Abstract
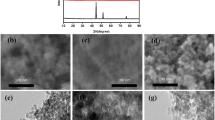
Mixed transition metallic sulfides have attracted researchers’ attention due to their unique electronic and electrochemical properties for energy storage devices. Herein, we have investigated nickel manganese sulfides (Nix–Mnx–S) based binary anode material for supercapattery devices. The hydrothermal method was used to synthesize the Nix–Mnx–S-based nanomaterials with different Ni to Mn ratios. Scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), energy dispersive X-ray (EDX), and Brunauer-Emmett-Teller spectroscopy (BETS) is used to examine surface characteristics, crystallinity, elemental analysis, and homogeneity. The electrochemical measurement of the Nix–Mnx–S-based electrode material is first explored in three electrodes assembly while maintaining a 1 M KOH electrolyte environment. Among all the electrodes, Ni0.50Mn0.50 S demonstrated exceptional performance with a specific capacity of 713 C/g or 1188 F/g at the current density of 1.0 A/g. Lastly, the Ni0.50Mn0.50 S based nanomaterials are used as working electrode and activated carbon (AC) as reference electrode for the two electrodes assembly test (Ni0.50 Mn0.50 S//AC). Which showing a high energy density of 35.24 (Wh/Kg), power density of 3200 (W/Kg), extraordinary specific capacity 158.6 C/g with coulomb efficiency 91.6% and capacity retention 70% after 11,000 galvanostatic charging/discharging (GCD) cycles. Our findings provide a platform to improve the performance of asymmetric energy storage devices.
Graphical abstract










Similar content being viewed by others
Data Availability
The data is available on request.
Change history
16 August 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10800-023-01953-5
References
Simon P, Gogotsi Y (2010) Materials for electrochemical capacitors. Nanosci Technol Collect Rev Nat J. https://doi.org/10.1142/9789814287005_0033
Wang Y, Song Y, Xia Y (2016) Electrochemical capacitors: mechanism, materials, systems, characterization and applications. Chem Soc Rev 45(21):5925–5950
Liu J et al (2008) Oriented nanostructures for energy conversion and storage. Chem Sustain Energy Mater 1(8–9):676–697
Hu C et al (2018) Carbon-based metal-free electrocatalysis for energy conversion, energy storage, and environmental protection. Electrochem Energy Rev 1(1):84–112
Bonaccorso F et al (2015) Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 347(6217):1246501
Ratajczak P et al (2019) Carbon electrodes for capacitive technologies. Energy Storage Mater 16:126–145
Zhang LL, Zhao X (2009) Carbon-based materials as supercapacitor electrodes. Chem Soc Rev 38(9):2520–2531
Qie L et al (2013) Synthesis of functionalized 3D hierarchical porous carbon for high-performance supercapacitors. Energy Environ Sci 6(8):2497–2504
Kalyani P, Anitha A (2013) Biomass carbon & its prospects in electrochemical energy systems. Int J Hydrog Energy 38(10):4034–4045
Zhong C et al (2015) A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem Soc Rev 44(21):7484–7539
Shi X et al (2018) Flexible, planar integratable and all-solid-state micro-supercapacitors based on nanoporous gold/manganese oxide hybrid electrodes via template plasma etching method. J Alloys Compd 739:979–986
Ghosh A, Lee YH (2012) Carbon-based electrochemical capacitors. Chemsuschem 5(3):480–499
Becker HI (1957) Low voltage electrolytic capacitor. Google Patents
Zhao Y et al (2018) Microgel-enhanced double network hydrogel electrode with high conductivity and stability for intrinsically stretchable and flexible all-gel-state supercapacitor. ACS Appl Mater Interfaces 10(23):19323–19330
Zhang Y et al (2009) Progress of electrochemical capacitor electrode materials: a review. Int J Hydrog Energy 34(11):4889–4899
Zeiger M et al (2016) Carbon onions for electrochemical energy storage. J Mater Chem A 4(9):3172–3196
Gilshteyn EP et al (2017) All-nanotube stretchable supercapacitor with low equivalent series resistance. Sci Rep 7(1):1–9
