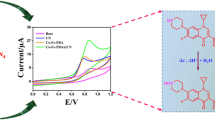
Abstract
Drug detection is crucial for monitoring the concentration of specific drugs in the human body or veterinary animals or the environment. In this study, the phyllosilicate Effenbergerite (BaCuSi4O10) was prepared by solid-state synthesis for electrochemical sensing of ciprofloxacin (CFX), one of the commonly used antibacterial drugs. Effenbergerite is naturally occurring and widely available and was used as a cost-effective material to fabricate a sensor for the rapid electrochemical detection and quantification of ciprofloxacin. The sensor was fabricated by modifying a glassy carbon electrode (GCE) with the synthesized Effenbergerite to obtain BaCuSi4O10/GCE. Powder X-ray diffraction (PXRD), Fourier-transform infra-red spectroscopy (FT-IR), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS) were used to characterize the Effenbergerite. Differential pulse voltammetry (DPV) showed that the optimum pH for the electrochemical detection of ciprofloxacin in phosphate buffer solution (PBS) electrolyte using the BaCuSi4O10/GCE sensor was pH 5.5. The linear range has been established to be 0.05–150 µM, and the limit of detection (LOD) was ca. 9 nM. The sensor was investigated in the presence of non-target interferents as well as in the presence of other drugs and was found to be selective toward ciprofloxacin. The pharmaceutical drug, Cifloc 500, analysis was done to establish the method’s reliability and a significantly low standard deviation of 1.74% was achieved. The results indicate that the sensor has application in detecting CFX in natural waters and wastewater effluents.
Graphic abstract









Similar content being viewed by others
Data Availability
Data obtained have been provided for transparency.
References
Rusch M, Spielmeyer A, Zorn H, Hamscher G (2019) Degradation and transformation of fluoroquinolones by microorganisms with special emphasis on ciprofloxacin. Appl Microbiol Biotechnol 103:6933–6948. https://doi.org/10.1007/s00253-019-10017-8
Campoli-Richards DM, Monk JP, Price A et al (1988) Ciprofloxacin. Drugs 35:373–447. https://doi.org/10.2165/00003495-198835040-00003
Farhangi M, Kobarfard F, Mahboubi A et al (2018) Preparation of an optimized ciprofloxacin-loaded chitosan nanomicelle with enhanced antibacterial activity. Drug Dev Ind Pharm 44:1273–1284. https://doi.org/10.1080/03639045.2018.1442847
Davis R, Markham A, Balfour JA (1996) Ciprofloxacin: An updated review of its pharmacology, therapeutic efficacy and tolerability. Drugs 51:1019–1074. https://doi.org/10.2165/00003495-199651060-00010
Phillips I, King A, Shannon K (2000) Comparative in-vitro properties of the Quinolones. In: Phillips I, King A, Shannon K (eds) The Quinolones. Elsevier, Amsterdam, pp 99–137
Gulen B, Demircivi P (2020) Adsorption properties of flouroquinolone type antibiotic ciprofloxacin into 2:1 dioctahedral clay structure: Box-Behnken experimental design. J Mol Struct. https://doi.org/10.1016/j.molstruc.2019.127659
Appelbaum PC, Hunter PA (2000) The fluoroquinolone antibacterials: past, present and future perspectives. Int J Antimicrob Agents 16:5–15. https://doi.org/10.1016/S0924-8579(00)00192-8
Jiang D (2018) 4-Quinolone derivatives and their activities against gram-negative pathogens. J Heterocycl Chem 55:2003–2018. https://doi.org/10.1002/jhet.3244
Wolfson JS, Hooper DC (1985) The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother 28:581. https://doi.org/10.1128/aac.28.4.581
Domagala JM (1994) Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J Antimicrob Chemother 33:685–706. https://doi.org/10.1093/jac/33.4.685
