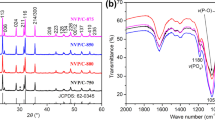
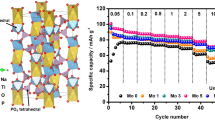
Abstract
The Na1+xAlxTi2−x(PO4)3/C (x = 0, 0.05, 0.10, 0.20) composites serving as anode for aqueous sodium ion battery are successfully synthesized through a facile sol–gel route. The results indicate that introduction of proper amount of aluminum has no obvious effect on the structure and morphology of NaTi2(PO4)3/C. Among the four synthesized samples, Na1.1Al0.1Ti1.9(PO4)3/C (NATP-0.10) exhibits the best electrochemical performance. NATP-0.10 delivers a discharge specific capacity of 115.8, 106.9, 98.4, and 89.1 mAh g−1 at 2, 5, 10, and 20 C rate, respectively, and still retains 114.7 mAh g−1 when the current density comes back to 2 C. Additionally, NATP-0.10 exhibits an initial discharge capacity of 102.9 mAh g−1 and still retains a reversible capacity of 90.1 mAh g−1 at 10 C rate after 200 cycles. Cyclic voltammetry and electrochemical impedance spectroscopy demonstrate the better electrochemical performance of NATP-0.10 is due to the faster sodium migration and enhanced electrochemical kinetics.
Graphical abstract
Al doping Na1+xAlxTi2−x(PO4)3/C (x = 0, 0.05, 0.10, 0.20) composites were firstly used as anodes in aqueous SIBs. The electrochemical performance of NaTi2(PO4)3/C has been improved by introducing a proper amount of Al.









Similar content being viewed by others
References
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367
Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652–657
Dunn B, Kamath H, Tarascon JM (2011) Electrical energy storage for the grid: a battery of choices. Science 334:928–935
Whitacre JF, Shanbhag S, Mohamed A, Polonsky A, Carlisle K, Gulakowski J, Wu W, Smith C, Cooney L, Blackwood D, Dandrea JC, Truchot C (2015) A polyionic, large-format energy storage device using an aqueous electrolyte and thick-format composite NaTi2(PO4)3/activated carbon negative electrodes. Energy Technol 3:796–798
Liu Y, Qiao Y, Zhang WX, Wang H, Chen KY, Zhu HP, Li Z, Huang YH (2015) Nanostructured alkali cation incorporated delta-MnO2 cathode materials for aqueous sodium-ion batteries. J Mater Chem A 3:7780–7785
Kim H, Hong J, Park KY, Kim H, Kim SW, Kang K (2014) Aqueous rechargeable Li and Na ion batteries. Chem Rev 114:11788–11827
Li Z, Young D, Xiang K, Carter WC, Chiang YM (2013) Towards high power high energy aqueous sodium-ion batteries: the NaTi2(PO4)3/Na0.44MnO2 system. Adv Energy Mater 3:290–294
Hou ZG, Li XN, Liang JW, Zhu YC, Qian YT (2015) An aqueous rechargeable sodium ion battery based on a NaMnO2–NaTi2(PO4)3 hybrid system for stationary energy storage. J Mater Chem A 3:1400–1404
Zhang XQ, Hou ZG, Li XN, Liang JW, Zhu YC, Qian YT (2016) Na-birnessite with high capacity and long cycle life for rechargeable aqueous sodium-ion battery cathode electrodes. J Mater Chem A 4:856–860
Whitacre JF, Tevar A, Sharma S (2010) Na4Mn9O18 as a positive electrode material for an aqueous electrolyte sodium-ion energy storage device. Electrochem Commun 12:463–466
Tevar AD, Whitacre JF (2010) Relating synthesis conditions and electrochemical performance for the sodium intercalation compound Na4Mn9O18 in aqueous electrolyte. J Electrochem Soc 157:A870–A875
Zhang Q, Liao CY, Zhai TY, Li HQ (2016) High performance LiNi0.5Mn1.5O4 cathode by Al-coating and Al3+-doping through a physical vapor deposition method. Electrochim Acta 191:470–478
Mason CW, Lange F (2015) Aqueous ion battery systems using sodium vanadium phosphate stabilized by titanium substitution. ECS Electrochem Lett 4:A79–A82
Wu XY, Cao YL, Ai XP, Qian JF, Yang HX (2013) A low-cost and environmentally benign aqueous rechargeable sodium-ion battery based on NaTi2(PO4)3–Na2NiFe(CN)6 intercalation chemistry. Electrochem Commun 31:145–148
Shao MM, Wang B, Liu MC, Wu C, Ke FS, Ai XP, Yang HX, Qian JF (2019) A high-voltage and cycle stable aqueous rechargeable Na-ion battery based on Na2Zn3[Fe(CN)6]2–NaTi2(PO4)3 intercalation chemistry. ACS Appl Energy Mater 2:5809–5815
Park SI, Gocheva I, Okada S, Yamaki J (2011) Electrochemical properties of NaTi2(PO4)3 anode for rechargeable aqueous sodium-ion batteries. J Electrochem Soc 158:A1067–A1070
