Abstract
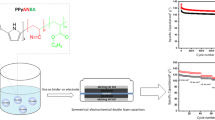
An eco-friendly conductive water-dispersed polymer binder was used in lithium-ion batteries (LIBs) for the first time. A conductive polypyrrole (PPy) was copolymerized successfully into an adhesive poly(acrylonitrile/butyl acrylate) (PANBA) emulsified binder to impart two important properties to the PANBA-PPy binder system simultaneously: adhesion and electronic conduction. These two properties ultimately help the active materials of the LIB to exert better electrochemical performance. The copolymers were synthesized via two-step polymerization with different weights of pyrrole monomer. With the incorporation of PPy, the electrical resistance of the copolymer was reduced significantly, and the increase in PPy content of the binder system also decreased the resistance. The electrochemical performance of the conductive copolymers as a binder for the Li4Ti5O12 (LTO) anodes of lithium-ion batteries was superior to the non-conductive PANBA binder. At relatively low charge/discharge current densities, the more conductive PANBA-PPy4 binder was favorable to the cyclic performance of the LTO electrodes, whereas the PANBA-PPy2 sample has prominent high rate performance at a current density of 10 C because of its adhesive properties and conductive characteristics.
Graphic Abstract







Similar content being viewed by others
Reference
Zuo X, Zhu J, Müller-Buschbaum P, Cheng Y-J (2017) Silicon based lithium-ion battery anodes: a chronicle perspective review. Nano Energy 31:113–143. https://doi.org/10.1016/j.nanoen.2016.11.013
Manthiram A (2017) An outlook on lithium ion battery technology. ACS Cent Sci 3:1063–1069. https://doi.org/10.1021/acscentsci.7b00288
Hu B, Shkrob IA, Zhang S et al (2018) The existence of optimal molecular weight for poly(acrylic acid) binders in silicon/graphite composite anode for lithium-ion batteries. J Power Sources 378:671–676. https://doi.org/10.1016/j.jpowsour.2017.12.068
Cao P-F, Naguib M, Du Z et al (2018) Effect of binder architecture on the performance of silicon/graphite composite anodes for lithium ion batteries. ACS Appl Mater Interfaces 10:3470–3478. https://doi.org/10.1021/acsami.7b13205
Gendensuren B, Oh E-S (2018) Dual-crosslinked network binder of alginate with polyacrylamide for silicon/graphite anodes of lithium ion battery. J Power Sources 384:379–386. https://doi.org/10.1016/j.jpowsour.2018.03.009
Magasinski A, Zdyrko B, Kovalenko I et al (2010) Toward efficient binders for Li-ion battery Si-based anodes: polyacrylic acid. ACS Appl Mater Interfaces 2:3004–3010. https://doi.org/10.1021/am100871y
Komaba S, Yabuuchi N, Ozeki T et al (2012) Comparative study of sodium polyacrylate and poly(vinylidene fluoride) as binders for high capacity Si–graphite composite negative electrodes in Li-ion batteries. J Phys Chem C 116:1380–1389. https://doi.org/10.1021/jp204817h
Hays KA, Ruther RE, Kukay AJ et al (2018) What makes lithium substituted polyacrylic acid a better binder than polyacrylic acid for silicon-graphite composite anodes? J Power Sources 384:136–144. https://doi.org/10.1016/j.jpowsour.2018.02.085
Zhu X, Zhang F, Zhang L et al (2018) A highly stretchable cross-linked polyacrylamide hydrogel as an effective binder for silicon and sulfur electrodes toward durable lithium-ion storage. Adv Funct Mater 28:1705015. https://doi.org/10.1002/adfm.201705015
Choi S, Kwon T, Coskun A, Choi JW (2017) Highly elastic binders integrating polyrotaxanes for silicon microparticle anodes in lithium ion batteries. Science 357:279–283. https://doi.org/10.1126/science.aal4373
Kovalenko I, Zdyrko B, Magasinski A et al (2011) A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 334:75–79. https://doi.org/10.1126/science.1209150
De Giorgio F, La Monaca A, Dinter A et al (2018) Water-processable Li4Ti5O12 electrodes featuring eco-friendly sodium alginate binder. Electrochim Acta 289:112–119. https://doi.org/10.1016/j.electacta.2018.09.017
Liu G, Xun S, Vukmirovic N et al (2011) Polymers with tailored electronic structure for high capacity lithium battery electrodes. Adv Mater 23:4679–4683. https://doi.org/10.1002/adma.201102421
Han S-W, Kim S-J, Oh E-S (2014) Significant performance enhancement of Li4Ti5O12 electrodes using a graphene-polyvinylidene fluoride conductive composite binder. J Electrochem Soc 161:A587–A592. https://doi.org/10.1149/2.035404jes
Ma X, Zou S, Tang A et al (2018) Three-dimensional hierarchical walnut kernel shape conducting polymer as water soluble binder for lithium-ion battery. Electrochim Acta 269:571–579. https://doi.org/10.1016/j.electacta.2018.03.031
Zeng W, Wang L, Peng X et al (2018) Enhanced ion conductivity in conducting polymer binder for high-performance silicon anodes in advanced lithium-ion batteries. Adv Energy Mater 8:1702314. https://doi.org/10.1002/aenm.201702314