El-Kommos ME, Saleh GA, El-Gizawi SM, Abou-Elwafa MA (2003) Spectrofluorometric determination of certain quinolone antibacterials using metal chelation. Talanta 60:1033–1050. https://doi.org/10.1016/S0039-9140(03)00171-1
Brighty KE, Gootz TD (2000) Chemistry and mechanism of action of the Quinolone antibacterials. In: Andriole VT (ed) The Quinolones. Elsevier, San Diego, pp 33–97
Reece RJ, Maxwell A (1991) DNA gyrase: structure and function. Crit Rev Biochem Mol Biol 26:335–375. https://doi.org/10.3109/10409239109114072
Blandeau JM (1999) Expanded activity and utility of the new fluoroquinolones: A review. Clin Ther 21:3–40. https://doi.org/10.1016/S0149-2918(00)88266-1
Hooper DC, Jacoby GA (2016) Topoisomerase inhibitors: Fluoroquinolone mechanisms of action and resistance. Cold Spring Harb Perspect Med 6:a025320. https://doi.org/10.1101/cshperspect.a025320
Mitton-Fry MJ, Brickner SJ, Hamel JC et al (2017) Novel 3-fluoro-6-methoxyquinoline derivatives as inhibitors of bacterial DNA gyrase and topoisomerase IV. Bioorg Med Chem Lett 27:3353–3358. https://doi.org/10.1016/j.bmcl.2017.06.009
Anderson VE, Osheroff N (2001) Type II topoisomerases as targets for quinolone antibacterials turning Dr. Jekyll into Mr. Hyde. Curr Pharm Des 7:337–353. https://doi.org/10.2174/1381612013398013
Menzel R, Gellert M (1994) The biochemistry and biology of DNA gyrase. Adv Pharmacol 29:39. https://doi.org/10.1016/s1054-3589(08)60539-6
Belal F, Al-Majed AA, Al-Obaid AM (1999) Methods of analysis of 4-quinolone antibacterials. Talanta 50:765–786. https://doi.org/10.1016/S0039-9140(99)00139-3
Mitsuhashi S, Kojima T, Nakanishi N et al (1992) Fluorinated quinolones — new quinolone antimicrobials. Progress in Drug Research / Fortschritte der Arzneimittelforschung / Progrès des recherches pharmaceutiques. Birkhäuser Basel, Basel, pp 9–147
Neu HC (1994) Major advances in antibacterial Quinolone therapy. Adv Pharmacol. 29A:227–262
Krumpe PE, Cohn S, Garreltes J et al (1999) Intravenous and oral mono-or combination-therapy in the treatment of severe infections: Ciprofloxacin versus standard antibiotic therapy. J Antimicrob Chemother 43:117–128. https://doi.org/10.1093/jac/43.suppl_1.117
Ball P, Fernald A, Tillotson G (1998) Therapeutic advances of new fluoroquinolones. Expert Opin Investig Drugs 7:761–783. https://doi.org/10.1517/13543784.7.5.761
Auquer F, Cordón F, Gorina E et al (2002) Single-dose ciprofloxacin versus 3 days of norfloxacin in uncomplicated urinary tract infections in women. Clin Microbiol Infect 8:50–54. https://doi.org/10.1046/j.1198-743x.2001.00279.x-i1
Richard GA, Mathew CP, Kirstein JM et al (2002) Single-dose fluoroquinolone therapy of acute uncomplicated urinary tract infection in women: results from a randomized, double-blind, multicenter trial comparing single-dose to 3-day fluoroquinolone regimens. Urology 59:334–339. https://doi.org/10.1016/S0090-4295(01)01562-X
Andersson MI, MacGowan AP (2003) Development of the quinolones. J Antimicrob Chemother 51:1–11. https://doi.org/10.1093/jac/dkg212
Andriole VT (2000) The Quinolones. In: Andriole VT (ed) The Quinolones. Elsevier, San Diego, pp 477–495
Ball P (2000) Quinolone generations: natural history or natural selection? J Antimicrob Chemother 46:17–24. https://doi.org/10.1093/oxfordjournals.jac.a020889
Andriole VT (2000) The quinolones. Elsevier, Amsterdam
Andriole CL, Andriole VT (2002) Are all quinolones created equal. Mediguide Infect Dis 21:1–5
