Abstract
Purpose
To determine the safety and efficacy of adding topical bromfenac 0.09% in the treatment of diabetic macular edema.
Methods
Seventy patients (70 eyes) with center involved diabetic macular edema with macular thickness (300–500 μm) were included. Patients were divided randomly into two groups: 35 eyes in each group. Both groups were treated with intravitreal ranibizumab monthly for three consecutive months. Bromfenac 0.09% eye drops twice daily was added to the treatment of study group for six months from commencement of treatment. The efficacy of topical bromfenac was evaluated by comparing both groups through follow-up period as regards to visual acuity, central and average thickness and the need for re-injection.
Results
Patients treated with topical bromfenac in addition to intravitreal ranibizumab revealed significant improvement in visual acuity, more reduction in central and average macular thickness and less tendency to need reinjection compared to those treated with ranibizumab alone (p 0.013, p 0.010 and p 0.022, respectively). No side effects was encountered with the use of topical bromfenac.
Conclusion
Topical bromfenac 0.09% twice a day could enhance and sustain the efficacy of intravitreal ranibizumab in the treatment of diabetic macular edema without increasing the incidence of corneal side effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy and consequently diabetic macular edema (DME) remains the leading cause of vision loss particularly among older patients with type 2 diabetes [1]. Being multifactorial, the pathogenesis of DME is not yet fully known with hypoxic state stimulates vascular endothelial growth factor (VEGF), causing more edema [2, 3].
Intravitreal injection of anti-VEGF agents has revolutionized the treatment of DME. Ranibizumab represents a monoclonal antibody antigen-binding fragment, which is administered intravitreally to neutralize all isoforms of vascular endothelial growth factor A [4]. But its consecutive monthly injections affect patient’s productivity, cost and time, in addition to the risk of endophthalmitis [5]. Hence, the need for adjuvant therapies is raised to enhance the effects of intravitreal anti-VEGFs or decrease the frequency of injections.
Inflammatory mediators may participate in the progression of vascular disease in patients with diabetic retinopathy. Therefore, nonsteroidal anti-inflammatory drugs (NSAIDs) were suggested to be beneficial in the reduction of vascular permeability and accordingly in the management of diabetic macular edema. Moreover, topical NSAIDs have fewer side effects [6, 7].
Bromfenac is an NSAID with a topical formulation. Its mechanism of action is binding to and inhibiting the activity of cyclooxygenase II (COX II), precluding conversion of arachidonic acid to cyclic endoperoxides, which are precursors of prostaglandins (PG). Its specific differences in the pharmacodynamics and tissue penetration are the reason for its superiority over other agents in its class, in addition to its capability to penetrate into the posterior segment [8].
Considering the high prevalence of DME and its challenging treatment, the present study was conducted to investigate whether addition of topical bromfenac to the intravitreal ranibizumab would promote its efficacy compared with intravitreal ranibizumab only.
Aim of the work
To determine the efficacy and safety of topical bromfenac 0.09% in addition to intravitreal ranibizumab for the treatment of diabetic macular edema.
Patients and methods
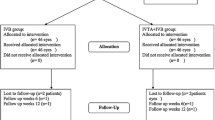
This prospective randomized clinical trial was performed from May 2021 to March 2022 at Aswan University Hospital. Informed consent was signed by all patients and approval for the study was obtained from the local Institutional Review Board (IRB). Also, the study was in line with the Declaration of Helsinki.
Sample size
The sample size was carried out using G*Power 3 software. A calculated minimum sample of 60 patients with diabetic macular edema was needed. However, to avoid missed or lost to follow-up patients, we enrolled 70 patients (divided into two groups, each of them including 35 patients). Group (I) received ranibizumab intravitreal injection, and group (II) received ranibizumab intravitreal injection plus additional topical bromfenac 0.09% twice daily for six months.
Inclusion criteria
Patients aged of 20–60 years old, with evidence of non-proliferative diabetic retinopathy, and centers involving macular edema (average macular thickness of 300–500 μm) who agreed to use additional topical treatments to the standard intravitreal injection of anti-VEGF were included.
