Abstract
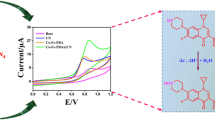
A needle-shaped perovskite, barium stannate (BaSnO3), was synthesized via a co-precipitation technique for the simultaneous electrochemical determination of antibiotic drug nitrofurantoin (NFTO) and pericardial drug nifedipine (NFP). The spectroscopic and microscopic result confirms that as-prepared BaSnO3 particles formed with desired crystalline nature, functional group, pore size, pore diameter, and fine needle-like morphology. The simultaneous electrochemical detection of the two pharmaceutical compounds was examined via cyclic voltammetry (CV) and differential pulse voltammetry (DPV) technique using BaSnO3-modified glassy carbon electrode (BaSnO3/GCE) at a potential range from +0.4 to − 1.2 V. The discrete and simultaneous detection of NFTO and NFP at the BaSnO3 sensor exhibits higher catalytic activity in terms of cathodic current and cathodic potential compared to bare GCE. DPV results of the BaSnO3 sensor provide improved linear ranges and limits of detection for NFTO (0.01–42.65 µM; 42.65–557.65 μM, 0.062 μM, respectively) and NFP (0.01–697.65 μM, 0.0168 μM, respectively). Besides, the BaSnO3-fabricated sensor exhibits good sensitivity, reproducibility, and repeatability. The modified electrode shows excellent recoveries of NFTO (97.0–100.7%) and NFP (98.7–101.3%) in plasma, urine, and milk samples with an acceptable relative standard deviation (RSD) of 1.6–4.8%.

Needle-shaped BaSnO3 perovskite material for simultaneous electrochemical sensing of pharmaceutical drugs.







Similar content being viewed by others
References
Xu JJ, Xu D, Wang ZL, Wang AG, Zhang LL, Zhang XB (2013) Synthesis of perovskite-based porous La0.75Sr0.25MnO3 nanotubes as a highly efficient electrocatalyst for rechargeable lithium-oxygen batteries. Angew Chem Int Ed 52:3887–3890
Grimaud A, May KL, Carlton CE, Lee YL, Risch M, Hong ET, Zhou J, Horn YS (2013) Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution. Nat Commun 4:2439
Kanhere P, Chen Z (2014) A review on visible light active perovskite-based photocatalysts. Molecules 19:19995–20022
Balamurugan C, Lee DW (2015) Perovskite hexagonal YMnO3 nanopowder as p-type semiconductor gas sensor for H2S detection. Sensors Actuators B Chem 221:857–886
Umari P, Mosconi M, Angelis FD (2014) Relativistic GW calculations on CH3NH3PbI3 and CH3NH3SnI3 perovskites for solar cell applications. Sci Rep 4:4467
Yang HM, Shi JX, Gong ML (2005) A novel red emitting phosphor Ca2SnO4: Eu3+. J Solid State Chem 178:917–920
Roth RS (1957) Classification of perovskite and other AB03-type compounds. J Res Natl Bur Stand 58:75–88
Muthukutty B, Karthik R, Chen SM, Abinaya M (2019) Designing novel perovskite-type strontium stannate (SrSnO3) and its potential as an electrode material for the enhanced sensing of anti-inflammatory drug mesalamine in biological samples. New J Chem 43:12264–12274
Liu HR, Yang JH, Xiang HJ, Gong XG, Wei SH (2013) Origin of the superior conductivity of perovskite Ba (Sr)SnO3. Appl Phys Lett 102(1–5):112109
Karuppiah C, Rani KK, Wang SF, Devasenathipathy R, Yang CC (2018) Dry particles coating preparation of highly conductive LaMnO3@C composite for the oxygen reduction reaction and hydrogen peroxide sensing. J Taiwan Inst Chem Eng 93:94–102
Wei CN, Karuppiah C, Yang CC, Shih JY, Lue SJ (2019) Bifunctional perovskite electrocatalyst and PVDF/PET/PVDF integrated split test cell for high performance Li-O2 battery. J Phys Chem Solids 133:67–78
Ahmadi E, Gholivand MB, Karami C (2020) Enzyme-less amprometric sensor manufactured using a NAfion-LaNiO3 nanocomposite for hydrogen peroxide. RSC Adv 10:23547–23465
Miao H, Wu X, Chen B, Wang Q, Wang F, Wang J, Zhang C, Zhang H, Yuan J, Zhang Q (2013) A-site deficient/excessive effect of LaMnO3 pervoskite as bifunctional oxygen catalyst for zinc-air batteries. Electrochim Acta:333–135566
Chang L, Li J, Le Z, Nie P, Guo Y, Wang H, Xu T, Xue X (2020) Pervoskite-type CaMnO3 anode material for highly efficient and stable lithium ion storage. J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2020.04.014
Liu Q, Li H, Li B, Wang W, Liu Q, Zhang Y, Dai J (2014) Structure and band gap engineering of Fe-doped SrSnO3 epitaxial films. EPL 108:37003
Doroftei C, Popa PD, Lacomi F (2012) Study of the influence of nickel ions substitutes in barium stannates used as humidity resistive sensors. Sensors Actuators A 173:24–29
