Abstract
Previously optimized anatase and nitrogen-doped anatase TiO2 coatings have been grown by low-frequency plasma-enhanced chemical vapor deposition (PECVD) on different kinds of substrates at low plasma power (64 W) and high plasma power (100 W) for photo-electrochemical studies. Nitrogen-doped TiO2 layers exhibit better photoactivity and also higher electronic conductivity under UV and visible irradiations than non-doped materials. The main reason is that nitrogen introduction induces TiO2 band gap tailoring towards higher wavelengths. In addition, films prepared at low plasma power present a ‘typical photo-material’ behavior (whose activity depends directly on the presence of light) while layers synthesized at higher plasma power contain an initial conductive phase giving them an activity that exists in the dark yet and can be slightly enhanced by illumination. Such conclusions are prominent in the field of photo-anodic thin films; indeed PECVD could constitute a promising approach for tailoring the efficiency of photo-electrochemical cells for hydrogen production under solar light.
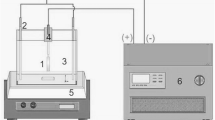
Graphical abstract














Similar content being viewed by others
References
Ribeiro H (2002) Fossil fuel energy impacts on health. Encycl Life Support Syst 1–9
Princen T, Manno J, Martin P (2015) The fossil fuel problem. In: Ending of fossil fuels era, pp 3–97
Holtsmark B (2015) A comparison of the global warming effects of wood fuels and fossil fuels taking albedo into account. GCB Bioenergy 7:984–997. https://doi.org/10.1111/gcbb.12200
Scientists U of C (2016) The hidden costs of fossil fuels. In: Science for a healthy planet and safer world
Chauhan NS, Singh VK (2015) Fundamentals and use of hydrogen as a fuel. J Mech Eng 6:63–68
Jechura J (2015) Hydrogen from natural gas via steam methane reforming (SMR)
Raggio G, Pettinau A, Orsini A et al (2005) Coal gasification pilot plant for hydrogen production. Part A: coal gasification and syngas desulphurization. In: Second international conference on clean coal technology our future
Deiana P, Pettinau A, Tola V (2007) Hydrogen production from coal gasification in updraft gasifier with syngas treatment line
Lensa W, Von, Verfondern K (2010) Coal gasification for hydrogen production using nuclear energy coal gasification for hydrogen production using nuclear energy. In: 18th World hydrogen energy conference, pp 191–198
Lan R, Jin H, Guo L et al (2014) Hydrogen production by catalytic gasification of coal in supercritical water. Energy Fuels 28:6911–6917
Van Niel EW (2016) Biological processes for hydrogen production. Adv Biochem Eng Biotechnol 156:155–193
Orozco-Pulido R (2011) Hydrogen production from biomass by integrating thermo-chemical and biological processes
Loubette N, Junker M, Jacques R, Forbach C (2006) State of the art of biological hydrogen production processes
Das D, Veziroǧlu TN (2001) Hydrogen production by biological processes: a survey of literature. Int J Hydrogen Energy 26:13–28
Carmo M, Fritz DL, Mergel J, Stolten D (2013) A comprehensive review on PEM water electrolysis. Int J Hydrogen Energy 38:4901–4934
Kasai S (2014) Hydrogen electrical energy storage by high-temperature steam electrolysis for next-millennium energy security. Int J Hydrogen Energy 39:21358–21370
Rashid MM, Al Mesfer MK, Naseem H, Danish M (2015) Hydrogen production by water electrolysis: a review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. Int J Eng Adv Technol 4:2249–8958
Bhandari R, Trudewind CA, Zapp P (2014) Life cycle assessment of hydrogen production via electrolysis e a review. J Clean Prod 85:151–163. https://doi.org/10.1016/j.jclepro.2013.07.048
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Kennedy JH, Frese K (1978) Photo-oxidation of water at a-Fe2O3 electrodes. J Electrochem Soc 125:709–714
Butler MA (1977) Photoelectrolysis and physical properties of the semiconducting electrode WO2. J Appl Phys 48:1914
Youssef L, Kinfack Leoga AJ, Roualdes S et al (2017) Optimization of N-doped TiO2 multifunctional thin layers by low frequency PECVD process. J Eur Ceram Soc 37:5289–5303. https://doi.org/10.1016/j.jeurceramsoc.2017.05.010
Fountaine K, Lewerenz HJ, Atwater H (2016) Efficiency limits for electrochemical water splitting. Nat Commun 7:13706
Duan J, Chen S, Zhao C (2017) Ultrathin metal-organic framework array for efficient electrocatalytic water splitting. Nat Commun 8:15341
Swierk J, Hernandez D, McCool N et al (2015) Metal-free organic sensitizers for use in water splitting dye sensitized photoelectrochemical cells. Proc Natl Acad Sci USA 112:1681–1686
Cohen A (1910) Studien über photochemische Gleichgewichte IV Das Ligchtgleichgewicht Knallgas-Wasserdampf. Berichte der Dtsch Chem Gesellschaft 43:880