Zhao H, Wei Y, Wang C et al (2018) Mussel-inspired conductive polymer binder for Si-alloy anode in lithium-ion batteries. ACS Appl Mater Interfaces 10:5440–5446. https://doi.org/10.1021/acsami.7b14645
Zhao H, Du A, Ling M et al (2016) Conductive polymer binder for nano-silicon/graphite composite electrode in lithium-ion batteries towards a practical application. Electrochim Acta 209:159–162. https://doi.org/10.1016/j.electacta.2016.05.061
Jeschull F, Brandell D, Wohlfahrt-Mehrens M, Memm M (2017) Water‐soluble binders for lithium‐ion battery graphite electrodes: slurry rheology, coating adhesion, and electrochemical performance. Energy Technol 5:2108–2118. https://doi.org/10.1002/ente.201700200
Wang R, Feng L, Yang W et al (2017) Effect of different binders on the electrochemical performance of metal oxide anode for lithium-ion batteries. Nanoscale Res Lett.https://doi.org/10.1186/s11671-017-2348-6
Zheng H, Liu G, Song X et al (2010) Optimization of ratio and amount of CMC/SBR binder for a graphite anode. 218th ECS Meeting Abstracts, 200
Nguyen MHT, Oh E-S (2013) Application of a new acrylonitrile/butylacrylate water-based binder for negative electrodes of lithium-ion batteries. Electrochem Commun 35:45–48. https://doi.org/10.1016/j.elecom.2013.07.042
Nguyen MHT, Oh E-S (2015) Improvement of the characteristics of poly(acrylonitrile–butylacrylate) water-dispersed binder for lithium-ion batteries by the addition of acrylic acid and polystyrene seed. J Electroanal Chem 739:111–114. https://doi.org/10.1016/j.jelechem.2014.12.026
Lee K, Kim T-H (2018) Poly(aniline-co-anthranilic acid) as an electrically conductive and mechanically stable binder for high-performance silicon anodes. Electrochim Acta 283:260–268. https://doi.org/10.1016/j.electacta.2018.06.175
Luo Y, Guo R, Li T et al (2018) Applications of polyaniline for Li-ion batteries, Li-sulfur batteries and supercapacitors. ChemSusChem. https://doi.org/10.1002/cssc.201802186
Gao H, Lu Q, Yao Y et al (2017) Significantly raising the cell performance of lithium sulfur battery via the multifunctional polyaniline binder. Electrochim Acta 232:414–421. https://doi.org/10.1016/j.electacta.2017.02.160
Das PR, Gräfenstein A, Ledwoch D et al (2014) Conducting polymers as binder additives for cathodes in Li ion battery. ECS Trans 63:31–43. https://doi.org/10.1149/06301.0031ecst
Zhong H, He A, Lu J et al (2016) Carboxymethyl chitosan/conducting polymer as water-soluble composite binder for LiFePO4 cathode in lithium ion batteries. J Power Sources 336:107–114. https://doi.org/10.1016/j.jpowsour.2016.10.041
Lee K, Lim S, Tron A et al (2016) Polymeric binder based on PAA and conductive PANI for high performance silicon-based anodes. RSC Adv 6:101622–101625. https://doi.org/10.1039/C6RA23805J
Vernitskaya TV, Efimov ON (1997) Polypyrrole: a conducting polymer; its synthesis, properties and applications. Russ Chem Rev 66:443. https://doi.org/10.1070/RC1997v066n05ABEH000261
Maruthamuthu S, Chandrasekaran J, Manoharan D, Magesh R (2015) Conductivity and dielectric analysis of Nanocolloidal polypyrrole particles functionalized with higher weight percentage of poly(styrene sulfonate) using the dispersion polymerization method. J Polym Eng 37:1–12. https://doi.org/10.1515/polyeng-2015-0321
Fu Y, Su Y-S, Manthiram A (2012) Sulfur-polypyrrole composite cathodes for lithium-sulfur batteries. J Electrochem Soc 159:A1420–A1424. https://doi.org/10.1149/2.027209jes
Li C, Tan J, Li H et al (2015) Fast magnetic-field-induced formation of one-dimensional structured chain-like materials via sintering of Fe3O4/poly(styrene-co-n-butyl acrylate-co-acrylic acid) hybrid microspheres. RSC Adv 5:28735–28742. https://doi.org/10.1039/C4RA16809G
Wu H, Bremner DH, Li H et al (2016) A novel multifunctional biomedical material based on polyacrylonitrile: preparation and characterization. Mater Sci Eng C 62:702–709. https://doi.org/10.1016/j.msec.2016.02.026
Geißler U, Hallensleben ML, Toppare L (1991) Conducting polymer composites of poly(N-methylpyrrole) and poly(bisphenol-A-carbonate). Adv Mater 3:104–106. https://doi.org/10.1002/adma.19910030207
Lee B-R, Oh E-S (2013) Effect of molecular weight and degree of substitution of a sodium-carboxymethyl cellulose binder on Li4Ti5O12 anodic performance. J Phys Chem C 117:4404–4409. https://doi.org/10.1021/jp311678p
Chou S-L, Wang J-Z, Liu H-K, Dou S-X (2011) Rapid synthesis of Li4Ti5O12 microspheres as anode materials and its binder effect for lithium-ion battery. J Phys Chem C 115:16220–16227. https://doi.org/10.1021/jp2039256
Acknowledgements
The study was supported by the 2019 research fund of the University of Ulsan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qi, Y., Nguyen, M.H.T. & Oh, ES. Enhancement of the lithium titanium oxide anode performance by the copolymerization of conductive polypyrrole with poly(acrylonitrile/butyl acrylate) binder. J Appl Electrochem 50, 431–438 (2020). https://doi.org/10.1007/s10800-020-01401-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-020-01401-8