Abstract
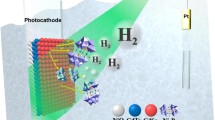
A comparative study of hydrogen evolution in devices based on cadmium chalcogenides quantum dots (CdS, CdSe and CdTe) and its combinations, sensitizing TiO2 was carried out. A maximum photocurrent of 2.7 mA cm−2 at 0 V bias, and a solar-to-hydrogen (STH) conversion efficiency of 0.9%, was obtained with CdSe QDs due to its wide absorption range. The co-sensitized device with CdS–CdSe QDs showed a higher photocurrent of 3.9 mA cm−2 with an STH of 1.2%. The improvement in hydrogen generation for electrodes sensitized with CdS in combination with CdSe or CdTe QDs, was attributed to the increased light absorption and appropriate band alignment for the enhanced charge transport.
Graphical abstract








Similar content being viewed by others
References
González-Pedro V, Zarazua I, Barea EM et al (2014) Panchromatic solar-to-H2 conversion by a hybrid quantum dots–dye dual absorber tandem device. J Phys Chem C 118:891–895. https://doi.org/10.1021/jp4109893
Turner J, Sverdrup G, Mann MK et al (2008) Renewable hydrogen production. Int J Energy Res 32:379–407. https://doi.org/10.1002/er.1372
Hisatomi T, Kubota J, Domen K (2014) Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem Soc Rev 43:7520–7535. https://doi.org/10.1039/C3CS60378D
Khan SUM (2002) Efficient photochemical water splitting by a chemically modified n-TiO2. Science 297:2243–2245. https://doi.org/10.1126/science.1075035
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Ni M, Leung MKH, Leung DYC, Sumathy K (2007) A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew Sustain Energy Rev 11:401–425. https://doi.org/10.1016/j.rser.2005.01.009
Sun J, Zhong DK, Gamelin DR (2010) Composite photoanodes for photoelectrochemical solar water splitting. Energy Environ Sci 3:1252. https://doi.org/10.1039/c0ee00030b
Gonell F, Haro M, Sánchez RS et al (2014) Photon up-conversion with lanthanide-doped oxide particles for solar H2 generation. J Phys Chem C 118:11279–11284. https://doi.org/10.1021/jp503743e
Han W, Ren L, Qi X et al (2014) Synthesis of CdS/ZnO/graphene composite with high-efficiency photoelectrochemical activities under solar radiation. Appl Surf Sci 299:12–18. https://doi.org/10.1016/j.apsusc.2014.01.170
Pihosh Y, Turkevych I, Mawatari K et al (2015) Photocatalytic generation of hydrogen by core-shell WO3/BiVO4 nanorods with ultimate water splitting efficiency. Sci Rep 5:11141. https://doi.org/10.1038/srep11141
Li X, Wang Z, Zhang Z et al (2015) Light illuminated α-Fe2O3/Pt nanoparticles as water activation agent for photoelectrochemical water splitting. Sci Rep 5:9130. https://doi.org/10.1038/srep09130
Haro M, Abargues R, Herraiz-Cardona I et al (2014) Plasmonic versus catalytic effect of gold nanoparticles on mesoporous TiO2 electrodes for water splitting. Electrochim Acta 144:64–70. https://doi.org/10.1016/j.electacta.2014.07.146
Simon T, Bouchonville N, Berr MJ et al (2014) Redox shuttle mechanism enhances photocatalytic H2 generation on Ni–decorated CdS nanorods. Nat Mater 13:1013–1018. https://doi.org/10.1038/nmat4049
Momeni MM, Ghayeb Y, Shafiei M (2017) Preparation and characterization of CrFeWTiO2 photoanodes and their photoelectrochemical activities for water splitting. Dalton Trans 46:12527–12536. https://doi.org/10.1039/c7dt01596h