Exclusion criteria
Patients with proliferative diabetic retinopathy, history of cataract surgery in less than one year, intravitreal injection or macular photocoagulation in less than six months and/or any other concomitant ocular pathologies causing vision loss were excluded from the study.
Methods
After obtaining complete medical and ocular history, all included patients were subjected to full ophthalmological examination including unaided and best-corrected visual acuity, slit-lamp examination for anterior segment, intraocular pressure (IOP) measuring using applanation tonometry and fundus examination using slit-lamp biomicroscopy using Volk 78D fundus lens. Fluorescein angiography was done, and optical coherence tomography (OCT) was performed using (Topcon 3D-OCT-2000 (FA Plus), Tokyo, Japan). Central macular thickness (CMT) represented thickness at the point of intersection of the six radial scans of the OCT, while average macular thickness (AMT) denoted thickness in the central 1000 μm diameter area of the macula.
All the included patients received three consecutive monthly injections of intravitreal ranibizumab 0.05 mL of 6 mg/mL solution (Lucentis™, Genentech, South San Francisco, CA). Patients were randomly distributed using the lottery method. They received either topical bromfenac 0.09% (Bromoflam™, Orchidia pharmaceutical, Al-Obour City, Egypt) in the first group or artificial tears twice a day over the study period (6 months) in the second group.
The patients were followed up every 4 weeks. At each visit, patients were undergone unaided and best-corrected visual acuity (BCVA) measured using LogMar, slit-lamp examination for anterior segment, IOP measuring using applanation tonometry and fundus examination. Central and average macular thickness were measured by OCT and reported at first, third and sixth months. Each patient had followed up chart divided into squares numbered by month days to ensure patient compliance with eye drops twice daily. Success was measured by improvement in BCVA, decrease signs of non-proliferative diabetic retinopathy (NPDR), decrease macular thickness in OCT, decrease number of intravitreal injections or increase duration of reinjection.
The number of intravitreal injections (IVR) was compared between the study groups and reinjection was administered based on the “as-needed” protocol if average macular thickness was 300 μm or more and visual acuity was not improved. Complications of injections as well as side effects of topical treatment during follow-up period were monitored and reported.
Statistical analysis
Data were verified, coded and analyzed using IBM SPSS 24.0 (IBM SPSS Inc., Chicago, IL, USA). Descriptive statistics: means, standard deviations, median, range and percentages were calculated. Chi-square/Fisher’s exact test was used to test significance. For continuous variables, independent t-test analysis and two-way repeated measure ANOVA (RM-ANOVA) tests were used appropriately. The clinical and demographic factors with proven statistical significance from the univariate analyses were further included in multivariable logistic regression models. A significant p value was considered when it is less than 0.05.
Results
A total number of 70 eyes from 70 patients who completed the study in two arms (35 eyes in each group) were included for final analysis. The baseline characteristics of the study groups regarding age, gender, duration of diabetes and glycosylated hemoglobin percentage (HbA1C) are demonstrated in Table 1 revealing no statistical differences between both study groups.
As regards to visual acuity, there was no significant difference between both groups at baseline and through follow-up period. Compared to baseline, improvement in visual acuity was noted after sixth months in both groups with statistically significant difference in ranibizumab plus bromfenac group (p < 0.001). For the interaction between time and group effect, the improvement in visual acuity was more evident in the study group with statistically significant (p = 0.013) (Table 2).
Although fluctuation of intraocular pressure was found, there was no significant difference between both groups at baseline and follow-ups. For the interaction between time and group effect, the reduction in IOP was slightly more evident in the study group with nonstatistically significant (p = 0.084) (Table 2).
Significant reduction of central macular thickness (CMT) was noted in both treated groups at the end of follow-up compared to baseline values. At baseline, first month and third months, there was nonstatistically significant difference between both groups (p = 0.214, 0.846 and 0.298, respectively). On the other hand, at sixth month, the CMT was significantly lower in ranibizumab plus bromfenac group (p = 0.013). For the interaction between time and group effect, the reduction in CMT was statistically significant in ranibizumab plus bromfenac group (p = 0.010) (Table 2).