Marikutsa A, Rumyantseva M, Baranchikov A, Gaskov A (2015) Nanocrystalline BaSnO3 as an alternative gas sensor material: surface reactivity and high sensitivity to SO2. Materials 8:6437–6454
Bhattacharya A, Jiang Y, Gao Q, Chu X, Dong Y, Liang S, Chakraborty AM (2019) Highly responsive and selective formaldehyde sensor based on La3+-doped barium stannate microtubes prepared by electrospinning. J Mater Res 34:2067–2077
Reddy GCV, Manorama SV, Rao VJ (2001) Preparation and characterization of barium stannate: application as a liquefied petroleum gas sensor. J Mater Sci Mater Electron 12:137–142
Cerda J, Arbiol J, Diaz R, Dezanneau G, Morante JR (2002) Synthesis of perovskite-type BaSnO3 particles obtained by a new simple wet chemical route based on a sol–gel process. Mater Lett 56:131–136
Moura KF, Chantelle L, Rosendo D, Longo E, Santos IMGD (2017) Effect of Fe3+ foping in the photocatalytic properties of BaSnO3 perovskite. Materials 20:317–324
Zhang W, Tang J, Ye J (2007) Structural, photocatalytic, and photophysical properties of perovskite MSnO3 (M = Ca, Sr, and Ba) Photocatalysts. J Mater Res 22:1859–1871
Kurre R, Bajpai S, Bajpai PK (2018) Synthesis, characterization, optical and transport properties of BaSnO3 synthesized by wet chemical route. Mater Sci Appl 09:92–110
Deepa AS, Vidya S, Manu PC, Solomon S, John A, Thomas JK (2012) Structural and optical characterization of BaSnO3 nanopowder synthesized through a novel combustion technique. J Alloys Compd 509:1830–1835
Lu W, Schmidt H (2007) Preparation and characterization of BaSnO3 powders by hydrothermal synthesis from tin oxide hydrate gel. J Mater Sci 42:10007–10013
Kovacic P, Somanathan R (2014) Nitroaromatic compounds: environmental toxicity, carcinogenicity, mutagenicity, therapy and mechanism. J Appl Toxicol 34:810–824
Yang T, Feng C, Zhao P, Wu Y, Ding Y, Wang G, Hu A (2020) Fluorescent electronic tongue supported with water-borne polyurethane for the discrimination of nitroaromatics in aqueous solution. J Mater Chem 8:2500–2506
Budi CS, Deka JR, Saikia D, Kao HM, Yang YC (2020) Ultrafine bimetallic Ag-doped Ni nanoparticles embedded in cage-type mesoporous silica SBA-16 as superior catalysts for conversion of toxic nitroaromatic compounds. J Hazard Mater 384:121270
Ritts RE (1990) Antibiotics as biological response modifiers. J Antimicrob Chemother 26:31–36
Wagenlehner FME, Naber KG (2006) Treatment of bacterial urinary tract infections: presence and future. Eur Urol 49:235–244
Tian YH, Kim HC, Cu JM, Kim YC (2005) Hepatoprotective constituents of cudrania tricuspidata. Arch Pharm Res 28(1):44–48
Kijima A, Ishii Y, Takasu S, Matsushita K, Kuroda K, Hibi D, Suzuki Y, Nohmi T, Umemura T (2015) Chemical structure-related mechanisms underlying in vivo genotoxicity induced by nitrofurantoin and its constituent moieties in gpt delta rats. Toxicology 331:125–135
McCalla DR (1983) Mutagenicity of nitrofuran derivatives. Environ Mutagen 5:745–765
Hoogenboom LAP, Kuiper HA (1997) The use of in vitro models for assessing the presence and safety of residues of xenobiotics in food. Trends Food Sci Technol 8(5):157–166
Sundaresan P, Karthik R, Chen SM, Kumar JV, Muthuraj V, Nagarajan ER (2019) Ultrasonication-assisted synthesis of sphere-like strontium cerate nanoparticles (SrCeO3 NPs) for the selective electrochemical detection of calcium channel antagonists nifedipine. Ultrason Sonochem 53:44–54
Jalili R, Amjadi M (2015) Surface molecular imprinting on silane-functionalized carbon dots for selective recognition of nifedipine. RSC Adv 5:74084–74090
Mokhtari B, Nematollahi D, Salehzadeh H (2018) Electrochemical simultaneous determination of nifedipine and its main metabolites dehydronifedepine using MWCNT modified glassy carbon electrode. J Mol Liq 264:543–549
Gaichore RR, Srivastava AK (2013) Voltammetric determination of nifedipine using a β-cyclodextrin modified multi-walled carbon nanotube paste electrode. Sensors Actuators B 188:1328–1337
Groom CA, Halasz A, Paquet L, Thiboutot S, Ampleman G, Hawari J (2005) Detection of nitroaromatic and cyclic nitramine compounds by cyclodextrin assisted capillary electrophoresis quadrupole ion trap mass spectrometry. J Chromatogr A 1072:73–82
Emmrich M (1993) Determination of RDX, 2,4,6-trinitrotoluene and other nitroaromatic compounds by high-performance liquid chromatography with photodiode-array detection. J Chromatogr 645:89–94
