Abstract
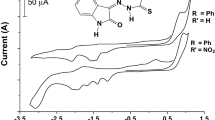
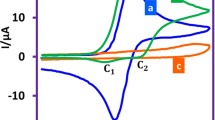
Two new sulfonylhydrazone derivatives with potentially antichagasic biological activity, analogues of nitrofural, were studied by cyclic voltammetry in aqueous medium using glassy carbon electrode aiming at a better understanding of their redox mechanism that can be related to the biological mechanism, being classically characterized by a charge transfer processes. In an acidic medium, an irreversible cathodic peak corresponding to the formation of a hydroxylamine derivative was observed, being linearly displaced with the decrease in the acidity of the medium to more negative potential values. In alkaline medium, the reduction was independent of the pH, registering the formation of the reversible R-NO2/R-NO2.− couple. The half-life of the radical nitro-anion was experimentally estimated and corroborated by simulation with Digisim.
Graphical abstract






Similar content being viewed by others
References
World Health Organization (2022) https://www.who.int/en/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) accessed July
Martín-Escolano J, Medina-Carmona E, Martín-Escolano R (2020) Chagas disease: current view of an ancient and global chemotherapy challenge. ACS Infect Dis 6:2830–2843. https://doi.org/10.1021/acsinfecdis.0c00353
Martín-Escolano J, Marín C, Rosales MJ, Tsaousis AD, Medina-Carmona E, Martín-Escolano R (2022) Chagas disease: current view of an ancient and global chemotherapy challenge. ACS Infect Dis 8:1107–1115. https://doi.org/10.1021/acsinfecdis.2c00123
Kratz JM (2019) Drug discovery for Chagas disease: a viewpoint. Acta Trop 19:8105107. https://doi.org/10.1016/j.actatropica.2019.105107v
Ribeiro V, Dias N, Paiva T, Hagström-Bex L, Nitz N, Pratesi R, Hecht M (2020) Current trends in the pharmacological management of Chagas disease. IJP Drugs Drug Resist 12:7–17. https://doi.org/10.1016/j.ijpddr.2019.11.004
Giarolla J, Ferreira EI (2015) Drug design for neglected disease in Brazil. Mini-reviews. Med Chem 15:219–241. https://doi.org/10.2174/138955751503150312122523
Scarim CB, Chin CM (2019) Nitroheterocyclic derivatives: privileged scaffold for drug development against Chagas disease. Med Chem Res 28:2099–2108. https://doi.org/10.1007/s00044-019-02453-y
Patterson S, Fairlamb AH (2019) Current and future prospects of nitro-compounds as drugs for trypanosomiasis and leishmaniasis. Curr Med Chem 26:4454–4475. https://doi.org/10.2174/0929867325666180426164352
Gatti FM (2015) Síntese e avaliação biológica de sulfonil-hidrazonas análogos do nitrofural como candidatos a antichagásico. https://www.teses.usp.br/teses/disponiveis/9/9138/tde-11092015-160545/publico/Fernando_de_Moura_Gatti_ME_corrigida.pdf
Gatti FM, Gomes RA, Fonseca AL, Lima EJC, Vital-Fujji DG, Taranto AG, Varotti FP, Trossini GHG (2021) Antiplasmodial activity of sulfonylhydrazones: in vitro and in silico approaches. Future Med Chem 13:233–250. https://doi.org/10.4155/fmc-2020-0229
Nepali K, Lee HY, Liou JP (2019) Nitro-group-containing drugs. J Med Chem 62:2851–2893. https://doi.org/10.1021/acs.jmedchem.8b00147
Jana S, Mandlekar S, Marathe P (2010) Prodrug design to improve pharmacokinetic and drug delivery properties: challenges to the discovery scientists. Curr Med Chem 17:3874–3908. https://doi.org/10.2174/092986710793205426
Choi-Sledeski YM, Wermuth CG (2015) Designing prodrugs and bioprecursors. In: Wermuth C, Aldous D, Raboisson P, Rognan D (eds) The practice of medicinal chemistry, 4th edn. Academic Press, Boca Raton, pp 677–696