Gootz TD, Brighty KE (1996) Fluoroquinolone antibacterials: SAR, mechanism of action, resistance, and clinical aspects. Med Res Rev 16:433–486
Danner M-C, Robertson A, Behrends V, Reiss J (2019) Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci Total Environ 664:. https://doi.org/10.1016/j.scitotenv.2019.01.406
Singer AC, Shaw H, Rhodes V, Hart A (2016) Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front Microbiol. https://doi.org/10.3389/fmicb.2016.01728
Mompelat S, Le Bot B, Thomas O (2009) Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ Int. https://doi.org/10.1016/j.envint.2008.10.008
Berendsen BJA, Wegh RS, Memelink J et al (2015) The analysis of animal faeces as a tool to monitor antibiotic usage. Talanta. https://doi.org/10.1016/j.talanta.2014.09.022
Gomes MP, Richardi VS, Bicalho EM et al (2019) Effects of ciprofloxacin and roundup on seed germination and root development of maize. Sci Total Environ 651:2671–2678. https://doi.org/10.1016/j.scitotenv.2018.09.365
Liu J, Li X, Wang X (2019) Toxicological effects of ciprofloxacin exposure to Drosophila melanogaster. Chemosphere 237:124542. https://doi.org/10.1016/j.chemosphere.2019.124542
Archundia D, Duwig C, Lehembre F et al (2017) Antibiotic pollution in the Katari subcatchment of the Titicaca Lake: Major transformation products and occurrence of resistance genes. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2016.10.129
Osińska A, Korzeniewska E, Harnisz M, Niestępski S (2017) The prevalence and characterization of antibiotic-resistant and virulent Escherichia coli strains in the municipal wastewater system and their environmental fate. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2016.10.203
Banipal TS, Kaur R, Banipal PK (2018) Effect of sodium chloride on the interactions of ciprofloxacin hydrochloride with sodium dodecyl sulfate and hexadecyl trimethylammonium bromide: Conductometric and spectroscopic approach. J Mol Liq 255:113–121. https://doi.org/10.1016/j.molliq.2018.01.089
Rahim MA, Mahbub S, Ahsan SMA et al (2021) Conductivity, cloud point and molecular dynamics investigations of the interaction of surfactants with ciprofloxacin hydrochloride drug: Effect of electrolytes. J Mol Liq 322:114683. https://doi.org/10.1016/j.molliq.2020.114683
Obaydo RH, Alhaj Sakur A (2019) Spectrophotometric strategies for the analysis of binary combinations with minor component based on isoabsorptive point’s leveling effect: An application on ciprofloxacin and fluocinolone acetonide in their recently delivered co-formulation. Spectrochim Acta - Part A Mol Biomol Spectrosc 219:186–194. https://doi.org/10.1016/j.saa.2019.04.036
Taghizade M, Ebrahimi M, Fooladi E, Yoosefian M (2021) Simultaneous spectrophotometric determination of the residual of ciprofloxacin, famotidine, and tramadol using magnetic solid phase extraction coupled with multivariate calibration methods. Microchem J 160:105627. https://doi.org/10.1016/j.microc.2020.105627
Vakh C, Pochivalov A, Koronkiewicz S et al (2019) A chemiluminescence method for screening of fluoroquinolones in milk samples based on a multi-pumping flow system. Food Chem 270:10–16. https://doi.org/10.1016/j.foodchem.2018.07.073
Pavão e Pavão D, Nascimento Botelho C, Nunes Fernandes R et al (2020) A simple, cost-effective, and environmentally friendly method for determination of ciprofloxacin in drugs and urine samples based on electrogenerated chemiluminescence. Electroanalysis 32:1498–1506. https://doi.org/10.1002/elan.201900355
Bosma R, Devasagayam J, Singh A, Collier CM (2020) Microchip capillary electrophoresis dairy device using fluorescence spectroscopy for detection of ciprofloxacin in milk samples. Sci Rep 10:13548. https://doi.org/10.1038/s41598-020-70566-1
