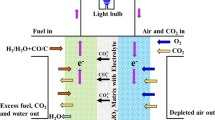
Abstract
Molten carbonate direct carbon fuel cells (MC-DCFCs) are among the most promising devices for high-efficiency energy conversion and clean power generation from coal. Many studies have focused on the anode performance, while little attention was paid to the cathode. In the present study, we comprehensively investigate the cathode polarization performance, revealing the reactions taken place and the impact of several important operation parameters on reaction rate. The results show that the reduction of inputted gases is the primary reaction at moderate cathodic polarization of a gold electrode, which could benefit from incorporation of an optimal O2/CO2 molar ratio of 1/2, increase of the gas flux or reaction temperature to a certain extent. Interestingly, with a sufficient overpotential applied, an unexpected carbon deposition phenomenon on the cathode is observed. To give an insight into this reaction, several material characterization techniques and electrochemical tests are conducted to analyze the composition and formation conditions of the deposit, respectively. It shows that the carbon deposition is resulted from the reduction of carbonate ions in the electrolyte, which occurs when the cathode potential reaches a critical value of −1.5 V, corresponding to a current density of −32 mA cm−2. To avoid the contamination of the cathode surface by the carbon deposition when operating an MC-DCFC, a feasible strategy is using a sufficiently large cathode to keep the cathodic current density as well as overpotential below the critical value.
Graphical Abstract












Similar content being viewed by others
References
Cao D, Sun Y, Wang G (2007) Direct carbon fuel cell: fundamentals and recent developments. J Power Sources 167:250–257. https://doi.org/10.1016/j.jpowsour.2007.02.034
Edison TA (1891) Process of and apparatus for generating electricity: Google Patents
Jacques WW (1896) Method of converting potential energy of carbon into electrical energy: Google Patents
Li X, Zhu Z, Chen J, De Marco R, Dicks A, Bradley J, Lu G (2009) Surface modification of carbon fuels for direct carbon fuel cells. J Power Sources 186:1–9. https://doi.org/10.1016/j.jpowsour.2008.09.070
Zecevic S, Patton EM, Parhami P (2004) Carbon–air fuel cell without a reforming process. Carbon 42:1983–1993. https://doi.org/10.1016/j.carbon.2004.03.036
Cherepy NJ, Krueger R, Fiet KJ, Jankowski AF, Cooper JF (2005) Direct conversion of carbon fuels in a molten carbonate fuel cell. J Electrochem Soc 152:A80–A87. https://doi.org/10.1149/1.1836129
Nabae Y, Pointon KD, Irvine JTS (2009) Ni/C slurries based on molten carbonates as a fuel for hybrid direct carbon fuel cells. J Electrochem Soc 156:B716–B720. https://doi.org/10.1149/1.3110862
Hackett GA, Zondlo JW, Svensson R (2007) Evaluation of carbon materials for use in a direct carbon fuel cell. J Power Sources 168:111–118. https://doi.org/10.1016/j.jpowsour.2007.02.021
Liu Q, Tian Y, Xia C, Thompson LT, Liang B, Li Y (2008) Modeling and simulation of a single direct carbon fuel cell. J Power Sources 185:1022–1029. https://doi.org/10.1016/j.jpowsour.2008.08.100
Cooper JF, Cherepy N, Krueger RL (2005) Tilted fuel cell apparatus: Google Patents
Cooper J (2003) Presented in direct carbon fuel cell workshop. NETL, Pittsburg
Cooper JF (2003) Reactions of the carbon anode in molten carbonate electrolyte direct carbon fuel cell workshop. NETL, Pittsburgh
Guo L, Calo JM, Kearney C, Grimshaw P (2014) The anodic reaction zone and performance of different carbonaceous fuels in a batch molten hydroxide direct carbon fuel cell. Appl Energy 129:32–38
Kacprzak A, Kobyłecki R, Włodarczyk R, Bis Z (2014) The effect of fuel type on the performance of a direct carbon fuel cell with molten alkaline electrolyte. J Power Sources 255:179–186. https://doi.org/10.1016/j.jpowsour.2014.01.012
Kacprzak A, Kobylecki R, Wlodarczyk R, Bis Z (2016) Efficiency of non-optimized direct carbon fuel cell with molten alkaline electrolyte fueled by carbonized biomass. J Power Sources 321:233–240
Tao T (2004) Carbon-oxygen fuel cell: Google Patents