Chung MC, Bosquesi PL, Santos JL (2011) A prodrug approach to improve the physico-chemical properties and decrease the genotoxicity of nitro compounds. Curr Pharm Des 17:3515–3526. https://doi.org/10.2174/138161211798194512
Mandic Z (2017) Electrochemical methods in drug discovery and development. Bulg Chem Commun 49:65–73
Aydina EB, Aydina M, Sezginturkb MK (2019) Biosensors in drug discovery and drug analysis. Curr Anal Chem 15:467–484. https://doi.org/10.2174/1573411014666180912131811
Ozkan SA, Uslu B (2016) From mercury to nanosensors: past, present and the future perspective of electrochemistry in pharmaceutical and biomedical analysis. J Pharm Biomed Anal 130:126–140. https://doi.org/10.1016/j.jpba.2016.05.006
Gulaboski R, Mirceski V, Bogeski I, Hoth M (2012) Protein film voltammetry: electrochemical enzymatic spectroscopy. A review on recent progress. J Solid State Electrochem 16:2315–2328. https://doi.org/10.1007/s10008-011-1397-5
Gulaboski R, Mirceski V (2020) Application of voltammetry in biomedicine—recent achievements in enzymatic voltammetry. Maced J Chem Chem Eng 39:153–166. https://doi.org/10.20450/mjcce.2020.2152
Gulaboski R (2020) Electrochemistry in the twenty-first century—future trends and perspectives. J Solid State Electrochem 24:2081. https://doi.org/10.1007/s10008-020-04550-0
Gulaboski R (2022) The future of voltammetry. Maced J Chem Chem Eng 41:151–162. https://doi.org/10.20450/mjcce.2022.2555
Squella JA, Bollo S, Núñez-Vergara LJ (2005) Recent developments in the electrochemistry of some nitro compounds of biological significance. Curr Org Chem 9:565–581. https://doi.org/10.2174/1385272053544380
Hammerich O (2015) Reduction of nitro compounds and related substrates. In: Hammerich O, Speiser B (eds) Organic electrochemistry, 5th edn. CRC Press, Boca Raton, pp 1149–1200
Uliana CV, Garbellini GS, Yamanaka H (2012) Electrochemical reduction of disperse orange 1 textile dye at a boron-doped diamond electrode. J Appl Electrochem 42:297–304. https://doi.org/10.1007/s10800-012-0403-7
La-Scalea MA, Menezes CM, Julião MSS, Chung MC, Serrano SHP, Ferreira EI (2005) Voltammetric behaviour of nitrofurazone and its hydroxymethyl prodrug with potential anti-chagasactivity. J Braz Chem Soc 16:744–782. https://doi.org/10.1590/S0103-50532005000500015
Brito CL, Trossini GHG, Ferreira EI, La-Scalea MA (2013) Nitrofurazone and its nitroheterocyclic analogues: a study of the electrochemical behaviour in aqueous medium. J Braz Chem Soc 24:1964–1973. https://doi.org/10.5935/0103-5053.20130246
Chiavassa LD, La-Scalea MA (2018) Square wave voltammetry of nitrofurans in aqueous media using a carbon fiber microelectrode. J Solid State Electrochem 22:1395–1402. https://doi.org/10.1007/s10008-017-3751-8
Brito CL, Lins RSO, Bertotti M, Ferreira EI, La-Scalea MA (2022) Free radical formation evidence from nimorazole electrochemical reduction in aqueous media. Electrochim Acta 403:139709. https://doi.org/10.1016/j.electacta.2021.139709
Lurie J (1978) Handbook of analytical chemistry. Mir Publishers, Moscow
Baur JE (2007) Diffusion coefficients. In: Zoski CG (ed) Handbook of electrochemistry. Elsevier, Amsterdam, pp 829–848
Roffel B, Van de Graff JJ (1977) The diffusion coefficient of ions in aqueous ferricyanide of potassium chloride with and without addition of poliethylene oxide. J Chem Eng Data 22:300–302. https://doi.org/10.1021/je60074a004
La-Scalea MA, Menezes CMS, Ferreira EI (2005) Molecular volume calculation using AM1 semi-empiric method toward diffusion coefficients and electrophoretic mobility estimates in aqueous solution. J Mol Struct Theochem 730:111–120. https://doi.org/10.1016/j.theochem.2005.05.030
www.molinspiration.com.br. Accessed January 2022.