Sharifi T, Ghayeb Y, Mohammadi T, Momeni MM (2018) Enhanced photoelectrochemical water splitting of CrTiO2 nanotube photoanodes by the decoration of their surface via the photodeposition of Ag and Au. Dalton Trans 47:11593–11604. https://doi.org/10.1039/c8dt02383b
Momeni MM, Mozafari AA (2016) The effect of number of SILAR cycles on morphological, optical and photo catalytic properties of cadmium sulfide–titania films. J Mater Sci Mater Electron 27:10658–10666. https://doi.org/10.1007/s10854-016-5163-4
Momeni MM, Ghayeb Y, Menati M (2018) Fabrication, characterization and photoelectrochemical properties of cuprous oxide-reduced graphene oxide photocatalysts for hydrogen generation. J Mater Sci Mater Electron 29:4136–4146. https://doi.org/10.1007/s10854-017-8358-4
Momeni MM, Ghayeb Y (2015) Visible light-driven photoelectrochemical water splitting on ZnO–TiO2 heterogeneous nanotube photoanodes. J Appl Electrochem 45:557–566. https://doi.org/10.1007/s10800-015-0836-x
Momeni MM, Ghayeb Y, Mozafari AA (2016) Optical and photo catalytic characteristics of Ag2S/TiO2 nanocomposite films prepared by electrochemical anodizing and SILAR approach. J Mater Sci Mater Electron 27:11201–11210. https://doi.org/10.1007/s10854-016-5240-8
Momeni MM, Ghayeb Y (2015) Fabrication, characterization and photoelectrochemical behavior of Fe-TiO2 nanotubes composite photoanodes for solar water splitting. J Electroanal Chem 751:43–48. https://doi.org/10.1016/j.jelechem.2015.05.035
Momeni MM, Ghayeb Y (2015) Photoelectrochemical water splitting on chromium-doped titanium dioxide nanotube photoanodes prepared by single-step anodizing. J Alloys Compd 637:393–400. https://doi.org/10.1016/j.jallcom.2015.02.137
Momeni MM, Ghayeb Y, Davarzadeh M (2015) Single-step electrochemical anodization for synthesis of hierarchical WO3-TiO2 nanotube arrays on titanium foil as a good photoanode for water splitting with visible light. J Electroanal Chem 739:149–155. https://doi.org/10.1016/j.jelechem.2014.12.030
Momeni MM, Ghayeb Y, Ezati F (2018) Fabrication, characterization and photoelectrochemical activity of tungsten-copper co-sensitized TiO2 nanotube composite photoanodes. J Colloid Interface Sci 514:70–82. https://doi.org/10.1016/j.jcis.2017.12.021
Trevisan R, Rodenas P, Gonzalez-Pedro V et al (2013) Harnessing infrared photons for photoelectrochemical hydrogen generation. A PbS quantum dot based “quasi-artificial leaf”. J Phys Chem Lett 4:141–146. https://doi.org/10.1021/jz301890m
Tanaka K, Jin-nouchi Y, Fujishima M, Tada H (2015) Lead selenide–titanium dioxide heteronanojunction formation by photocatalytic current doubling-induced two-step photodeposition technique. J Colloid Interface Sci 457:248–253. https://doi.org/10.1016/j.jcis.2015.03.008
Yu Z, Li F, Sun L (2015) Recent advances in dye-sensitized photoelectrochemical cells for solar hydrogen production based on molecular components. Energy Environ Sci 8:760–775. https://doi.org/10.1039/C4EE03565H
Jang JS, Li W, Oh SH, Lee JS (2006) Fabrication of CdS/TiO2 nano-bulk composite photocatalysts for hydrogen production from aqueous H2S solution under visible light. Chem Phys Lett 425:278–282. https://doi.org/10.1016/j.cplett.2006.05.031
