Abstract
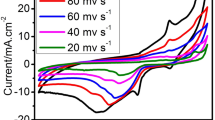
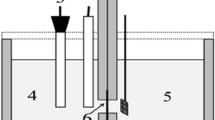
In this paper, we report the successful synthesis of multiwalled carbon nanotubes supported by gold-cobalt (AuxCo100 − x/MWCNT) nanoparticles to develop a novel electrocatalyst for anodic application in direct borohydride fuel cells. X-ray diffraction spectroscopy, X-ray photoelectron spectroscopy, field emission scanning electron microscopy and transmission electron microscopy are employed to examine the crystalline structure, chemical composition and morphology of the prepared electrocatalysts. Cyclic voltammetry, electrochemical impedance spectroscopy and chronoamperometry tests are used for electrocatalytic characterizations of developed electrocatalysts. Using AuxCo100 − x/MWCNT electrocatalyst, the fundamental kinetics parameters of electrocatalytic performance (current density, exchanged electrons number and apparent activation energy) for borohydride electrooxidation are investigated. Results reveal that among all bimetallic electrocatalysts, the Au74Co26/MWCNT electrocatalyst exhibits the highest specific activity (24.15 mA.cm− 2) and Au49Co51/MWCNT shows the highest mass activity (1127.03 mA.mg− 1) for \({BH}_{4}^{-}\) electrooxidation. The lowest apparent activation energy (8.22 kJmol− 1) and smallest charge transfer resistance (134.9 Ω) suggest the best electrocatalytic activity of Au74Co26/MWCNT electrocatalyst toward borohydride oxidation. The exchanged electron number for the Au74Co26/MWCNT electrocatalyst for borohydride electrooxidation at \(303 \text{K}\)is estimated as 4.70.
Graphical Abstract













Similar content being viewed by others
Data availability
Data will be made available on reasonable request.
References
de Leon CP, Walsh FC, Rose A, Lakeman JB, Browning DJ, Reeve RW (2007) A direct borohydride—acid peroxide fuel cell. J Power Sour 164:441–448. https://doi.org/10.1016/j.jpowsour.2006.10.069
Cheng H, Scott K (2006) Influence of operation conditions on direct borohydride fuel cell performance. J Power Sour 160:407–412. https://doi.org/10.1016/j.jpowsour.2006.01.097
Atwan MH, Macdonald CL, Northwood DO, Gyenge EL (2006) Colloidal au and Au-alloy catalysts for direct borohydride fuel cells: electrocatalysis and fuel cell performance. J Power Sour 158(1):36–44. https://doi.org/10.1016/j.jpowsour.2005.09.054
Li ZP, Liu BH, Arai K, Asaba K, Suda S (2004) Evaluation of alkaline borohydride solutions as the fuel for fuel cell. J Power Sour 158:36–44. https://doi.org/10.1016/j.jpowsour.2003.08.017
Dey S, Pramanik S, Chakraborty P, Rana DK, Basu S (2022) An easy synthesis of carbon-supported silver–cobalt bimetallic nanoparticles to study the electrocatalytic performance in alkaline borohydride fuel cell. J Appl Electrochem 52:247–258. https://doi.org/10.1007/s10800-021-01641-2
Indig ME, Snyder RN (1962) Sodium borohydride, an interesting anodic fuel(1). J Electrochem Soc 109(11):1104. https://doi.org/10.1149/1.2425247
Duan D, Feng J, You X, Zhou X, Wang Y, Chen L, Liu S (2021) Evaluation of Co–Au bimetallic nanoparticles as anode electrocatalyst for direct borohydride-hydrogen peroxide fuel cell. Ionics 27:3521–3532. https://doi.org/10.1007/s11581-021-04115-9
Yi L, Liu L, Liu X, Wang X, Yi W, He P, Wang X (2012) Carbon-supported Pt–Co nanoparticles as anode catalyst for direct borohydride-hydrogen peroxide fuel cell: electrocatalysis and fuel cell performance. Int J Hydrog Energy 37:12650–12658. https://doi.org/10.1016/j.ijhydene.2012.06.065
Gyenge E (2004) Electrooxidation of borohydride on platinum and gold electrodes: implications for direct borohydride fuel cells. Electrochim Acta 49:965–978. https://doi.org/10.1016/j.electacta.2003.10.008
Xu CW, Wang H, Shen PK, Jiang SP (2007) Highly ordered pd nanowire arrays as effective electrocatalysts for ethanol oxidation in direct alcohol fuel cells. Adv Mater 19(23):4091–4304. https://doi.org/10.1002/adma.200602911
