z, Côté AP, Choi JY, Huang R, Uribe-Romo FJ, Chae HK, O’Keeffe M, Yaghi OM (2006) Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc Natl Acad Sci USA 103:10186–10191. https://doi.org/10.1073/pnas.0602439103
Qiu M, He C (2019) Efficient removal of heavy metal ions by forward osmosis membrane with a polydopamine modified zeolitic imidazolate framework incorporated selective layer. J Hazard Mater 367:339–347. https://doi.org/10.1016/j.jhazmat.2018.12.096
Chen L, Guo H, Fujita T, Hirata A, Zhang W, Inoue A, Chen M (2011) Nanoporous PdNi bimetallic catalyst with enhanced electrocatalytic performances for electro-oxidation and oxygen reduction reactions. Electrochim Acta 21:4364–4370. https://doi.org/10.1002/adfm.201101227
Wu H, Li M, Wang Z, Yu H, Han J, Xie G, Chen S (2019) Highly stable Ni-MOF comprising triphenylamine moieties as a high-performance redox indicator for sensitive aptasensor construction. Anal Chim Acta 1049:74–81. https://doi.org/10.1016/j.aca.2018.10.022
Liu XZ, Yang HP, Diao YY, He Q, Lu CY, Singh A, Kumar A, Liu JQ, Lan Q (2022) Recent advances in the electrochemical applications of Ni-based metal organic frameworks (Ni-MOFs) and their derivatives. Chemosphere 307:18. https://doi.org/10.1016/j.chemosphere.2022.135729
Drachuk I, Harbaugh S, Chávez JL, Kelley-Loughnane N (2020) Improving the activity of DNA-encoded sensing elements through confinement in silk microcapsules. ACS Appl Mater Interfaces 12:48329–48339. https://doi.org/10.1021/acsami.0c13713
Sayhi M, Ouerghi O, Belgacem K, Arbi M, Tepeli Y, Ghram A, Anik Ü, Österlund L, Laouini D, Diouani MF (2018) Electrochemical detection of influenza virus H9N2 based on both immunomagnetic extraction and gold catalysis using an immobilization-free screen printed carbon microelectrode. Biosens Bioelectron 107:170–177. https://doi.org/10.1016/j.bios.2018.02.018
Wang Y, Wang XP, Gao TG, Lou CX, Wang HF, Liu YF, Cao AN (2022) Folding of flexible protein fragments and design of nanoparticle-based artificial antibody targeting lysozyme. J Phys Chem B 126(27):5045–5054. https://doi.org/10.1021/acs.jpcb.2c03200
Li R, Ren X, Feng X, Li X, Hu C, Wang B (2014) A highly stable metal- and nitrogen-doped nanocomposite derived from Zn/Ni-ZIF-8 capable of CO2 capture and separation. Chem Commun 50:6894–6897. https://doi.org/10.1039/C4CC01087F
Jiang X, He S, Han G, Long J, Li S, Lau CH, Zhang S, Shao L (2021) Aqueous one-step modulation for synthesizing monodispersed ZIF-8 nanocrystals for mixed-matrix membrane. ACS Appl Mater Interfaces 13:11296–11305. https://doi.org/10.1021/acsami.0c22910
Kim H, Trinh BT, Kim KH, Moon J, Kang H et al (2021) Au@ ZIF-8 SERS paper for food spoilage detection. Biosens Bioelectron 179:113063. https://doi.org/10.1016/j.bios.2021.113063
Zhang Y, Zhang Z, Rong S, Yu H, Gao H, Ding P, Chang D, Pan H (2020) Electrochemical immunoassay for the carcinoembryonic antigen based on Au NPs modified zeolitic imidazolate framework and ordered mesoporous carbon. Microchim Acta 187:1–9. https://doi.org/10.1007/s00604-020-04235-5
Kaur H, Mohanta GC, Gupta V, Kukkar D, Tyagi S (2017) Synthesis and characterization of ZIF-8 nanoparticles for controlled release of 6-mercaptopurine drug. J Drug Deliv Sci Technol 41:106–112. https://doi.org/10.1016/j.jddst.2017.07.004
Zhang W, Liu S, Zhang Y, Ding X, Jiang B, Zhang Y (2019) An electrochemical sensor based on electro-polymerization of caffeic acid and Zn/Ni-ZIF-8-800 on glassy carbon electrode for the sensitive detection of acetaminophen. Biosens Bioelectron 131:200–206. https://doi.org/10.1016/j.bios.2019.01.069
