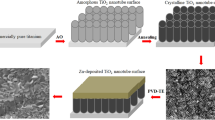
Abstract
Coating systems for titanium implants comprising TiO2 nanotubes and Se alloys (Cu2Se-pTNT and Ag2Se-pTNT) were developed in our previous work to prevent bacterial infections at the bone-implant interface. TiO2 nanotubes were grown on a Ti-based material by anodization and Cu2Se or Ag2Se alloys were incorporated by pulse electrodeposition. Preliminary in vitro investigations identified promising antibacterial properties. Two possible mechanisms of antibacterial activity are the release of bactericide metal ions and/or the formation of reactive oxygen species (ROS). In this work, the activity of the Se alloys for both effects was investigated by electrochemical measurements. Cu2Se and Ag2Se alloys were characterized by rotating disk electrode (RDE) measurements to investigate the reaction pathway for the oxygen reduction reaction (ORR) which may lead to peroxide species. RDE measurements, performed at potentials between 0 and − 0.8 V versus Ag/AgCl (sat. KCl) in an isotonic electrolyte (9 g L−1 NaCl, pH 7) at different oxygen partial pressures, showed that both, Cu2Se and Ag2Se, catalyze the two-electron transfer indicative of an indirect ORR with formation of H2O2 as an intermediate product. For comparison, bare Cu and Ag electrodes were also investigated. Under anodic conditions, selenide alloys slowly release antibacterial metal ions in a controllable and healthy amount for the body. The results demonstrate that these coatings can trigger antibacterial activity by two different mechanisms under both reducing and oxidizing conditions.
Graphic abstract












Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Lenguerrand E, Whitehouse MR, Beswick AD, Toms AD, Porter ML, Blom AW (2017) BMJ Open 7(7):e014056–e014056. https://doi.org/10.1136/bmjopen-2016-014056
Oliveira WF, Silva PMS, Silva RCS, Silva GMM, Machado G, Coelho LCBB, Correia MTS (2018) Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J Hosp Infect 98(2):111–117. https://doi.org/10.1016/j.jhin.2017.11.008
Weber M, Renkawitz T, Voellner F, Craiovan B, Greimel F, Worlicek M, Grifka J, Benditz A (2018) Revision surgery in total joint replacement is cost-intensive. Biomed Res Int 2018:8987104–8987104. https://doi.org/10.1155/2018/8987104
Shiels SM, Mangum LH, Wenke JC (2020) Revisiting the “race for the surface” in a pre-clinical model of implant infection. Eur Cell Mater 39:77–95. https://doi.org/10.22203/eCM.v039a05
Pham N-D, Duong M-M, Le M-V, Hoang HA, Pham L-K-O (2019) Preparation and characterization of antifungal colloidal copper nanoparticles and their antifungal activity against Fusarium oxysporum and Phytophthora capsici. C R Chim 22(11):786–793. https://doi.org/10.1016/j.crci.2019.10.007
Pauksch L, Hartmann S, Rohnke M, Szalay G, Alt V, Schnettler R, Lips KS (2014) Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater 10(1):439–449. https://doi.org/10.1016/j.actbio.2013.09.037
Gugala N, Lemire J, Chatfield-Reed K, Yan Y, Chua G, Turner RJ (2018) Using a chemical genetic screen to enhance our understanding of the antibacterial properties of silver. Genes 9(7):344. https://doi.org/10.3390/genes9070344
Rezvani Amin Z, Khashyarmanesh Z, Fazly Bazzaz BS (2016) Different behavior of Staphylococcus epidermidis in intracellular biosynthesis of silver and cadmium sulfide nanoparticles: more stability and lower toxicity of extracted nanoparticles. World J Microbiol Biotechnol 32(9):140. https://doi.org/10.1007/s11274-016-2110-8
Albers CE, Hofstetter W, Siebenrock KA, Landmann R, Klenke FM (2013) In vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrations. Nanotoxicology 7(1):30–36. https://doi.org/10.3109/17435390.2011.626538
Yoshida Y, Furuta S, Niki E (1993) Effects of metal chelating agents on the oxidation of lipids induced by copper and iron. Biochim Biophys Acta Lipids Lipid Metab 1210(1):81–88. https://doi.org/10.1016/0005-2760(93)90052-B
Grass G, Rensing C, Solioz M (2011) Metallic copper as an antimicrobial surface. Appl Environ Microbiol 77(5):1541–1547. https://doi.org/10.1128/aem.02766-10
Macomber L, Rensing C, Imlay JA (2007) Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J Bacteriol 189(5):1616–1626. https://doi.org/10.1128/jb.01357-06
Ladomersky E, Petris MJ (2015) Copper tolerance and virulence in bacteria. Metallomics 7(6):957–64. https://doi.org/10.1039/c4mt00327f