He YW, Yuan H, Wu YX, Chen C, Yang S, Ai CC (2016) NaTi2(PO4)3/carbon and NaTi2(PO4)3/graphite composites as anode materials for aqueous rechargeable Na-ion batteries. Electrochemistry 84:705–708
Mohamed AI, Whitacre JF (2017) Capacity fade of NaTi2(PO4)3 in aqueous electrolyte solutions: relating pH increases to long term stability. Electrochim Acta 235:730–739
Wu W, Mohamed A, Whitacre JF (2013) Microwave synthesized NaTi2(PO4)3 as an aqueous sodium-ion negative electrode. J Electrochem Soc 160:A497–A504
Wu W, Yan JY, Wise A, Rutt A, Whitacre JF (2014) Using intimate carbon to enhance the performance of NaTi2(PO4)3 anode materials: carbon nanotubes vs graphite. J Electrochem Soc 161:A561–A567
Pang G, Yuan CZ, Nie P, Ding B, Zhu JJ, Zhang XG (2014) Synthesis of NASICON-type structured NaTi2(PO4)3–graphene nanocomposite as an anode for aqueous rechargeable Na-ion batteries. Nanoscale 6:6328–6334
Pang G, Nie P, Yuan CZ, Shen LF, Zhang XG, Zhu JJ, Ding B (2014) Enhanced performance of aqueous sodium-ion batteries using electrodes based on the NaTi2(PO4)3/MWNTs-Na0.44MnO2 system. Energy Technol 2:705–712
Zhao BD, Lin B, Zhang S, Deng C (2015) A frogspawn-inspired hierarchical porous NaTi2(PO4)3–C array for high-rate and long-life aqueous rechargeable sodium batteries. Nanoscale 7:18552–18560
Zhao BD, Wang QY, Zhang S, Deng C (2015) Self-assembled wafer-like porous NaTi2(PO4)3 decorated with hierarchical carbon as a high-rate anode for aqueous rechargeable sodium batteries. J Mater Chem A 3:12089–12096
Yao XL, Luo YX, Li Y, Li WW, Fang MH, Shui M, Shu J, Ren YL (2018) The investigation of NaTi2(PO4)3@C/Ag as a high-performance anode material for aqueous rechargeable sodium-ion batteries. Mater Res Bull 104:194–201
Liu ZX, An YF, Pang G, Dong SY, Xu CY, Mi CH, Zhang XG (2018) TiN modified NaTi2(PO4)3 as an anode material for aqueous sodium ion batteries. Chem Eng J 353:814–823
Mohamed AI, Sansone NJ, Kuei B, Washburn NR, Whitacre JF (2015) Using polypyrrole coating to improve cycling stability of NaTi2(PO4)3 as an aqueous Na-ion anode. J Electrochem Soc 162:A2201–A2207
Li XN, Zhu XB, Liang JW, Hou ZG, Wang Y, Lin N, Zhu YC, Qian YT (2014) Graphene-supported NaTi2(PO4)3 as a high rate anode material for aqueous sodium ion batteries. J Electrochem Soc 161(6):A1181–A1187
Zhang F, Li WF, Xiang XD, Sun ML (2017) Nanocrystal-assembled porous Na3MgTi(PO4)3 aggregates as highly stable anode for aqueous sodium-ion batteries. Chem Eur J 23:12944–12948
Huang ZQ, Yao M, Jiang Z, Meng WW, Li B, Li C, Li CC, He ZX, Meng W, Dai L, Wang L (2018) Impact of Fe doping on performance of NaTi2(PO4)3/C anode for aqueous lithium ion battery. Solid State Ion 327:123–128
Pérez-Estébanez M, Isasi-Marín J, Többens DM, Rivera-Calzada A, León C (2014) A systematic study of NASICON-type Li1+xMxTi2−x(PO4)3 (M: Cr, Al, Fe) by neutron diffraction and impedance spectroscopy. Solid State Ion 266:1–8
Zajac W, Tarach M, Trenczek-Zajac A (2017) Towards control over redox behaviour and ionic conductivity in LiTi2(PO4)3 fast lithium-ion conductor. Acta Mater 140:417–423
Arbi K, Mandal S, Rojo JM, Sanz J (2002) Dependence of ionic conductivity on composition of fast ionic conductors Li1+xTi2−xAlx(PO4)3, 0 ≤ x ≤ 0.7. A parallel NMR and electric impedance study. Chem Mater 14:1091–1097
Luo JY, Cui WJ, He P, Xia YY (2010) Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nat Chem 2:760–765
Wang DX, Liu Q, Chen CJ, Lia ML, Meng X, Bie XF, Wei YJ, Huang YH, Du F, Wang CZ, Chen G (2016) NASICON-structured NaTi2(PO4)3@C nanocomposite as the low operation voltage anode material for high-performance sodium-ion batteries. ACS Appl Mater Interfaces 8:2238–2246
Acknowledgments
The project was financially supported by the Guangdong Basic and Applied Basic Research Foundation, China (No. 2019A1515110825).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, J., Yang, L., Liu, H. et al. Effect of Al doping on electrochemical performance of NaTi2(PO4)3/C anode for aqueous sodium ion battery. J Appl Electrochem 52, 1563–1572 (2022). https://doi.org/10.1007/s10800-022-01726-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01726-6