Wu Y, Xu W, Wang Y, Yuan Y, Yuan R (2013) Silver–graphene oxide nanocomposites as redox probes for electrochemical determination of α-1-fetoprotein. Electrochim Acta 88:135–140. https://doi.org/10.1016/j.electacta.2012.10.081
Rouf TB, Díaz-Amaya S, Stanciu L, Kokini J (2020) Application of corn zein as an anchoring molecule in a carbon nanotube enhanced electrochemical sensor for the detection of gliadin. Food Control 117:107350. https://doi.org/10.1016/j.electacta.2012.10.081
Rahemi V, Vandamme J, Garrido J, Borges F, Brett C, Garrido E (2012) Enhanced host–guest electrochemical recognition of herbicide MCPA using a β-cyclodextrin carbon nanotube sensor. Talanta 99:288–293. https://doi.org/10.1016/j.talanta.2012.05.053
Saum A, Cumming R, Rowell FJ (2000) Detection of protease activity in the wetted surface of gelatin-coated electrodes in air by AC impedance spectroscopy. Biosens Bioelectron 15:305–313. https://doi.org/10.1016/S0956-5663(00)00068-3
Codex Standard 118-1979. https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B118-1979%252FCXS_118e_2015.pdf. Accessed 2 Aug 2023
Valdes I, Garcia E, Llorente M, Mendez E (2003) Innovative approach to low-level gluten determination in foods using a novel sandwich enzyme-linked immunosorbent assay protocol. Eur J Gastroenterol Hepatol 15:465–474. https://doi.org/10.1097/01.meg.0000059119.41030.df
Garcia-Garcia A, Madrid R, Sohrabi H, de la Cruz S, Garcia T, Martin R, Gonzalez I (2019) A sensitive and specific real-time PCR targeting DNA from wheat, barley and rye to track gluten contamination in marketed foods. LWT Food Sci Technol 114:108378. https://doi.org/10.1016/j.lwt.2019.108378
Benitez M, Zubiate P, Socorro-Leranoz AB, Matias IR (2023) Lossy mode resonance-based optical immunosensor towards detecting gliadin in aqueous solutions. Food Control 147:109624. https://doi.org/10.1016/j.foodcont.2023.109624
Liao Y-S, Kuo J-H, Chen B-L, Tsuei H-W, Lin C-Y, Lin HY, Cheng H-F (2017) Development and validation of the detection method for wheat and barley glutens using mass spectrometry in processed foods. Food Anal Methods 10:2839–2847. https://doi.org/10.1007/s12161-017-0827-0
Hnasko RM, Jackson ES, Lin AV, Haff RP, McGarvey JA (2021) A rapid and sensitive lateral flow immunoassay (LFIA) for the detection of gluten in foods. Food Chem 355:129514. https://doi.org/10.1016/j.foodchem.2021.129514
Svigelj R, Zuliani I, Grazioli C, Dossi N, Toniolo R (2022) An effective label-free electrochemical aptasensor based on gold nanoparticles for gluten detection. Nanomaterials 12(6):987. https://doi.org/10.3390/nano12060987
Corpuz A, Khumsap T, Bamrungsap S, Thu VT, Nguyen LT (2023) Epitope-imprinted polydopamine and reduced graphene oxide-based sensing interface for label-free detection of gliadin. J Food Compos Anal 51(3):366–373. https://doi.org/10.1016/j.jfca.2022.105090
Funding
This research was funded by Open Project of Key Laboratory of Animal Immunology of the Ministry of Agriculture (PKLA20170606).
Author information
Authors and Affiliations
Contributions
KZ, NW and QL conceived and designed the experiments. KZ, YD, CC and SL performed the experiments. YD, GX, HR and CX contributed data analysis. KZ wrote the manuscript. KZ, NW, FW and CX revised the manuscript content. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhan, K., Du, Y., Liu, Q. et al. A label-free electrochemical immunosensor based on AuNPs/Zn/Ni-ZIF-8-800@graphene for the detection of wheat gliadin. J Appl Electrochem 54, 669–685 (2024). https://doi.org/10.1007/s10800-023-01978-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-01978-w