Huijsmans JPP, Van Berkel FPF, Christie GM (1998) Intermediate temperature SOFC - a promise for the 21st century. J Power Sources 71:107–110. https://doi.org/10.1016/s0378-7753(97)02789-4
Huang KQ, Goodenough JB (2000) A solid oxide fuel cell based on Sr- and Mg-doped LaGaO3 electrolyte: the role of a rare-earth oxide buffer. J Alloys Compd 303:454–464. https://doi.org/10.1016/s0925-8388(00)00626-5
Huang KQ, Wan JH, Goodenough JB (2001) Increasing power density of LSGM-based solid oxide fuel cells using new anode materials. J Electrochem Soc 148:A788–A794. https://doi.org/10.1149/1.1378289
Xia C, Li Y, Tian Y, Liu Q, Zhao Y, Jia L, Li Y (2009) A high performance composite ionic conducting electrolyte for intermediate temperature fuel cell and evidence for ternary ionic conduction. J Power Sources 188:156–162
Jiang C, Ma J, Arenillas A, Bonaccorso AD, Irvine JTS (2016) Comparative study of durability of hybrid direct carbon fuel cells with anthracite coal and bituminous coal. Int J Hydrogen Energy 41:18797–18806. https://doi.org/10.1016/j.ijhydene.2016.04.047
Elleuch A, Boussetta A, Yu J, Halouani K, Li Y (2013) Experimental investigation of direct carbon fuel cell fueled by almond shell biochar: part I. Physico-chemical characterization of the biochar fuel and cell performance examination. Int J Hydrogen Energy 38:16590–16604. https://doi.org/10.1016/j.ijhydene.2013.08.090
Gür TM, Huggins RA (1992) Direct electrochemical conversion of carbon to electrical energy in a high temperature fuel cell. J Electrochem Soc 139:L95–L97
Xu H, Chen B, Zhang H, Tan P, Yang G, Irvine JTS, Ni M (2018) Experimental and modeling study of high performance direct carbon solid oxide fuel cell with in situ catalytic steam-carbon gasification reaction. J Power Sources 382:135–143. https://doi.org/10.1016/j.jpowsour.2018.02.033
Xu H, Chen B, Zhang H, Sun Q, Yang G, Ni M (2017) Modeling of direct carbon solid oxide fuel cells with H2O and CO2 as gasification agents. Int J Hydrogen Energy 42:15641–15651. https://doi.org/10.1016/j.ijhydene.2017.05.075
Li X, Zhu ZH, De Marco R, Dicks A, Bradley J, Liu SM, Lu GQ (2008) Factors that determine the performance of carbon fuels in the direct carbon fuel cell. Ind Eng Chem Res 47:9670–9677. https://doi.org/10.1021/ie800891m
Elleuch A, Yu J, Boussetta A, Halouani K, Li Y (2013) Electrochemical oxidation of graphite in an intermediate temperature direct carbon fuel cell based on two-phases electrolyte. Int J Hydrogen Energy 38:8514–8523. https://doi.org/10.1016/j.ijhydene.2012.11.070
Xu X, Zhou W, Liang F, Zhu Z (2013) A comparative study of different carbon fuels in an electrolyte-supported hybrid direct carbon fuel cell. Appl Energy 108:402–409. https://doi.org/10.1016/j.apenergy.2013.03.053
Li X, Chen JL, Zhu ZH, De Marco R, Bradley J, Dicks A (2009) Carbon nanofibers synthesized by catalytic decomposition of methane and their electrochemical performance in a direct carbon fuel cell. Energy Fuels 23:3721–3731. https://doi.org/10.1021/ef900203h
Yu J, Yu B, Li Y (2013) Electrochemical oxidation of catalytic grown carbon fiber in a direct carbon fuel cell using Ce0.8Sm0.2O1.9-carbonate electrolyte. Int J Hydrogen Energy 38:16615–16622. https://doi.org/10.1016/j.ijhydene.2013.02.113
Chen J, Yang X, Li Y (2010) Investigation on the structure and the oxidation activity of the solid carbon produced from catalytic decomposition of methane. Fuel 89:943–948. https://doi.org/10.1016/j.fuel.2009.08.017
Li P, Zhao Y, Yu B, Li J, Li Y (2015) Improve electrical conductivity of reduced La2Ni0.9Fe0.1O4 + δ as the anode of a solid oxide fuel cell by carbon deposition. Int J Hydrogen Energy 40:9783–9789. https://doi.org/10.1016/j.ijhydene.2015.06.026
Zhang J, Zhong Z, Shen D, Zhao J, Zhang H, Yang M, Li W (2011) Preparation of bamboo-based activated carbon and its application in direct carbon fuel cells. Energy Fuels 25:2187–2193. https://doi.org/10.1021/ef200161c
Zhang J, Zhong Z, Zhao J, Yang M, Li W, Zhang H (2012) Study on the preparation of activated carbon for direct carbon fuel cell with oak sawdust. Can J Chem Eng 90:762–768. https://doi.org/10.1002/cjce.20549