At baseline and first month, there was nonstatistically significant difference in AMT between both study groups (p = 0.934 and 0.288, respectively). On the other hand, at third and sixth months, the AMT was significantly lower in ranibizumab plus bromfenac compared with the other one (p = 0.044 and 0.027, respectively). Additionally, there was significant reduction in AMT only in ranibizumab plus bromfenac group at the end of study period (p < 0.001). For interaction between time and group effect, the reduction in AMT was more evident in ranibizumab plus bromfenac group with statistically nonsignificant difference (p = 0.060) (Table 2).
There were eight eyes from ranibizumab group needed reinjection from the fourth to sixth months compared to only two eyes, which needed reinjection in ranibizumab plus bromfenac group (p = 0.022) (Table 3). Self-limited ocular discomfort was reported by two patients (5.7%) in ranibizumab group and in five patients (14.2%) in ranibizumab plus bromfenac group, while subconjunctival hemorrhage was reported by three patients (8.6%) in ranibizumab group compared to two patients (5.7%) in ranibizumab plus bromfenac group with nonstatistical differences. No serious complications as endophthalmitis or corneal ulceration have been encountered in both study groups (Table 3).
Table 4 shows the multivariable logistic regression model of the independent effect of adding topical bromfenac as an adjunctive treatment with intravitreal ranibizumab for diabetic macular edema. After adjusting for the basic demographic and clinical data, the final model contained two factors, i.e., CMT and AMT.
It was found that one µm decrease in the central macular thickness was associated with 7% (OR = 0.993, 95% CI: 0.987–0.999) increase in the chance of adding bromfenac, and this was statistically significant (p = 0.018). Likewise, one µm decrease in the average macular thickness was associated with 12% (OR = 0.989, 95% CI: 0.978–0.999) increase in the chance of adding bromfenac, and this was statistically significant (p = 0.031).
Discussion
Anti-vascular endothelial growth factor (Anti-VEGF) drugs have resulted in improved visual outcomes, while treating diabetic macular edema. To maintain the initial gains in VA, repeated intravitreal injections are needed with subsequent increase of economic burden as well as possible occurrence of serious complications [9, 10].
Although VEGF upregulation plays an essential role in DME, several studies have demonstrated elevated inflammatory factors in the vitreous cavity of patients with diabetic retinopathy [11]. Inflammatory mediators and prostaglandins, such as interleukins and prostaglandin E2, facilitate leukocyte migration, induce blood–retinal barrier disruption and increase vascular permeability leading to exaggeration of edema [12].
Bromfenac is indicated for the treatment of ocular inflammatory diseases and postoperative inflammation. It is more lipophilic than other NSAIDs, facilitating corneal penetration, increasing duration of action and enhancing COX II inhibitory activity, thereby reducing production of prostaglandins and inflammatory cytokines [13, 14].
Due to the paucity of studies reported about using of topical bromfenac after intravitreal ranibizumab for the treatment of DME, our results will be compared with studies reported about using this combination in the treatment of other causes of macular edema or about using other NSAIDs such as topical nepafenac or ketorolac after intravitreal ranibizumab in treatment of DME.
Both bromfenac and nepafenac may be more beneficial in the treatment of DME compared to ketorolac due to their higher levels of posterior segment penetration [15].
The present randomized clinical trial showed that adding topical bromfenac to intravitreal ranibizumab produces additive effects on DME treatment. Both study groups revealed improvement of visual acuity after sixth month follow-up compared to the baseline. Although statistically not significant, the beneficial effect of visual acuity was more evident in patients who received ranibizumab plus topical bromfenac. This was found also to be correlated with the decrease in CMT and AMT at the sixth months of follow-up.
Nikkhah et al. [16] found that using topical ketorolac 0.5% three times a day after intravitreal bevacizumab in the management of diabetic macular edema, the mean change of visual acuity was greater in bevacizumab plus ketorolac group. Grant also suggested a synergistic effect of topical bromfenac on anti-VEGF treatment in inhibiting choroidal neovascularization (CNV) secondary to age-related macular degeneration (AMD). Patients with topical bromfenac had a significant improvement in visual outcomes [17]. Garcia-Gonzalez et al. [18] suggested that patients with diabetic macular edema treated with topical nepafenac, showed statistically significant improvement in visual acuity.