Shi J et al (2015) Electrodeposition of high-capacitance 3D CoS/graphene nanosheets on nickel foam for high-performance aqueous asymmetric supercapacitors. J Mater Chem A 3(41):20619–20626
Liu P et al (2018) Synthesis of poly (m-phenylenediamine)-coated hexagonal Co9 S8 for high-performance supercapacitors. J Mater Sci 53(1):759–773
Hu H et al (2016) Metal–organic-framework-engaged formation of Co nanoparticle-embedded carbon@ Co9 S8 double-shelled nanocages for efficient oxygen reduction. Energy Environ Sci 9(1):107–111
Peng S et al (2014) MS2 (M = co and ni) hollow spheres with tunable interiors for high-performance supercapacitors and photovoltaics. Adv Funct Mater 24(15):2155–2162
Yu XY et al (2014) General formation of MS (M = ni, Cu, Mn) Box-in‐Box hollow structures with enhanced pseudocapacitive properties. Adv Funct Mater 24(47):7440–7446
Ruan Y et al (2016) Rapid self-assembly of porous square rod-like nickel persulfide via a facile solution method for high-performance supercapacitors. J Power Sources 301:122–130
Long L et al (2017) Ni 3 S 2@ polypyrrole composite supported on nickel foam with improved rate capability and cycling durability for asymmetric supercapacitor device applications. J Mater Sci 52(7):3642–3656
Tang Y, Chen T, Yu S (2015) Morphology controlled synthesis of monodispersed manganese sulfide nanocrystals and their primary application in supercapacitors with high performances. Chem Commun 51(43):9018–9021
Song X et al (2018) Highly active and porous M3S4 (M = Ni, Co) with enriched electroactive edge sites for hybrid supercapacitor with better power and energy delivery performance. Electrochim Acta 283:121–131
Han X et al (2019) Identifying the activation of bimetallic sites in NiCo2S4@ g-C3N4‐CNT hybrid electrocatalysts for synergistic oxygen reduction and evolution. Adv Mater 31(18):1808281
Han X et al (2019) Template synthesis of NiCo2S4/Co9S8 hollow spheres for high-performance asymmetric supercapacitors. Chem Eng J 368:513–524
Yang Y et al (2019) Effective synthetic strategy for Zn 0.76 Co 0.24 S encapsulated in stabilized N-doped carbon nanoarchitecture towards ultra-long-life hybrid supercapacitors. J Mater Chem A 7(24):14670–14680
Balamurugan J et al (2018) Hierarchical Ni Mo S and Ni Fe S nanosheets with ultrahigh energy density for flexible all solid-state supercapacitors. Adv Funct Mater 28(35):1803287
Li S et al (2018) Hierarchical layer-by-layer porous FeCo 2 S 4@ Ni (OH) 2 arrays for all-solid-state asymmetric supercapacitors. J Mater Chem A 6(41):20480–20490
Cui Y et al (2019) Ionic liquid-controlled growth of NiCo2S4 3D hierarchical hollow nanoarrow arrays on Ni foam for superior performance binder free hybrid supercapacitors. Small 15(3):1804318
Ranjith KS et al (2021) Lignin-derived carbon nanofibers‐laminated redox‐active‐mixed metal sulfides for high‐energy rechargeable hybrid supercapacitors. Int J Energy Res 45(5):8018–8029
Ahmed N et al (2019) Three-dimensional interconnected binder-free Mn–Ni–S nanosheets for high performance asymmetric supercapacitor devices with exceptional cyclic stability. ACS Appl Energy Mater 2(5):3717–3725
Afzal AM et al (2022) Enhanced the electrochemical performance and stability of supercapattery device with carbon nanotube/cobalt-manganese sulfide-based composite electrode material. Int J Energy Res. https://doi.org/10.1002/er.8745
Zaka A et al (2022) Facile synthesis of strontium copper phosphate (SrCuPO4) binary composite for the high-performance supercapattery devices. J Mater Sci Mater Electron. https://doi.org/10.1007/s10854-022-09363-7
Chen H et al (2013) Highly conductive NiCo 2 S 4 urchin-like nanostructures for high-rate pseudocapacitors. Nanoscale 5(19):8879–8883
Faisal MM et al (2022) Redox-active anomalous electrochemical performance of mesoporous nickel manganese sulfide nanomaterial as an anode material for supercapattery devices. Ceram Int 48(19):28565
Chen S et al (2017) Tuning the electrochemical behavior of Co x Mn3 – x sulfides by varying different Co/Mn ratios in supercapacitor. J Mater Sci 52(11):6687–6696