Turkie NS, Munshid HQ (2019) New approach for the determination of ciprofloxacin hydrochloride using fluorescence resonance energy transfer (FRET) and continuous flow injection analysis via ISNAG-fluorimeter. J Pharm Sci Res 11:1563–1570
Peixoto PS, Tóth IV, Barreiros L et al (2019) Screening of fluoroquinolones in environmental waters using disk-based solid-phase extraction combined to microplate fluorimetric determination and LC-MS/MS. Int J Environ Anal Chem 99:258–269. https://doi.org/10.1080/03067319.2019.1588969
Yu L, Liu M, Wang T, Wang X (2019) Development and application of a lateral flow colloidal gold immunoassay strip for the rapid quantification of ciprofloxacin in animal muscle. Anal Methods 11:3244–3251. https://doi.org/10.1039/c9ay00657e
Gezahegn T, Tegegne B, Zewge F, Chandravanshi BS (2019) Salting-out assisted liquid-liquid extraction for the determination of ciprofloxacin residues in water samples by high performance liquid chromatography-diode array detector. BMC Chem. https://doi.org/10.1186/s13065-019-0543-5
Karimi-Maleh H, Karimi F, Alizadeh M, Sanati AL (2020) Electrochemical sensors, a bright future in the fabrication of portable kits in analytical systems. Chem Rec 20:682–692
Burzo E (2007) Phyllosilicates. Springer, Berlin
Brown G (1984) Crystal structures of clay minerals and related phyllosilicates. Philos Trans R Soc London Ser A Math Phys Sci 311:221–240. https://doi.org/10.1098/rsta.1984.0025
Bailey SW, Brindley GW, Johns WD et al (1971) Summary of national and international recommendations on clay mineral nomenclature. Clays Clay Miner 19:129–132. https://doi.org/10.1346/CCMN.1971.0190210
Tadanaga K, Morita K, Mori K, Tatsumisago M (2013) Synthesis of monodispersed silica nanoparticles with high concentration by theStöber process. J Solgel Sci Technol 68:341–345. https://doi.org/10.1007/s10971-013-3175-6
Johnson-McDaniel D, Salguero TT (2014) Exfoliation of Egyptian blue and Han blue, two alkali earth copper silicate-based pigments. J Vis Exp. https://doi.org/10.3791/51686
Zanello P, Nervi C, de Biani FF (2007) Inorganic electrochemistry. Royal Society of Chemistry, Cambridge
Wang S, Peng T, Yang CF (2003) Electrochemical determination of interaction parameters for DNA and mitoxantrone in an irreversible redox process. Biophys Chem 104:239–248. https://doi.org/10.1016/S0301-4622(02)00371-X
Fotouhi L, Atoofi Z, Heravi MM (2013) Interaction of ciprofloxacin with DNA studied by spectroscopy and voltammetry at MWCNT/DNA modified glassy carbon electrode. Talanta 103:194–200. https://doi.org/10.1016/j.talanta.2012.10.032
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19–28. https://doi.org/10.1016/S0022-0728(79)80075-3
Bard AJ, Faulkner LR, Leddy J, Zoski CG (1980) Electrochemical methods: fundamentals and applications. John Wiley & Sons, New York
Pabst A (1959) Structures of some tetragonal sheet silicates. Acta Crystallogr 12:733–739
Saksena BD (1961) Infra-red absorption studies of some silicate structures. Trans Faraday Soc 57:242–258. https://doi.org/10.1039/TF9615700242
Wiedemann H-G, Berke H (2001) Chemical and physical investigations of Egyptian and Chinese blue and purple. Monum Sites 3:154–171. https://doi.org/10.11588/MONSTITES.2001.0.22351
McMillan PF, Piriou B (1983) Raman spectroscopic studies of silicate and related glass structure: a review. Bull Mineral 106:57–75. https://doi.org/bulmi_0180-9210_1983_act_106_1_7668
