Abstract
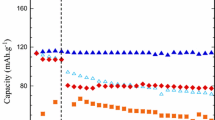
The electrochemical performance of lithium–sulfur batteries with LiClO4 DOL/DME as electrolyte was investigated. Impedance and SEM analysis indicated that too high content of DME(Dimethoxy ethane) in electrolyte could raise the interfacial resistance of battery due to the impermeable layer formed on the surface of the sulfur cathode, which led to bad cycle performance, while the increase of DOL(1,3-dioxolane) could change those phenomena. The optimal composition of electrolyte was DME:DOL = 2:1 (v/v). With this electrolyte, the lithium–sulfur battery obtained a high initial discharge capacity of 1,200 mA h g−1 and still remained 800 mA h g−1 after 20 cycles.




Similar content being viewed by others
References
Tischer RP (1983) The sulfur electrode. Academic Press, New York
Besenhard JO (1998) Handbook of battery materials. Wiley-VCH, New York
Marmorstein D, Yu TH, Striebel KA, McLarnon FR, Hou J, Cairns EJ (2000) Electrochemical performance of lithium/sulfur cells with three different polymer electrolytes. J Power Sources 89:219–226
Choi J-W, Kim J-K, Cheruvally G, Ahn J-H, Ahn H-J, Kim K-W (2007) Rechargeable lithium/sulfur battery with suitable mixed liquid electrolytes. Electrochem Acta 52:2075–2082
Aurbach D, Youngman Q, Gofer Y, Meitav A (1990) Electrochem Acta 35:625
Aurbach D (2000) J Power Sources 89:206
Sun J, Huang Y, Wang W, Yu Z, Wang A, Yuan K (2008) Application of gelatin as a binder for the sulfur cathode in lithium–sulfur batteries. Electrochem Acta 53:7084–7088
Sun J, Huang Y, Wang W, Yu Z, Wang A, Yuan K (2008) Preparation and electrochemical characterization of the porous sulfur cathode using a gelatin binder. Electrochem Commun 10:930–933
Kim S, Jung Y, Lim HS (2004) The effect of solvent component on the discharge performance of Lithium-sulfur cell containing various organic electrolytes. Electrochem Acta 50:889–892
Choi J-W, Cheruvally G, Kim D-S, Ahn J-H, Kim K-W, Ahn H-J (2008) Dischargeable lithium/sulfur battery with liquid electrolytes containing toluene as additive. J Power Sources 183:441–445
Wang Y, Huang Y, Wang W, Huang C, Yu Z, Zhang H, Sun J, Wang A, Yuan K (2009) Structural change of the porous sulfur cathode using gelatin as a binder during discharge and charge. Electrochem Acta 54(16):4062–4066
Cheon S-E, Ko K-S, Cho J-H, Kim S-W, Chin E-Y, Kim H-T (2003) Rechargeable lithium sulfur battery I. J Electrochem Soc 150(6):A796–A799
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, W., Wang, Y., Huang, Y. et al. The electrochemical performance of lithium–sulfur batteries with LiClO4 DOL/DME electrolyte. J Appl Electrochem 40, 321–325 (2010). https://doi.org/10.1007/s10800-009-9978-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-009-9978-z