Mozo JD, Carbajo J, Sturm JC, Núñez-Vergara LJ, Moscoso R, Squella JA (2011) The use of digital simulation to improve the cyclic voltammetric determination of rate constants for homogeneous chemical reactions following charge transfers. Anal Chim Acta 699:33–43. https://doi.org/10.1016/j.aca.2011.04.060
Olmstead ML, Nicholson RS (1969) Cyclic voltammetry theory for the disproportionation reaction and spherical diffusion. Anal Chem 41:862–864. https://doi.org/10.1021/ac60275a004
Zoubir J, Assabbane A, Bakas I (2022) Electroanalysis of nirofurazone at silver particles and graphite powder composite electrode. Carbon Lett 32:767–780. https://doi.org/10.1007/s42823-021-00307-5
Cai S, Jiao T, Wang L, Wang F, Chen Q (2022) Electrochemical sensing of nitrofurazone on Ru(bpy)32+ functionalized polyoxometalate combined with graphene modified electrode. Food Chem 378:132084. https://doi.org/10.1016/j.foodchem.2022.132084
Rani R, Deep A, Mizaikoff B, Singh S (2022) Zirconium metal organic framework based opto-electrochemical sensor for nitrofurazone detection. J Electroanal Chem 909:116124. https://doi.org/10.1016/j.jelechem.2022.116124
Radulescu MC, Bucur MP, Bucur B, Radu GL (2021) Rapid determination of 5-nitrofuran ring antibiotics in complex samples using a boron-doped diamond electrode and differential pulse voltammetry. Anal Lett 54:2363–2375. https://doi.org/10.1080/00032719.2020.1862140
Guzmán A, Agüí L, Pedrero M, Yáñez-Sedeño P, Pingarrón JM (2004) Voltammetric determination of antibacterial nitro-compounds at activated carbon fibre microelectrodes. Electroanalysis 16:1763–1770. https://doi.org/10.1002/elan.200303020
Bollo S, Nuñez-Vergara LJ, Martinez C, Chauviere G, Périé J, Squella JA (2003) Voltammetric study of nitro radical anion generated from some nitrofuran compounds of pharmacological significance. Electroanalysis 15:19–25. https://doi.org/10.1002/elan.200390000
Julião MSS, Almeida EC, La-Scalea MA, Ferreira NG, Compton RG, Serrano SHP (2005) Voltammetric behaviour of nitrofurazone at highly boron doped diamond electrode. Electroanalysis 17:269–274. https://doi.org/10.1002/elan.200403093
Ni Y, Wang P, Kokot S (2012) Voltammetric investigation of DNA damage induced by nitrofurazone and short-lived nitro-radicals with the use of an electrochemical DNA biosensor. Biosens Bioelectron 38:245–251. https://doi.org/10.1016/j.bios.2012.05.034
Brito CL, Ferreira EI, La-Scalea MA (2020) Multi-walled carbon nanotube functionalization and the dispersing agents study applied for the glassy carbon electrode modification and voltammetric reduction of nitrofurazone. J Solid State Electrochem 24:1969–1980. https://doi.org/10.1007/s10008-020-04621-2
Alemu H, Hlalele L (2007) Voltammetric determination of chloramphenicol at electrochemically preatreated glassy carbon electrode. Bull Chem Soc Ethiop 21:1–12. https://doi.org/10.4314/bcse.v21i1.61361
Kor K, Zarei K (2014) Electrochemical determination of chloramphenicol on glassy carbon electrode modified with multi-walled carbon nanotube–cetyltrimethylammonium bromide–poly(diphenylamine). J Electroanal Chem 733:39–46. https://doi.org/10.1016/j.jelechem.2014.09.013
Peng R, Gao Y, Chen W (2021) Determination of chloramphenicol by a new electrochemically activated glassy carbon electrode in sodium sulfate medium. J Electrochem Soc 168:067509. https://doi.org/10.1149/1945-7111/ac04ef
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19–28. https://doi.org/10.1016/S0022-0728(79)80075-3
Nikodimos Y, Amare M (2016) Electrochemical determination of metronidazole in tablet samples using carbon paste electrode. J Anal Meth Chem 2016:3612943. https://doi.org/10.1155/2016/3612943