On the other hand, Shimura et al. [19] did not find any improvement in visual acuity when using combination of ranibizumab plus topical bromfenac in treating macular edema secondary to branch retinal vein occlusion.
In a case series, Pinna et al. [20] applied only topical bromfenac twice a day to treat center involved DME. After 30 days, they found nonsignificant improvement in visual acuity. The same result was also reported by other investigators [5]. Other researchers concluded that visual acuity did not’ improved in patients with neovascular AMD, they could not detect a beneficial effect of adding topical bromfenac (0.09%) twice daily over two months [21].
Both study arms showed macular thickness reduction after sixth month of follow-up compared to the baseline. Furthermore, patients who received ranibizumab plus topical bromfenac demonstrated a more statistically significant reduction of central and average macular thickness compared to patients treated by ranibizumab alone.
Gomi concluded that reduction in CMT in exudative age-related macular degeneration between baseline and month 6 tended to be greater in eyes treated with topical bromfenac. Thus, combined bromfenac may strengthen and extend the effect of an anti-VEGF drug [5]. Also, Warren et al. [22] reported that bromfenac reduced the macular thickness in eyes with chronic pseudophakic cystoid macular edema.
Nikkhah et al. [16] concluded that reduction of mean CMT was significantly greater in bevacizumab plus ketorolac group compared with bevacizumab group in the management of diabetic macular edema.
Garcia-Gonzalez et al. [18] also showed statistically significant reduction of macular thickness when using topical nepafenac alone in the treatment of DME. Also, Pinna et al. [20] also found that the difference between the initial and posttreatment mean macular thickness in DME fell just short of statistical significance (p = 0.06) after applying topical bromfenac only. While others found that in eyes with noncentral DME, topical nepafenac 0.1% for one year likely does not have a significant effect on OCT-measured retinal thickness [23].
Intraocular pressure was found to be reduced in the ranibizumab plus bromfenac group (although not significant) compared to group treated with ranibizumab only. This finding could be due to the effect of bromfenac on prostaglandin (PG) production particularly prostaglandin E2 (PGE2), which increases the IOP by local vasodilation and increased permeability of blood–aqueous barrier [24, 25].
In the present study, the need for reinjection was significantly less among patients in ranibizumab plus bromfenac group compared to those treated with ranibizumab alone. Grant also noticed significant decrease in the number of ranibizumab injections after using of topical bromfenac with anti-VEGF for inhibiting CNV secondary to AMD [17]. Moreover, other investigators reported the same advantage of this combination during the treatment of macular edema secondary to branch retinal vein occlusion [19]. On the other hand, Nikkhah et al. [16] found that bevacizumab group and bevacizumab plus ketorolac group were comparable regarding number of intravitreal bevacizumab injections.
Both study groups revealed no serious side effects and adding of topical bromfenac to intravitreal ranibizumab did not alternate this finding. On the contrary, decrease corneal sensation as well as corneal melting were reported after using topical bromfenac. Cumulative application of topical bromfenac may cause a neurolytic keratitis like situation, which may trigger the formation of corneal ulcer [26].
The major limitation of this study was the small sample size and shorter study period. Follow-up for longer period and inclusion of larger number of patients is recommended.