Chovet A, Masson P (2007) Cours de physique des semi-conducteurs
Ngô C, Ngô H (2012) Physique des semi-conducteurs
Ma G, Domen K, Hisatomi T (2015) Semiconductors for photocatalytic and photoelectrochemical solar water splitting. In: From molecules to materials. pp 1–56
Würfel P, Würfel U (2016) From basic principles to advanced concepts
McKevoy A, Markvart T, Castaner L (2013) Materials, manufacture and operation. Sol Cells 1–615
Ajmal A, Majeed I, Malik R et al (2014) Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: a comparative overview. RSC Adv 4:37003–37026
Tayade RJ, Surolia PK, Kulkarni RG, Jasra RV (2007) Photocatalytic degradation of dyes and organic contaminants in water using nanocrystalline anatase and rutile TiO2. Sci Technol Adv Mater 8:455–462
Khataee AR, Kasiri MB (2010) Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: Influence of the chemical structure of dyes. J Mol Catal A Chem 238
Grätzel M (2001) Photoelectrochemical cells. Nature 414:338–343
Liu B, Zhao X, Terashima C et al (2014) Thermodynamic and kinetic analysis of heterogeneous photocatalysis for semiconductor systems. Phys Chem Chem Phys 16:8751
Zhou M (2015) Novel photocatalytic TiO2-based porous membranes prepared by plasma-enhanced chemical vapor deposition (PECVD) for organic pollutant degradation in water
Cho HJ, Hwang PG, Jung D (2011) Preparation and photocatalytic activity of nitrogen-doped TiO2 hollow nanospheres. J Phys Chem Solids 72:1462–1466. https://doi.org/10.1016/j.jpcs.2011.08.026
Guimond S, Schütz U, Hanselmann B et al (2011) Influence of gas phase and surface reactions on plasma polymerization. Surf Coatings Technol 205:S447–S450. https://doi.org/10.1016/j.surfcoat.2010.08.131
Nurfani E, Kurniawan R, Muhammady S et al (2016) Effect of Ta concentration on the refractive index of TiO2:Ta studied by spectroscopic ellipsometry. AIP Conf Proc 1725:1–4
Liu H-Y, Hsu Y-L, Su H-Y et al (2018) A comparative study of amorphous, anatase, rutile, and mixed phase TiO2 films by mist chemical vapor deposition and ultraviolet photodetectors applications. IEEE Sens J 1748:1–1. https://doi.org/10.1109/JSEN.2018.2819700
Woo S-H, Hwangbo CK (2006) Optical anisotropy of microstructure-controlled TiO2 films fabricated by glancing-angle deposition (GLAD). J Korean Phys Soc 48:1199–1204
Lazzarelli HN (2010) Blue chart gem identification
Lee A, Van Der (2013) Diffusion, diffraction, réflectométrie et fluorescence de rayons-X à la PAC. In: International year of crystallography, pp 1–35
Chen H, Nambu A, Graciani J et al (2007) Reaction of NH3 with Titania: N-doping of the oxide and TiN formation. J Phys Chem C 111:1366–1372
Swihart MT, Allendorf MD, Meyyapan M (2001) Process control, diagnostics, and modeling in semiconductor manufacturing IV. In: Fundamental gas-phase and surface chemistry of vapor-phase deposition II. pp 1–496
Rahul M (2010) Photoelectrochemical (PEC) and electrical studies on tungsten and molybdenum heteropolyoxometalate thin films
Muhibbullah M, Abdel Haleem A (2015) Estimation of the open circuit voltage of a pn junction based on photo-electrochemical measurements. Trans Mater Res Soc Japan 40:247–252
Muhibbullah M, Golam Mowla Choudhury M, Mominuzzaman SM (2012) An equation of the width of the depletion layer for a step heterojunction. Trans Mater Res Soc Japan 37:405–408
Jie H, Song-Tao W, Xiao-Li C et al (2004) Photoelectrochemical characteristics of nano-titanium dioxide thin films prepared by RF magnetron sputtering. Acta Phys Chim Sin 20:1191–1195
Ji Y, Guo W, Chen H et al (2015) Surface Ti3+/ Ti4+ redox shuttle enhancing photocatalytic H2 production in ultrathin TiO2 nanosheets/CdSe quantum dots. J Phys Chem C 119:27053–27059
Qiu M, Tian Y, Chen Z et al (2016) Synthesis of Ti3+ self-doped TiO2 nanocrystals based on Le Chatelier’s principle and their application in solar light photocatalysis. RSC Adv 78:74376–74383
Acknowledgements
This project has been funded with support from Lebanese University (UL) in collaboration with AZM&SAADÉ foundation. We also thank Arie VAN DER LEE (IEM, Montpellier) for XRR analysis, Didier COT (IEM, Montpellier) for SEM observations, and Valérie FLAUD (ICGM, Montpellier) for thin films profile investigation by XPS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Youssef, L., Roualdès, S., Bassil, J. et al. Effect of plasma power on the semiconducting behavior of low-frequency PECVD TiO2 and nitrogen-doped TiO2 anodic thin coatings: photo-electrochemical studies in a single compartment cell for hydrogen generation by solar water splitting. J Appl Electrochem 49, 135–150 (2019). https://doi.org/10.1007/s10800-018-1265-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-018-1265-4