Zhang Y, Janyasupab M, Liu CW, Li X, Xu J, Liu CC (2012) Three dimensional PtRh alloy porous nanostructures: tuning the atomic composition and controlling the morphology for the application of direct methanol fuel cells. Adv Funct Mater 22(17):3570–3575. https://doi.org/10.1002/adfm.201200678
Evans GE, Kordesch KV (1967) Hydrazine-air fuel cells: hydrazine-air fuel cells emerge from the laboratory. Science 158(3805):1148–1152. https://doi.org/10.1126/science.158.3805.1148
Han SB, Song YJ, Lee YW, Ko AR, Oh JK, Park KW (2011) High-performance hydrogen fuel cell using nitrate reduction reaction on a non-precious catalyst. Chem Commun 47(12):3496–3498. https://doi.org/10.1039/C0CC05534D
Yu SS, Lee TH, Oh TH (2022) Ag–Ni nanoparticles supported on multiwalled carbon nanotubes as a cathode electrocatalyst for direct borohydride–hydrogen peroxide fuel cells. Fuel 315:123151. https://doi.org/10.1016/j.fuel.2022.123151
Santos DMF, Sequeira CAC (2010) Cyclic voltammetry investigation of borohydride oxidation at a gold electrode. Electrochim Acta 55:6775–6781. https://doi.org/10.1016/j.electacta.2010.05.091
Nagle LC, Rohan JF (2011) Nanoporous gold anode catalyst for direct borohydride fuel cell. Int J Hydrog Energy 36(16):10319–10326. https://doi.org/10.1016/j.ijhydene.2010.09.077
Martins M, Šljukić B, Metin Ö, Sevim M, Sequeira CA, Şener T, Santos DM (2017) Bimetallic PdM (M = fe, ag, au) alloy nanoparticles assembled on reduced graphene oxide as catalysts for direct borohydride fuel cells. J Alloys Compd 718:204–214. https://doi.org/10.1016/j.jallcom.2017.05.058
Oh TH (2021) Gold-based bimetallic electrocatalysts supported on multiwalled carbon nanotubes for direct borohydride–hydrogen peroxide fuel cell. Renew Energy 163:930–938. https://doi.org/10.1016/j.renene.2020.09.028
He P, Wang X, Liu Y, Liu X, Yi L (2012) Comparison of electrocatalytic activity of carbon-supported Au–M (M = fe, Co, Ni, Cu and Zn) bimetallic nanoparticles for direct borohydride fuel cells. Int J Hydrog Energy 37(16):11984–11993. https://doi.org/10.1016/j.ijhydene.2012.05.054
Oh TH, Jang B, Kwon S (2014) Electrocatalysts supported on multiwalled carbon nanotubes for direct borohydride–hydrogen peroxide fuel cell. Int J Hydrog Energy 39(13):6977–6986. https://doi.org/10.1016/j.ijhydene.2014.02.117
Yi L, Wei W, Zhao C, Tian L, Liu J, Wang X (2015) Enhanced activity of Au–Fe/C anodic electrocatalyst for direct borohydride-hydrogen peroxide fuel cell. J Power Sour 285:325–333. https://doi.org/10.1016/j.jpowsour.2015.03.118
He P, Wang X, Fu P, Wang H, Yi L (2011) The studies of performance of the au electrode modified by Zn as the anode electrocatalyst of direct borohydride fuel cell. Int J Hydrog Energy 36(15):8857–8863. https://doi.org/10.1016/j.ijhydene.2011.04.128
Yan S, Gao L, Zhang S, Gao L, Zhang W, Li Y (2013) Investigation of AuNi/C anode catalyst for direct methanol fuel cells. Int J Hydrog Energy 38(29):12838–12846. https://doi.org/10.1016/j.ijhydene.2013.07.102
Yin D, Tang J, Bai R, Yin S, Jiang M, Kan Z, Li H, Wang F, Li C (2021) Cobalt phosphide (Co2P) with notable electrocatalytic activity designed for sensitive and selective enzymeless bioanalysis of hydrogen peroxide. Nanoscale Res Lett 16(1):1–10. https://doi.org/10.1186/s11671-020-03469-9
Pourbaix M (1974) Atlas of electrochemical equilibria in aqueous solution. NACE
Pei F, Wang Y, Wang X, He P, Chen Q, Wang X, Wang H, Yi L, Guo J (2010) Performance of supported Au–Co alloy as the anode catalyst of direct borohydride-hydrogen peroxide fuel cell. Int J Hydrog Energy 35(15):8136–8142. https://doi.org/10.1016/j.ijhydene.2010.01.016
Duan D, Liang J, Liu H, You X, Wei H, Wei G, Liu S (2015) The effective carbon supported core–shell structure of Ni@ au catalysts for electro-oxidation of borohydride. Int J Hydrog Energy 40(1):488–500. https://doi.org/10.1016/j.ijhydene.2014.10.101
Duan D, Liu H, You X, Wei H, Liu S (2015) Anodic behavior of carbon supported Cu@ Ag core–shell nanocatalysts in direct borohydride fuel cells. J Power Sour 293:292–300. https://doi.org/10.1016/j.jpowsour.2015.05.086