Seabold J, Shankar K, Wilke RHT et al (2008) Photoelectrochemical properties of heterojunction CdTe/TiO2 electrodes constructed using highly ordered TiO2 nanotube arrays. Chem Mater 20:5266–5273. https://doi.org/10.1021/cm8010666
Sreedhar G, Sivanantham A, Venkateshwaran S et al (2015) Enhanced photoelectrochemical performance of CdSe quantum dot sensitized SrTiO3. J Mater Chem A 3:13476–13482. https://doi.org/10.1039/C5TA00304K
Cui W, Ma S, Liu L, Liang Y (2012) PbS-sensitized K2Ti4O9 composite: preparation and photocatalytic properties for hydrogen evolution under visible light irradiation. Chem Eng J 204–206:1–7. https://doi.org/10.1016/j.cej.2012.07.075
Chen HM, Chen CK, Chang Y-C et al (2010) Quantum dot monolayer sensitized ZnO nanowire-array photoelectrodes: true efficiency for water splitting. Angew Chem 122:6102–6105. https://doi.org/10.1002/ange.201001827
Liu L, Hensel J, Fitzmorris RC et al (2010) Preparation and photoelectrochemical properties of CdSe/TiO2 hybrid mesoporous structures. J Phys Chem Lett 1:155–160. https://doi.org/10.1021/jz900122u
Jin-Nouchi Y, Hattori T, Sumida Y et al (2010) PbS quantum dot-sensitized photoelectrochemical cell for hydrogen production from water under illumination of simulated sunlight. ChemPhysChem 11:3592–3595. https://doi.org/10.1002/cphc.201000593
Vaneski A, Schneider J, Susha AS, Rogach AL (2014) Colloidal hybrid heterostructures based on II–VI semiconductor nanocrystals for photocatalytic hydrogen generation. J Photochem Photobiol C 19:52–61. https://doi.org/10.1016/j.jphotochemrev.2013.12.001
Peng X (2009) An essay on synthetic chemistry of colloidal nanocrystals. Nano Res 2:425–447. https://doi.org/10.1007/s12274-009-9047-2
Gür TM, Bent SF, Prinz FB (2014) Nanostructuring materials for solar-to-hydrogen conversion. J Phys Chem C 118:21301–21315. https://doi.org/10.1021/jp500966u
Shin K, Yoo J-B, Park JH (2013) Photoelectrochemical cell/dye-sensitized solar cell tandem water splitting systems with transparent and vertically aligned quantum dot sensitized TiO2 nanorod arrays. J Power Sources 225:263–268. https://doi.org/10.1016/j.jpowsour.2012.10.036
Wang G, Yang X, Qian F et al (2010) Double-sided CdS and CdSe quantum dot co-sensitized ZnO nanowire arrays for photoelectrochemical hydrogen generation. Nano Lett 10:1088–1092. https://doi.org/10.1021/nl100250z
Kim DH, Han HS, Cho IS et al (2015) CdS-sensitized 1-D single-crystalline anatase TiO2 nanowire arrays for photoelectrochemical hydrogen production. Int J Hydrogen Energy 40:863–869. https://doi.org/10.1016/j.ijhydene.2014.09.174
Hensel J, Wang G, Li Y, Zhang JZ (2010) Synergistic effect of CdSe quantum dot sensitization and nitrogen doping of TiO2 nanostructures for photoelectrochemical solar hydrogen generation. Nano Lett 10:478–483. https://doi.org/10.1021/nl903217w
Berr MJ, Wagner P, Fischbach S et al (2012) Hole scavenger redox potentials determine quantum efficiency and stability of Pt-decorated CdS nanorods for photocatalytic hydrogen generation. Appl Phys Lett 100:223903. https://doi.org/10.1063/1.4723575
Seol M, Jang J, Cho S et al (2013) Highly efficient and stable cadmium chalcogenide quantum Dot/ZnO nanowires for photoelectrochemical hydrogen generation. Chem Mater 25:184–189. https://doi.org/10.1021/cm303206s