Osman D, Waldron KJ, Denton H, Taylor CM, Grant AJ, Mastroeni P, Robinson NJ, Cavet JS (2010) Copper homeostasis in Salmonella is atypical and copper-CueP is a major periplasmic metal complex. J Biol Chem 285(33):25259–68. https://doi.org/10.1074/jbc.M110.145953
Macomber L, Imlay JA (2009) The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. PNAS 106(20):8344–8349. https://doi.org/10.1073/pnas.0812808106
Caza M, Kronstad JW (2013) Shared and distinct mechanisms of iron acquisition by bacterial and fungal pathogens of humans. Front Cell Infect Microbiol 3:80. https://doi.org/10.3389/fcimb.2013.00080
Puig S, Askeland E, Thiele DJ (2005) Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell 120(1):99–110. https://doi.org/10.1016/j.cell.2004.11.032
Mchugh JP, Rodríguez-Quiñones F, Abdul-Tehrani H, Svistunenko DA, Poole RK, Cooper CE, Andrews SC (2003) Global iron-dependent gene regulation in Escherichia coli: a new mechanism for iron homoestasis. J Biol Chem 278(32):29478–29486. https://doi.org/10.1074/jbc.M303381200
Steunou AS, Bourbon M-L, Babot M, Durand A, Liotenberg S, Yamaichi Y, Ouchane S (2020) Increasing the copper sensitivity of microorganisms by restricting iron supply, a strategy for bio-management practices. Microb Biotechnol 13(5):1530–1545. https://doi.org/10.1111/1751-7915.13590
Šulce A, Bulke F, Schowalter M, Rosenauer A, Dringen R, Kunz S (2016) Reactive oxygen species (ROS) formation ability and stability of small copper (Cu) nanoparticles (NPs). RSC Adv 6(80):76980–76988. https://doi.org/10.1039/C6RA16599K
Flores-López LZ, Espinoza-Gómez H, Somanathan R (2019) Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J Appl Toxicol 39(1):16–26. https://doi.org/10.1002/jat.3654
Sun J, Boiadjieva-Scherzer T, Kronberger H, Staats K, Holinka J, Windhager R (2019) Surface modification of Ti6Al4V alloy for implants by anodization and electrodeposition. AIMS Mater Sci 6(5):713–729. https://doi.org/10.3934/matersci.2019.5.713
Rayman MP (2005) Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc 64(04):527–542. https://doi.org/10.1079/PNS2005467
Ravn-Haren G, Krath BN, Overvad K, Cold S, Moesgaard S, Larsen EH, Dragsted LO (2008) Effect of long-term selenium yeast intervention on activity and gene expression of antioxidant and xenobiotic metabolising enzymes in healthy elderly volunteers from the Danish Prevention of Cancer by Intervention by Selenium (PRECISE) Pilot Study. Br J Nutr 99(06):1190–1198. https://doi.org/10.1017/S0007114507882948
Lu J, Jiang C (2001) Antiangiogenic activity of selenium in cancer chemoprevention: metabolite-specific effects. Nutr Cancer 40(1):64–73. https://doi.org/10.1207/S15327914NC401_12
Chen X, Cai K, Fang J, Lai M, Hou Y, Li J, Luo Z, Hu Y, Tang L (2013) Fabrication of selenium-deposited and chitosan-coated titania nanotubes with anticancer and antibacterial properties. Colloids Surf B 103:149–157. https://doi.org/10.1016/j.colsurfb.2012.10.022
Holinka J, Pilz M, Kubista B, Presterl E, Windhager R (2013) Effects of selenium coating of orthopaedic implant surfaces on bacterial adherence and osteoblastic cell growth. Bone Joint J 95(5):678–682. https://doi.org/10.1302/0301-620x.95b5.31216
Liu W, Golshan NH, Deng X, Hickey DJ, Zeimer K, Li H, Webster TJ (2016) Selenium nanoparticles incorporated into titania nanotubes inhibit bacterial growth and macrophage proliferation. Nanoscale 8(34):15783–15794. https://doi.org/10.1039/c6nr04461a
Xu Z, Lai Y, Wu D, Huang W, Huang S, Zhou L, Chen J (2015) Increased mesenchymal stem cell response and decreased staphylococcus aureus adhesion on titania nanotubes without pharmaceuticals. Biomed Res Int 2015:9. https://doi.org/10.1155/2015/172898
Zhou R, Zheng Y, Jaroniec M, Qiao S-Z (2016) Determination of the electron transfer number for the oxygen reduction reaction: from theory to experiment. ACS Catal 6(7):4720–4728. https://doi.org/10.1021/acscatal.6b01581
Dotel UR, Sydnes MO, Urkedal H, Hemmingsen T (2018) The effects of acidic, alkaline, and neutral anolytes on electrochemical seawater deoxygenation. Appl Sci 8(11):2280
Van Stroe AJ, Janssen LJJ (1993) Determination of the diffusion coefficient of oxygen in sodium chloride solutions with a transient pulse technique. Anal Chim Acta 279(2):213–219. https://doi.org/10.1016/0003-2670(93)80320-K
