Abstract
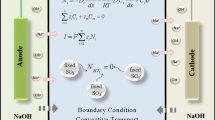
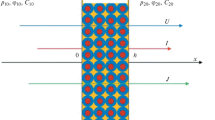
A previously published macrohomogeneous mathematical model of the simultaneous transport of multiple ions across an ion-selective membrane under current load based on the Nernst–Planck equation was extended. A significantly more realistic model is proposed and realised. The change with the most significant impact on the results of the model is consideration of convective mass transfer in the external diffusion layers adjacent to the membrane surfaces. This change results in a reduction of the concentration maximum previously observed in the membrane interior and highlights the importance of the external diffusion layers for ion transport across an ion-selective membrane. Hitherto this has often been underestimated.













Similar content being viewed by others
Abbreviations
- a :
-
Activity [1]
- A ca :
-
Clarke’s equation constant [m3 mol−1]
- c :
-
Molar concentration related to the volume of the solution or to the volume of the wet membrane [mol m−3]
- d e :
-
Equivalent diameter [m]
- D :
-
Diffusivity [m2 s−1]
- F :
-
Faraday number [96,487 C mol−1]
- j :
-
Current density [A m−2]
- k :
-
Membrane permeability [m2]
- \(\bar k\) :
-
Mass transfer coefficient [m s−1]
- L :
-
Membrane length [m]
- M :
-
Molar weight [kg mol−1]
- \(\overline M \) :
-
Mean molar weight of mixture [kg mol−1]
- N :
-
Molar flux [mol m−2 s−1]
- p :
-
Pressure [Pa]
- R :
-
Universal gas constant [8.314 J K−1 mol−1]
- T :
-
Temperature [K]
- v:
-
Fluid flow velocity [m s−1]
- V :
-
Volume [m3]
- V ∞ ca :
-
Clarke’s equation constant [m3 mol−1]
- x :
-
Molar fraction
- z :
-
Charge number [1]
- Re :
-
Reynolds number \(Re=\frac{vd_e \rho }{\eta }\)
- Sc :
-
Schmidt number \(Sc=\frac{\eta }{D\rho }\)
- Sh :
-
Sherwood number \(Sh=\frac{\bar{k}d_e }{D}\)
- δ:
-
Thickness of Nernst diffusion or membrane layer [m]
- ϕ:
-
Source [mol m−3 s−1]
- γ:
-
Activity coefficient [1]
- η:
-
Electrolyte dynamic viscosity [kg m−1 s−1]
- φ:
-
Galvani potential [V]
- μ:
-
Chemical potential [1]
- \(\tilde {\mu }\) :
-
Electrochemical potential [1]
- ρ:
-
Density [kg m−3]
- τ:
-
Time [s]
- a :
-
Anion
- c :
-
Cation
- ca :
-
Apparent component
- Don :
-
Donnan
- l i :
-
Length
- m :
-
Molar
- M :
-
Membrane
- x :
-
Axial coordinate
- s :
-
Solid phase
- tot :
-
All components (including water)
- a :
-
Apparent
- l :
-
Liquid phase
- l i :
-
Length
- M :
-
Membrane
- N ions :
-
Number of ions
- N tot :
-
Number of all components
- 0:
-
Standard state
- t :
-
True
References
Fíla V, Bouzek K (2003) J Appl Electrochem 33:675
Pletcher D, Walsh F (1990) Industrial electrochemistry. Chapman and Hall, London
Kordesch K, Simander G (1996) Fuel cells and their applications. VCH, Weinheim
Helfferich F (1962) Ion exchange. McGraw-Hill, New York
Yeager HL, Steck A (1981) J Electrochem Soc 128:1880
Yeager HL, Twardowski Z, Clarke LM (1982) J Electrochem Soc 129:324
Eisenberg A and Yeager HL (eds) (1982) Perfluorinated ionomer membranes, ACS Symp. Ser. 180, American Chemical Society, Washington DC
Yeo RS (1983) J Electrochem Soc 130:533
Mulder M (1991) Basic principles of membrane technology. Kluwer Academic Publishers, Dordrecht
Schlick S (ed) (1996) Ionomers characterization theory and applications. CRC Press, New York
Divisek J, Eikerling M, Mazin V, Schmitz H, Stimming U, Volfkovich Yu M (1998) J Electrochem Soc 145:2677
Haubold H.-G, Vad Th, Jungbluth H, Hiller P (2001). Electrochim Acta 46:1559
Buck RP (1984) J Membr Sci 17:1
Newcombe DT, Cardwell TJ, Cattrall RW, Kolev SD (1998) J Membr Sci 141:155
Palatý Z, Žáková A, Doleček P (2000) J Membr Sci 165:237
Kuppinger FF, Neubrand W, Rapp HJ, Eigenberger G (1995) Chem Ing Tech 67:441
Sistat P, Pourcelly G (1999) J Electroanal Chem 460:53
Weber AZ, Newman J (2004) Chem Rev 104:4679
Kreuer K-D, Paddison SJ, Spohr E, Schuster M (2004) Chem Rev 104:4637
Verbrugge MW, Hill RF (1990) J Electrochem Soc 137:886