Warowna-Grześkiewicz M, Chodkowski J, Fijałek Z (1995) Electrochemical studies of some quinolone antibiotics. Part I. Qualitative analysis on mercury and carbon electrodes. Acta Pol Pharm 52:187–192
Zhang S, Wei S (2007) Electrochemical determination of ciprofloxacin based on the enhancement effect of sodium dodecyl benzene sulfonate. Bull Korean Chem Soc 28:543–546. https://doi.org/10.5012/bkcs.2007.28.4.543
Uslu B, Bozal B, Kuscu ME (2010) Anodic voltammetry of ciprofloxacin and its analytical applications. Open Chem Biomed Methods J 3:108–114. https://doi.org/10.2174/18750389010030100108
Osman NSE, Thapliyal N, Alwan WS et al (2015) Synthesis and characterization of Ba0.5Co0.5Fe2O4 nanoparticle ferrites: application as electrochemical sensor for ciprofloxacin. J Mater Sci Mater Electron 26:5097–5105. https://doi.org/10.1007/s10854-015-3036-x
Radi A, El-Sherif Z (2002) Determination of levofloxacin in human urine by adsorptive square-wave anodic stripping voltammetry on a glassy carbon electrode. Talanta 58:319–324. https://doi.org/10.1016/S0039-9140(02)00245-X
Girousi ST, Gherghi IC, Karava MK (2004) DNA-modified carbon paste electrode applied to the study of interaction between Rifampicin (RIF) and DNA in solution and at the electrode surface. J Pharm Biomed Anal 36:851–858. https://doi.org/10.1016/j.jpba.2004.08.034
Berg H, Horn G, Luthardt U, Ihn W (1981) Interaction of anthracycline antibiotics with biopolymers. Part V. Polarographic behavior and complexes with DNA. J Electroanal Chem 128:537–553. https://doi.org/10.1016/S0022-0728(81)80246-X
Anuar K, Tan WT, Atan MS et al (2007) Cyclic voltammetry study of copper tin sulfide compounds. Pacific J Sci Technol 8:252–260
Zhang F, Gu S, Ding Y et al (2013) A novel sensor based on electropolymerization of β-cyclodextrin and l-arginine on carbon paste electrode for determination of fluoroquinolones. Anal Chim Acta 770:53–61. https://doi.org/10.1016/j.aca.2013.01.052
Yuphintharakun N, Nurerk P, Chullasat K et al (2018) A nanocomposite optosensor containing carboxylic functionalized multiwall carbon nanotubes and quantum dots incorporated into a molecularly imprinted polymer for highly selective and sensitive detection of ciprofloxacin. Spectrochim Acta Part A Mol Biomol Spectrosc 201:382–391. https://doi.org/10.1016/j.saa.2018.05.034
Radičová M, Behúl M, Marton M et al (2017) Heavily boron doped diamond electrodes for ultra sensitive determination of ciprofloxacin in human urine. Electroanalysis 29:1612–1617. https://doi.org/10.1002/elan.201600769
Funding
The authors gratefully acknowledge research funds provided by the University of KwaZulu Natal and for the research supported in part by the National Research Foundation of South Africa (Grant Number: 132014), and the Eskom TESP program.
Author information
Authors and Affiliations
Contributions
GM: Conceptualization, methodology, experimental investigation, and writing original draft manuscript. VM: Conceptualization and draft manuscript editing. WEZ: Conceptualization, supervision, revised the manuscript, and research funding.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the research work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Sensors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muungani, G., Moodley, V. & van Zyl, W.E. Solid-state synthesis of the phyllosilicate Effenbergerite (BaCuSi4O10) for electrochemical sensing of ciprofloxacin antibiotic in pharmaceutical drug formulation. J Appl Electrochem 52, 285–297 (2022). https://doi.org/10.1007/s10800-021-01633-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-021-01633-2