Ahn SY, Eom SY, Rhie YH, Sung YM, Moon CE, Choi GM, Kim DJ (2013) Application of refuse fuels in a direct carbon fuel cell system. Energy 51:447–456. https://doi.org/10.1016/j.energy.2012.12.025
Hao WB, Mi YL (2016) Evaluation of waste paper as a source of carbon fuel for hybrid direct carbon fuel cells. Energy 107:122–130. https://doi.org/10.1016/j.energy.2016.04.012
Cao D, Wang G, Wang C, Wang J, Lu T (2010) Enhancement of electrooxidation activity of activated carbon for direct carbon fuel cell. Int J Hydrogen Energy 35:1778–1782. https://doi.org/10.1016/j.ijhydene.2009.12.133
Xu K, Chen C, Liu H, Tian Y, Li X, Yao H (2014) Effect of coal based pyrolysis gases on the performance of solid oxide direct carbon fuel cells. Int J Hydrogen Energy 39:17845–17851
Zhong Y, Su C, Cai R, Tade MO, Shao Z (2016) Process investigation of a solid carbon-fueled solid oxide fuel cell integrated with a CO2-permeating membrane and a sintering-resistant reverse boudouard reaction catalyst. Energy Fuels 30:1841–1848. https://doi.org/10.1021/acs.energyfuels.5b02198
Yang B, Ran R, Zhong Y, Su C, Tade MO, Shao Z (2015) A carbon-air battery for high power generation. Angew Chem Int Ed 54:3722–3725. https://doi.org/10.1002/anie.201411039
Li X, Zhu Z, De Marco R, Bradley J, Dicks A (2010) Evaluation of raw coals as fuels for direct carbon fuel cells. J Power Sources 195:4051–4058. https://doi.org/10.1016/j.jpowsour.2010.01.048
Dudek M, Tomczyk P, Socha R, Hamaguchi M (2014) Use of ash-free “Hyper-coal” as a fuel for a direct carbon fuel cell with solid oxide electrolyte. Int J Hydrogen Energy 39:12386–12394. https://doi.org/10.1016/j.ijhydene.2014.04.057
Eom S, Cho J, Ahn S, Sung Y, Choi G, Kim D (2016) Comparison of the electrochemical reaction parameter of graphite and sub-bituminous coal in a direct carbon fuel cell. Energy Fuels 30:3502–3508. https://doi.org/10.1021/acs.energyfuels.5b02904
Dudek M, Tomczyk P (2011) Composite fuel for direct carbon fuel cell. Catal Today 176:388–392. https://doi.org/10.1016/j.cattod.2010.11.029
Yamaura H, Ikuta T, Yahiro H, Okada G (2005) Cathodic polarization of strontium-doped lanthanum ferrite in proton-conducting solid oxide fuel cell. Solid State Ionics 176:269–274. https://doi.org/10.1016/j.ssi.2004.08.008
Jia L, Tian Y, Liu Q, Xia C, Yu J, Wang Z, Zhao Y, Li Y (2010) A direct carbon fuel cell with (molten carbonate)/(doped ceria) composite electrolyte. J Power Sources 195:5581–5586
Godula-Jopek A, Suski L (2000) Wetting of Ni and NiO by alternative molten carbonate fuel cell electrolytes II. Influence of the electrode overpotential. J Electrochem Soc 147:910–915. https://doi.org/10.1149/1.1393291
Peele WHA, Hemmes K, De Wit JHW (1998) CO2 reduction in molten 62/38 mol% Li/K carbonate mixture. Electrochim Acta 43:763–769. https://doi.org/10.1016/S0013-4686(97)00141-2
White S, Twardoch U (1989) The solubility and electrochemistry of alkali metal oxides in the molten eutectic mixture of lithium carbonate-sodium carbonate-potassium carbonate. J Appl Electrochem 19:901–910
Wickramasinghe M, Kiss IZ (2016) Nonlinear behavior of nickel dissolution in sulfuric acid in a cathode-anode cell configuration: effect of cathode area. J Electrochem Soc 163:H1171–H1178. https://doi.org/10.1149/2.0471614jes
Zhang J, Zhong Z, Shen D, Xiao J, Fu Z, Zhang H, Zhao J, Li W, Yang M (2011) Characteristics of a fluidized bed electrode for a direct carbon fuel cell anode. J Power Sources 196:3054–3059. https://doi.org/10.1016/j.jpowsour.2010.11.130
Acknowledgments
This work was supported by the National Natural Science Fund Program of China (51576082), the National Key Research and Development Project (2018YFB0604100-2), and the Foundation of State Key Laboratory of Coal Combustion. The authors are also grateful to the Analytical and Testing Center of Huazhong University of Science and Technology for the experimental measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bie, K., Zhou, H., Fu, P. et al. Investigation of the cathode polarization and carbon deposition in a molten carbonate direct carbon fuel cell. J Appl Electrochem 49, 585–597 (2019). https://doi.org/10.1007/s10800-019-01307-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-019-01307-0