Wang Y, Lu X, Liu Y, Deng Y (2013) Silver supported on Co3O4 modified carbon as electrocatalyst for oxygen reduction reaction in alkaline media. Electrochem Commun 31:108–111. https://doi.org/10.1016/j.elecom.2013.03.017
Yang F, Cheng K, Wang G, Cao D (2015) Preparation of au nanosheets supported on ni foam and its electrocatalytic performance towards NaBH4 oxidation. Electrochim Acta 159:111–115. https://doi.org/10.1016/j.electacta.2015.01.171
Tamašauskaitė-Tamašiūnaitė L, Balčiūnaitė A, Šimkūnaitė D, Selskis A (2012) Self-ordered titania nanotubes and flat surfaces as a support for the deposition of nanostructured Au–Ni catalyst: enhanced electrocatalytic oxidation of borohydride. J Power Sour 202:85–91. https://doi.org/10.1016/j.jpowsour.2011.11.030
Chen D, Liu S, Li J, Zhao N, Shi C, Du X, Sheng J (2009) Nanometre Ni and core/shell Ni/Au nanoparticles with controllable dimensions synthesized in reverse microemulsion. J Alloys Compd 475(1–2):494–500. https://doi.org/10.1016/j.jallcom.2008.07.115
Dey S, Chakraborty P, Rana DK, Pramanik S, Basu S (2021) Surfactant-free synthesis of carbon-supported silver (Ag/C) nanobars as an efficient electrocatalyst for alcohol tolerance and oxidation of sodium borohydride in alkaline medium. SN Appl Sci 3(6):1–12. https://doi.org/10.1007/s42452-021-04601-9
Hansu TA, Caglar A, Sahin O, Kivrak H (2020) Hydrolysis and electrooxidation of sodium borohydride on novel CNT supported CoBi fuel cell catalyst. Mater Chem Phys 239:122031. https://doi.org/10.1016/j.matchemphys.2019.122031
Cui N, Luo JL (1998) Effects of oxide additions on electrochemical hydriding and dehydriding behavior of Mg2Ni-type hydrogen storage alloy electrode in 6 M KOH solution. Electrochim Acta 44:711–720. https://doi.org/10.1016/S0013-4686(98)00235-7
Abdel-Rehim SS, Khaled KF, Abd-Elshafi NS (2006) Electrochemical frequency modulation as a new technique for monitoring corrosion inhibition of iron in acid media by new thiourea derivative. Electrochim Acta 51(16):3269–3277. https://doi.org/10.1016/j.electacta.2005.09.018
Liu BH, Li ZP (2009) A review: hydrogen generation from borohydride hydrolysis reaction. J Power Sour 187(2):527–534. https://doi.org/10.1016/j.jpowsour.2008.11.032
Zhang D, Ye K, Cheng K, Cao D, Yin J, Xu Y, Wang G (2014) High electrocatalytic activity of cobalt–multiwalled carbon nanotubes–cosmetic cotton nanostructures for sodium borohydride electrooxidation. Int J Hydrog Energy 39(18):9651–9657. https://doi.org/10.1016/j.ijhydene.2014.04.113
Birry L, Lasia A (2004) Studies of the hydrogen evolution reaction on raney nickel—molybdenum electrodes. J Appl Electrochem 34(7):735–749. https://doi.org/10.1023/B:JACH.0000031161.26544.6a
Wang K, Lu J, Zhuang L (2005) Direct determination of diffusion coefficient for borohydride anions in alkaline solutions using chronoamperometry with spherical au electrodes. J Electroanal Chem 585(2):191–196. https://doi.org/10.1016/j.jelechem.2005.08.009
Acknowledgements
The authors acknowledge the centre of excellence (COE), NIT Durgapur, for the FESEM facility.
Funding
The authors acknowledge the DST&BT project (Project no. 326(Sanc.)/ST/P/S&T 16G-21/2018), Govt. of West Bengal, for financial support during this work.
Author information
Authors and Affiliations
Contributions
CKR: Investigation, Formal analysis, Writing—original draft, Validation. SD: Resources, Formal analysis, Validation. MH: Writing—original draft, Validation. RK: Resources, Validation. SB: Resources, Validation AKM: Supervision, Conceptualization, Methodology, Visualization, Validation, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This declaration is “not applicable”.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raul, C.K., Dey, S., Halder, M. et al. Synthesis of AuxCo100 − x/MWCNT nanoparticles as an efficient anode electrocatalyst for borohydride oxidation in alkaline medium. J Appl Electrochem 53, 977–990 (2023). https://doi.org/10.1007/s10800-022-01824-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01824-5