Kim H, Seol M, Lee J, Yong K (2011) Highly efficient photoelectrochemical hydrogen generation using hierarchical ZnO/WOx nanowires cosensitized with CdSe/CdS. J Phys Chem C 115:25429–25436. https://doi.org/10.1021/jp2093115
Ali Z, Shakir I, Kang DJ (2014) Highly efficient photoelectrochemical response by sea-urchin shaped ZnO/TiO2 nano/micro hybrid heterostructures co-sensitized with CdS/CdSe. J Mater Chem A 2:6474. https://doi.org/10.1039/c3ta15439d
Wang H, Zhu W, Chong B, Qin K (2014) Improvement of photocatalytic hydrogen generation from CdSe/CdS/TiO2 nanotube-array coaxial heterogeneous structure. Int J Hydrog Energy 39:90–99. https://doi.org/10.1016/j.ijhydene.2013.10.048
Gao X-F, Sun W-T, Ai G, Peng L-M (2010) Photoelectric performance of TiO2 nanotube array photoelectrodes cosensitized with CdS/CdSe quantum dots. Appl Phys Lett 96:153104. https://doi.org/10.1063/1.3386525
Rodenas P, Song T, Sudhagar P et al (2013) Quantum dot based heterostructures for unassisted photoelectrochemical hydrogen generation. Adv Energy Mater 3:176–182. https://doi.org/10.1002/aenm.201200255
Zeng T-W, Liu S, Hsu F-C et al (2010) Effects of bifunctional linker on the performance of P3HT/CdSe quantum dot-linker-ZnO nanocolumn photovoltaic device. Opt Express 18:A357. https://doi.org/10.1364/OE.18.00A357
Zhao Y, Yan Z, Liu J, Wei A (2013) Synthesis and characterization of CdSe nanocrystalline thin films deposited by chemical bath deposition. Mater Sci Semicond Process 16:1592–1598. https://doi.org/10.1016/j.mssp.2013.04.027
Emin S, Singh SP, Han L et al (2011) Colloidal quantum dot solar cells. Sol Energy 85:1264–1282. https://doi.org/10.1016/j.solener.2011.02.005
De La Fuente MS, Sánchez RS, González-Pedro V et al (2013) Effect of organic and inorganic passivation in quantum-dot-sensitized solar cells. J Phys Chem Lett 4:1519–1525. https://doi.org/10.1021/jz400626r
Cerdán-Pasarán A, Esparza D, Zarazúa I et al (2016) Photovoltaic study of quantum dot-sensitized TiO2/CdS/ZnS solar cell with P3HT or P3OT added. J Appl Electrochem 46:975–985. https://doi.org/10.1007/s10800-016-0972-y
Peng ZA, Peng X (2002) Nearly monodisperse and shape-controlled CdSe nanocrystals via alternative routes: nucleation and growth. J Am Chem Soc 124:3343–3353
Cerdán A, López-Luke T, Esparza D et al (2015) Photovoltaic properties of multilayered quantum dot/quantum rod-sensitized TiO2 solar cells fabricated by SILAR and electrophoresis. Phys Chem Chem Phys. https://doi.org/10.1039/C5CP02541A
Esparza D, Zarazúa I, López-Luke T et al (2015) Effect of different sensitization technique on the photoconversion efficiency of CdS quantum dot and CdSe quantum rod sensitized TiO2 solar cells. J Phys Chem C. https://doi.org/10.1021/acs.jpcc.5b01525
Diguna LJ, Shen Q, Kobayashi J, Toyoda T (2007) High efficiency of CdSe quantum-dot-sensitized TiO2 inverse opal solar cells. Appl Phys Lett 91:023116. https://doi.org/10.1063/1.2757130
Giménez S, Lana-Villarreal T, Gómez R et al (2010) Determination of limiting factors of photovoltaic efficiency in quantum dot sensitized solar cells: correlation between cell performance and structural properties. J Appl Phys 108:064310. https://doi.org/10.1063/1.3477194