Bard AJ, Faulkaner LF (2001) Electrochemical methods, fundamentals and applications, 2nd edn. Wiley, New Work
Morales A, Richter P, Toral I (1987) Voltammetric behaviour of nitrofurazone, furazolidone and other nitro derivatives of biological importance. Analyst 112:965–970. https://doi.org/10.1039/AN9871200965
Zuman P, Fijalek Z, Dumanovic D, Sužnjević D (1992) Polarographic and electrochemical studies of some aromatic and heterocyclic nitro compounds, part I: general mechanistic aspects. Electroanalysis 4:783–794. https://doi.org/10.1002/elan.1140040808
Batchelor-McAuley C, Compton RG (2012) Voltammetry of multi-electron electrode processes of organic species. J Electroanal Chem 669:73–81. https://doi.org/10.1016/j.jelechem.2012.01.016
Bacil RP, Chen L, Serrano SHP, Compton RG (2020) Dopamine oxidation at gold electrodes: mechanism and kinetics near neutral pH. Phys Chem Chem Phys 22:607–614. https://doi.org/10.1039/c9cp05527d
Gosser DK Jr (1994) Cyclic voltametry, simulation and analysis of reaction mechanism. VCH, New York
La-Scalea MA, Trossini GHG, Menezes CMS, Chung MC, Ferreira EI (2009) Electrochemical reduction using glassy carbon electrode in aqueous medium of a potential anti-Chagas drug: NFOH. J Electrochem Soc 156:F93–F97. https://doi.org/10.1149/1.3130082
Lazarova S, Apostoloski P, Gulaboski R (2023) Electrochemically induced dimerization of lipophilic redox proteins: theoretical insights in protein-film square-wave voltammetry. Monatsh Chem 154:595–603. https://doi.org/10.1007/s00706-023-03065-4
Olmstead ML, Hamilton R, Nicholson RS (1969) Theory of cyclic voltammetry for a dimerization reaction initiated electrochemically. Anal Chem 41:260–266. https://doi.org/10.1021/ac60271a032
Maya JD, Bollo S, Nuñez-Vergara LJ, Squella JA, Repetto Y, Morello A, Périé J, Chauvière G (2003) Trypanosoma cruzi: effect and mode of action of nitroimidazole and nitrofuran derivatives. Biochem Pharmacol 65:999–1006. https://doi.org/10.1016/S0006-2952(02)01663-5
Mandal PC (2004) Reactions of the nitro radical anion of metronidazole in aqueous and mixed solvent: a cyclic voltammetric study. J Electroanal Chem 570:55–61. https://doi.org/10.1016/j.jelechem.2004.02.030
Tocher JH, Edwards DI (1989) Electrochemical characteristics of nitro-heterocyclic compounds of biological interest IV. Lifetime of the metronidazole radical anion. Free Radic Res Commun 6:3945. https://doi.org/10.3109/10715768909073426
Tocher JH, Edwards DI (1990) Electrochemical characteristics of nitroheterocyclic compounds of biological interest V. Measurement and comparison of nitro radical lifetimes. Int J Radiat Biol 57:45–53. https://doi.org/10.1080/09553009014550331
Brito CL, Ferreira EI, La-Scalea MA (2020) Multi-walled carbon nanotube functionalization and the dispersing agents study applied for the glassy carbon electrode modification and voltammetric reduction of nitrofurazone. J Sol State Electrochem 24:1969–1980. https://doi.org/10.1007/s10008-020-04621-2
Author information
Authors and Affiliations
Contributions
LSA, LDC and CLB had the conception and design of study, involving acquisition of data, analysis and/or interpretation of data. FMG synthesized the derivatives and performed the biological tests. GHGT revised the manuscript critically for important intellectual content. MAL-S wrote and revised the manuscript text critically for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Almeida, L.S., Chiavassa, L.D., de Lima Brito, C. et al. Voltammetric study in aqueous media of two new sulfonylhydrazone derivatives as candidates for antichagasic drugs. J Appl Electrochem 54, 41–52 (2024). https://doi.org/10.1007/s10800-023-01945-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-01945-5