Jamnongwong M, Loubiere K, Dietrich N, Hébrard G (2010) Experimental study of oxygen diffusion coefficients in clean water containing salt, glucose or surfactant: consequences on the liquid-side mass transfer coefficients. Chem Eng J 165(3):758–768. https://doi.org/10.1016/j.cej.2010.09.040
Zhang H-L, Han S-J (1996) Viscosity and Density of Water + Sodium Chloride + Potassium Chloride Solutions at 298.15 K. J Chem Eng Data 41(3):516–520. https://doi.org/10.1021/je9501402
Zoski CG (2007) Handbook of electrochemistry. Elsevier, Amsterdam
King F, Litke CD, Tang Y (1995) Effect of interfacial pH on the reduction of oxygen on copper in neutral NaClO4 solution. J Electroanal Chem 384(1):105–113. https://doi.org/10.1016/0022-0728(94)03704-7
Whipple GC, Whipple MC (1911) Solubility of oxygen in sea water. J Am Chem Soc 33(3):362–365. https://doi.org/10.1021/ja02216a012
Fabregat-Santiago F, Mora-Seró I, Garcia-Belmonte G, Bisquert J (2003) Cyclic voltammetry studies of nanoporous semiconductors. capacitive and reactive properties of nanocrystalline TiO2 electrodes in aqueous electrolyte. J Phys Chem B 107(3):758–768. https://doi.org/10.1021/jp0265182
King F, Quinn MJ, Litke CD (1995) Oxygen reduction on copper in neutral NaCl solution. J Electroanal Chem 385(1):45–55. https://doi.org/10.1016/0022-0728(94)03705-8
Blizanac BB, Ross PN, Marković NM (2006) Oxygen reduction on silver low-index single-crystal surfaces in alkaline solution: rotating ring DiskAg(hkl) studies. J Phys Chem B 110(10):4735–4741. https://doi.org/10.1021/jp056050d
Blizanac BB, Ross PN, Marković NM (2007) Oxygen electroreduction on Ag(111): the pH effect. Electrochim Acta 52:2264–2271. https://doi.org/10.1016/j.electacta.2006.06.047
Šepa D, Vojnovíc M, Damjanovic A (1970) Oxygen reduction at silver electrodes in alkaline solutions. Electrochimi Acta 15(8):1355–1366. https://doi.org/10.1016/0013-4686(70)80055-X
Lützenkirchen-Hecht D, Strehblow HH (1998) Surface analytical investigations of the electrochemical double layer on silver electrodes in alkaline media. Electrochim Acta 43(19):2957–2968. https://doi.org/10.1016/S0013-4686(98)00036-X
Hecht D, Strehblow HH (1997) XPS investigations of the electrochemical double layer on silver in alkaline chloride solutions. J Electroanal Chem 440(1):211–217. https://doi.org/10.1016/S0022-0728(97)80058-7
Massaccesi S (1993) Cathodic deposition of copper selenide films on tin oxide in sulfate solutions. J Electrochem Soc 140(9):2540. https://doi.org/10.1149/1.2220858
Rong F, Bai Y, Chen T, Zheng W (2012) Chemical synthesis of Cu2Se nanoparticles at room temperature. Mater Res Bull 47(1):92–95. https://doi.org/10.1016/j.materresbull.2011.09.026
Wei W, Zhang S, Fang C, Zhao S, Jin B, Wu J, Tian Y (2008) Electrochemical behavior and electrogenerated chemiluminescence of crystalline CuSe nanotubes. Solid State Sci 10(5):622–628. https://doi.org/10.1016/j.solidstatesciences.2007.10.010
Riha SC, Johnson DC, Prieto AL (2011) Cu2Se nanoparticles with tunable electronic properties due to a controlled solid-state phase transition driven by copper oxidation and cationic conduction. J Am Chem Soc 133(5):1383–1390. https://doi.org/10.1021/ja106254h
Institute of Medicine (2006) Dietary reference intakes: the essential guide to nutrient requirements. The National Academies Press, Washington
Petrov GV, Belenkii AM (2005) Interactions in the silver selenide-water system. Russ J Appl Chem 78(1):53–56. https://doi.org/10.1007/s11167-005-0230-2
World Health Organization (2003) Silver in drinking-water. World Health Organization, Geneva
Lafuente B, Downs RT, Yang H, Stone N (2015) The power of databases: the RRUFF project. In: Highlights in mineralogical crystallography. W. De Gruyter, Berlin
Acknowledgements
The authors want to thank Voestalpine and Roland Steger for the ICP-MS measurements.
Funding
These investigations were performed with support of the Austrian Science Foundation FFG (Grant 4253627) and the governments of Lower Austria and Upper Austria in the COMET program framework.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, J., Hämmerle, A., Fafilek, G. et al. Electrochemical investigation for understanding the bactericidal effect of Cu2Se and Ag2Se for biomedical applications. J Appl Electrochem 52, 1–15 (2022). https://doi.org/10.1007/s10800-021-01617-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-021-01617-2