Jung SW, Kim JH, Kim H et al (2012) ZnS overlayer on in situ chemical bath deposited CdS quantum dot-assembled TiO2 films for quantum dot-sensitized solar cells. Curr Appl Phys 12:1459–1464. https://doi.org/10.1016/j.cap.2012.04.012
Lee H, Wang M, Chen P et al (2009) Efficient CdSe quantum dot-sensitized solar cells prepared by an improved successive ionic layer adsorption and reaction process. Nano Lett 9:4221–4227. https://doi.org/10.1021/nl902438d
Liu Y, Li Z, Yu L, Sun S (2015) Effect of the nature of cationic precursors for SILAR deposition on the performance of CdS and PbS/CdS quantum dot-sensitized solar cells. J Nanoparticle Res 17:132. https://doi.org/10.1007/s11051-015-2940-6
Ai G, Mo R, Xu H et al (2015) Vertically aligned TiO2/(CdS, CdTe, CdSTe) core/shell nanowire array for photoelectrochemical hydrogen generation. J Power Sources 280:5–11. https://doi.org/10.1016/j.jpowsour.2015.01.071
Luo J, Karuturi SK, Liu L et al (2012) Homogeneous photosensitization of complex TiO2 nanostructures for efficient solar energy conversion. Sci Rep 2:451. https://doi.org/10.1038/srep00451
Seol M, Kim H, Kim W, Yong K (2010) Highly efficient photoelectrochemical hydrogen generation using a ZnO nanowire array and a CdSe/CdS co-sensitizer. Electrochem Commun 12:1416–1418. https://doi.org/10.1016/j.elecom.2010.07.035
Yue D, Qian X, Zhang Z et al (2016) CdTe/CdS Core/shell quantum dots cocatalyzed by sulfur tolerant [Mo3S13]2-nanoclusters for efficient visible-light-driven hydrogen evolution. ACS Sustain Chem Eng 4:6653–6658. https://doi.org/10.1021/acssuschemeng.6b01520
Banerjee S, Mohapatra SK, Das PP, Misra M (2008) Synthesis of coupled semiconductor by filling 1D TiO2 nanotubes with CdS. Chem Mater 20:6784–6791. https://doi.org/10.1021/cm802282t
Li Z, Luo W, Zhang M et al (2013) Photoelectrochemical cells for solar hydrogen production: current state of promising photoelectrodes, methods to improve their properties, and outlook. Energy Environ Sci 6:347–370. https://doi.org/10.1039/C2EE22618A
Chen X, Shen S, Guo L, Mao SS (2010) Semiconductor-based photocatalytic hydrogen generation. Chem Rev 110:6503–6570. https://doi.org/10.1021/cr1001645
Cendula P, Tilley SD, Gimenez S et al (2014) Calculation of the energy band diagram of a photoelectrochemical water splitting cell. J Phys Chem C 118:29599–29607. https://doi.org/10.1021/jp509719d
Gelderman K, Lee L, Donne SW (2007) Flat-band potential of a semiconductor: using the Mott–Schottky equation. J Chem Educ 84:685. https://doi.org/10.1021/ed084p685
Mora-Seró I, Bisquert J (2012) Impedance characterization of quantum dot sensitized solar cells. In: Frontiers of Quantum Dot Solar Cells. CMC Publishing Co., Ltd, Japan, pp 162–175
Acknowledgements
We acknowledge financial support to CONACYT through Grant 259192 and CEMIE-Solar (207450) consortium projects P27 and P28. A. Cerdán-Pasarán acknowledges to CONACYT for the Doctoral fellowship. We thank to Christian Albor for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cerdán-Pasarán, A., López-Luke, T., Zarazúa, I. et al. Co-sensitized TiO2 electrodes with different quantum dots for enhanced hydrogen evolution in photoelectrochemical cells. J Appl Electrochem 49, 475–484 (2019). https://doi.org/10.1007/s10800-019-01299-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-019-01299-x