Chen H et al (2015) Bimetallic nickel cobalt selenides: a new kind of electroactive material for high-power energy storage. J Mater Chem A 3(47):23653–23659
Chen W, Xia C, Husam N (2014) Alshareef ‘one-step electrodeposited nickel cobalt sulfide nano sheet arrays for high-performance asymmetric Super capacitors.’ ACS Nano 8:9531–9541
Singh I, Birajdar B (2017) Synthesis, characterization and photocatalytic activity of mesoporous Na-doped TiO 2 nano-powder prepared via a solvent-controlled non-aqueous sol–gel route. RSC Adv 7(85):54053–54062
Hegazy H et al (2022) Synthesis of MXene and design the high-performance energy harvesting devices with multifunctional applications. Ceram Int 49:1710
Iqbal MW et al (2022) Facile hydrothermal synthesis of high-performance binary silver-cobalt-sulfide for supercapattery devices. J Energy Storage 52:104847
Hassan Hu et al (2022) Highly stable binary composite of nickel silver sulfide (NiAg2S) synthesized using the hydrothermal approach for high-performance supercapattery applications. Int J Energy Res 46(8):11346–11358
Iqbal MZ et al (2020) Capacitive and diffusion-controlled mechanism of strontium oxide based symmetric and asymmetric devices. J Energy Storage 27:101056
Zhang Y et al (2019) Fabrication of ZnCoS nanomaterial for high energy flexible asymmetric supercapacitors. Chem Eng J 374:347–358
Li H et al (2019) Zinc cobalt sulfide nanoparticles as high performance electrode material for asymmetric supercapacitor. Electrochim Acta 319:716–726
Zhang Z et al (2019) Free-standing NiCo2S4@ VS2 nanoneedle array composite electrode for high performance asymmetric supercapacitor application. J Alloys Compd 771:274–280
Chen H et al (2014) Facilely synthesized porous NiCo2O4 flowerlike nanostructure for high-rate supercapacitors. J Power Sources 248:28–36
Chen H et al (2016) Hierarchical NiCo2S4 nanotube@ NiCo2S4 nanosheet arrays on ni foam for high-performance supercapacitors. Chem Asian J 11(2):248–255
Li Y et al (2014) Ni–Co sulfide nanowires on nickel foam with ultrahigh capacitance for asymmetric supercapacitors. J Mater Chem A 2(18):6540–6548
Cao J et al (2018) One-pot synthesis of porous nickel–manganese sulfides with tuneable compositions for high-performance energy storage. J Solgel Sci Technol 85(3):629–637
Huang X et al (2017) Novel fabrication of Ni3S2/MnS composite as high performance supercapacitor electrode. J Alloys Compd 722:662–668
Wan H et al (2014) Direct formation of hedgehog-like hollow Ni–Mn oxides and sulfides for supercapacitor electrodes. Part Part Syst Charact 31(8):857–862
Cheng C et al (2017) Self-template synthesis of hollow ellipsoid Ni–Mn sulfides for supercapacitors, electrocatalytic oxidation of glucose and water treatment. Dalton Trans 46(16):5406–5413
Pan Q et al (2018) Synthesis of a MnS/Ni x S y composite with nanoparticles coated on hexagonal sheet structures as an advanced electrode material for asymmetric supercapacitors. RSC Adv 8(32):17754–17763
Acknowledgements
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R184), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
MI, MWI, and AMA worked on experiments, data collection, analysis, and interpretation of results. AMA, MI, and MMF performed the calculation and wrote the manuscript. AMA, MMF, MI, and MWI and HAA helped with the analysis and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Consent to publication
The submitted work should be original and should not have been published elsewhere in any form or language.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Figure 1 is replaced.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Imran, M., Iqbal, M.W., Afzal, A.M. et al. Synergetic electrochemical performance of Nix–Mnx sulfide-based binary electrode material for supercapattery devices. J Appl Electrochem 53, 1125–1136 (2023). https://doi.org/10.1007/s10800-022-01837-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01837-0