Moscoso FG, Almeida J, Sousaraei A, Costa TL, Silva AMG, Gonzalez JC, Silva LC, Pedrosa JM (2020) A lanthanide MOF immobilized in PMMA transparent flims as a selective fluorescence sensor for nitroaromatic explosive vapours. J Mater Chem C 8:3626–3630
Zyryanov GV, Kopchuk DS, Kovalev IS, Nosova EV, Rusinov VL, Chupakhin ON (2014) Chemosenoer for the detection of nitroaromatic compounds (explosives). Russ Chem Rev 83(9):783–819
Rohaizad N, Sofer Z, Pumera M (2020) Boron and nitrogen dopants in graphene have opposite effects on the electrochemical detection of explosive nitroaromatic compounds. Electrochem Commun 112:106660
Doroftei C, Popa PD, Iacomi F (2012) Preparation and study of structural properties of zinc doped barium stannate. J Optoelectron Adv Mater 14:413–417
Zhu L, Shao Z, Ye J, Zhang X, Pan X, Dai S (2016) Mesoporous BaSnO3 layer based perovskite solar cell. Chem Commun 52:970–973
Sukanya R, Ramki S, Chen SM, Karthik R (2020) Ultrasound treated cerium oxide/tin oxide (CeO2/SnO2) nanocatalyst: a feasible approach and enhanced electrode material for sensing of anti-inflammatory drug 5-aminosalicylic acid in biological samples. Anal Chim Acta 1096:76–88
Kumar JV, Karthik R, Chen SM, Chen KH (2018) Design of novel 3D flower-like neodymium molybdate: an efficient and challenging catalyst for sensing and destroying pulmonary toxicity antibiotic drug nitrofurantoin. Chem Eng J 346:11–23
Vangala VR, Chow PA, Tan RBH (2013) The solvates and salt of antibiotics agent, nitrofurantoin: structural, thermochemical and desolvation studies. CrystEngcomm 15:878–889
Babu VR, Rao KSVK, Lee YI (2010) Preparation and characterization of Nifedepine-loaded cellulose acetate butyrate based microspheres and their controlled release behaviour. Polym Bull 65:157–167
Velmurugan S, Palanisamy S, Yang TCK, Gochoo M, Chen SM (2020) Ultrasonic assisted functionalization of MWCNT and synergistic electrocatalytic effect of nano-hydroxyapatite incorporated MWCNT chitosan scaffolds for sensing nitrofurantoin. Ultrason Sonochem 62:104863
Jain R, Dwivedi A, Mishra R (2009) Stripping voltammetric dehaviour of toxic drug nitrofurantoin. J Hazard Mater 169:667–672
Aydogdu G, Gunendi G, Zeybek DK, Zeybek B, Pekyardimci S (2014) A novel electrochemical DNA biosensor on poly-(5-amino-2-mercapto-1,3,4-thiadiazole) modified glassy carbon electrode for the determination of nitrofurantoin. Sensors Actuators B 197:211–219
Figueroa SP, Ulloa PJ, Lueje AA, Squella JA (2013) Sensitive determination of nitrofurantoin by flow injection analysis using carbon nanofibre screen printed electrodes. Electroanalysis 25(6):1433–1438
Kor K, Zarei K (2013) β-Cyclodextrin incorporated carbon nanotube paste electrode as electrochemical sensor for nifedipine. Electroanalysis 25(6):1497–1504
Khairy M, Khorshed AA, Rashwan FA, Salah GA, Abdel-Wadood HM, Banks CE (2017) Simultaneous voltammetric determination of antihypertensive drugs nifedipine and atenolol utilising MgO nanoplatelet modified screen-printed electrodes in pharmaceuticals and human fluids. Sensors Actuators B 252:1045–1054
Senturk Z, Ozkan SA, Ozkan Y (1998) Electroanalytical study of nifedipine using activated glassy carbon electrode. J Pharm Biomed Anal 16:801–807
Shang L, Zhao F, Zeng B (2015) Highly dispersive hollow PdAg alloy nanoparticles modified ionic liquid functionalized graphene nanoribbons for electrochemical sensing of nifedipine. Electrochim Acta 168:330–336
Roy A, Das PP, Selvaraj P, Sundaram S, Devi P (2018) Perforated BaSnO3 nanorods exhibiting enhanced efficiency in dye sensitized solar cells. ACS Sustainable Chem. Eng 6:3299–3310
Funding
This project was supported by the Ministry of Science and Technology (MOST 107-2113-M-027-005-MY3), Taiwan. This work is also jointly supported by the projects from NTUT-NUST-109-01 and NSFC51872141, National Taipei University of Technology, and Nanjing University of Science and Technology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1636 kb).
Rights and permissions
About this article
Cite this article
Balamurugan, M., Alagumalai, K., Chen, TW. et al. Simultaneous electrochemical determination of nitrofurantoin and nifedipine with assistance of needle-shaped perovskite structure: barium stannate fabricated glassy carbon electrode. Microchim Acta 188, 19 (2021). https://doi.org/10.1007/s00604-020-04645-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04645-5