Weber AZ, Newman J (2003) J Electrochem Soc 150:A1008
Weber AZ, Newman J (2004) J Electrochem Soc 151:A311
Weber AZ, Newman J (2004) J Electrochem Soc 151:A326
Mazumder S (2005) J Electrochem Soc 152:A1633
Fimrite J, Struchtrup H, Djilali N (2005) J Electrochem Soc 152:A1804
Fimrite J, Struchtrup H, Djilali N (2005) J Electrochem Soc 152:A1815
St-Pierre J (2007) J Electrochem Soc 154:B88
Millet P (1994) Electrochim Acta 39:2501
Görgün H (2006) Int J Hydrogen Energy 31:29
Seko M, Yomiyama A, Ogawa A (1984) In: White RE (ed) Electrochemical cell design, Plenum Press, New York, p 135
Curlin C, Bommaraju TV, Hansson CB (1991) Kirk-Othmer encyclopedia of chemical technology, vol 1. Wiley, New York, p 938
Chen C-P, Tilak BV (1996) J Appl Electrochem 26:235
Ogata Y, Kojima T, Uchiyama S, Yasuda M, Fine H (1989) J Electrochem Soc 136:91
Pillay G (1993) Mathematical modelling of macrohomogeneous transport phenomena in ion-exchange membranes. Ph.D. Thesis, New Mexico State University
Kimoto K (1983) J Electrochem Soc 130:334
Chandran RR, Yeo RS, Chin D-T (1985) Electrochim Acta 30:1585
Schlögl R (1966) Ber Bunsenges Phys Chem 70:400
Brumleve TR, Buck RP (1978) J Electroanal Chem 90:1
Verbrugge MW, Pintauro PN (1989) In: Conway BE, Bockris JO’M, White RE (eds) Modern aspects of electrochemistry 19. Plenum Press, New York
Verbrugge MW, Hill RF (1992) Electrochim Acta 37:221
Pintauro PN, Bennion DN (1984) Ind Eng Chem Fundam 23:230
Hogendoorn JA, van der Veen AJ, van der Stegen JHG, Kuipers JAM, Versteeg GF (2001) Comput Chem Eng 25:1251
Auclair B, Nikonenko V, Larchet C, Métayer M, Dammak L (2002) J Membr Sci 195:89
Sasidhar V, Ruckenstein E (1982) J Coll Interface Sci 85:332
Yang Y, Pintauro PN (2000) AIChE J 46:1177
Cwirko EH, Carbonell RG (1992) J Membr Sci 67:227
Din XD, Michaelides EE (1998) AIChE J 44:35
Mologin D, Khalatur P, Khokhlov A (2002) Macromol Theory Simul 11:587
Jinnouchi R (2003) Microscale Thermophys Eng 7:15
Paddison SJ, Paul R, Zawodzinski TA (2001) J Chem Phys 115:7753
Paul R, Paddison SJ (2001) J Chem Phys 115:7762
Paddison SJ, Paul R, Zawodzinski TA (2000) J Electrochem Soc 147:617
Eikerling M, Kornyshev AA, Stimming U (1997) J Phys Chem B 101:10807
Khalatur P, Talitskikh S, Khokhlov A (2002) Macromol Theory Simul 11:566
Pismenskiy A, Nikonenko V, Urtenov M, Pourcelly G (2006) Desalination 192:374
Leah RT, Brandon NP, Vesovic V, Kelsall GH (2000) J Electrochem Soc 147:4173
Pitzer KS (1991) Activity coefficients in electrolyte solutions. CRC Press, Boca Raton
van der Stegen JHG, Weerdenburg H, van der Veen AJ, Hogendoorn JA, Versteeg GF (1999) Fluid Phase Equilibria 157:181
Roušar I, Hostomský J, Cezner V, Štverák B (1971) J Electrochem Soc 118:881
Chen CC, Britt HI, Boston JF, Clarke WM (1983) Thermodynamic property evaluation in computer-based flowsheet simulation for aqueous electrolyte systems. AIChE Meeting, Denver
Chen CC, Britt HI, Boston JF, Evans LB (1982) AIChE J 28:588
Lide DR (1997) CRC handbook of chemistry and physics, 78th edn. CRC Press, Boca Raton
Yakimenko LM, Pasmanik MI (1976) Spravochnik po Proizvodstvu Khlora, Kausticheskoi Sody i Khlorproduktov. Khimiya, Moscow, pp 197–201
Brown PN, Hindmarsh AC, Petzold LR (1995) Consistent initial condition calculation for differential-algebraic systems, LLNL Report UCRL-JC-122175
IMSL Math/Library, FORTRAN Subroutines for Mathematical Applications, vol 2, Visual Numerics
Acknowledgements
Financial support by the Grant Agency of the Czech Republic under project number 203/05/0080 and by the Ministry of Education, Youth and Sports of teh Czech Republic under project number MSM6046137301 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fíla, V., Bouzek, K. The effect of convection in the external diffusion layer on the results of a mathematical model of multiple ion transport across an ion-selective membrane. J Appl Electrochem 38, 1241–1252 (2008). https://doi.org/10.1007/s10800-008-9545-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-008-9545-z