Conclusion
Topical bromfenac 0.09% twice a day could enhance and sustain the efficacy of intravitreal ranibizumab in the treatment of diabetic macular edema without increasing the incidence of corneal side effects.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Lee R, Wong TY, Sabanayagam C (2015) Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2:17–28
Singh A, Stewart JM (2009) Pathophysiology of diabetic macular edema. Int Ophthalmol Clin 49:1–11
Musat O, Cernat C, Labib M et al (2015) Diabetic macular edema. Rom J Ophthalmol 59(3):133–136
Ferrara N, Damico L, Shams N et al (2006) Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 26(8):859–870
Gomi F, Sawa M, Tsujikawa M et al (2012) Topical bromfenac as an adjunctive treatment with intravitreal ranibizumab for exudative age-related macular degeneration. Retina 32(9):1804–1810
Ke TL, Graff G, Spellman JM et al (2000) Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: II. In vitro bioactivation and permeation of external ocular barriers. Inflammation 24:371–384
Meleth AD, Agrón E, Chan CC et al (2005) Serum inflammatory markers in diabetic retinopathy. Invest Ophthalmol Vis Sci 46:4295–4301
Donnenfeld ED, Donnenfeld A (2006) Global experience with Xibrom (bromfenac ophthalmic solution) 0.09%: the first twice-daily ophthalmic nonsteroidal anti-inflammatory drug. Int Ophthalmol Clin 46(4):20–32
Regillo CD, Brown DM, Abraham P et al (2008) Randomized, double- masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol 145:239–248
Sampat KM, Garg SJ (2010) Complications of intravitreal injections. Curr Opin Ophthalmol 21:178–183
Zhou J, Wang S, Xia X (2012) Role of intravitreal infammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res 37:416–420
Gonzalez VH, Campbell J, Holekamp NM et al (2016) Early and long-term responses to anti–vascular endothelial growth factor therapy in diabetic macular edema: analysis of protocol I data. Am J Ophthalmol 172:72–79
Kim SJ, Flach AJ, Jampol LM (2010) Nonsteroidal anti-infammatory drugs in ophthalmology. Surv Ophthalmol 55:108–133
Schechter BA, Trattler W (2010) Efficacy and safety of bromfenac for the treatment of corneal ulcer pain. Adv Ther 27(10):756–761. https://doi.org/10.1007/s12325-010-0066-x
Heier JS, Awh CC, Busbee BG et al (2009) Vitreous non-steroidal anti-inflammatory drug concentrations and prostaglandin E2 levels in vitrectomy patients treated with ketorolac 0.4%, bromfenac 0.09%, and nepafenac 0.1%. Retina 29(9):1310–1313
Nikkhah H, Niazpour R, Entezari M et al (2021) Topical ketorolac as an adjunctive treatment with intravitreal bevacizumab in the management of diabetic macular edema: a double-masked placebo-controlled randomized clinical trial. Graefes Arch Clin Exp Ophthalmol 259(10):2949–2959
Grant CA (2008) Combination therapy: Lucentis (ranibizumab injection) and Xibrom (bromfenac ophthalmic solution) 0.09% in the treatment of choroidal neovascular membrane secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci 49(suppl):563
Garcia-Gonzalez J, Emanuelli A, Berrocal M (2009) Topical nepafenac 0.1% for the treatment of macular edema secondary to diabetic retinopathy and retinal vascular occlusions. Invest Ophthalmol Vis Sci 50(13):13–49
Shimura M, Yasuda K (2015) Topical bromfenac reduces the frequency of intravitreal bevacizumab in patients with branch retinal vein occlusion. Br J Ophthalmol 99(2):215–219
Pinna A, Blasetti F, Ricci GDA et al (2017) Bromfenac eyedrops in the treatment of diabetic macular edema: a pilot study. Eur J Ophthalmol 27:326–330
Zweifel SA, Engelbert M, Khan S et al (2009) Retrospective review of the efficacy of topical bromfenac (0.09%) as an adjunctive therapy for patients with neovascular age-related macular degeneration. Retina 29:1527–1531
Warren KA, Bahrani H, Fox JE (2010) NSAIDs in combination therapy for the treatment of chronic pseudophakic cystoid macular edema. Retina 30:260–266
Friedman S, Almukhtar T, Baker C, Diabetic Retinopathy Clinical Research Network et al (2015) Topical nepafenec in eyes with noncentral diabetic macular edema. Retina 35(5):944–956
Ahuja M, Dhake A, Sharma S et al (2008) Topical ocular delivery of NSAIDs. AAPS Journal 10(2):229–241
Schoenberger SD, Kim SJ (2013) Nonsteroidal anti-inflammatory drugs for retinal disease. Int J Inflam Article 281981:8
Asai T, Nakagami T, Mochizuki M et al (2006) Three cases of corneal melting after instillation of a new nonsteroidal anti-inflammatory drug. Cornea 25(2):224–227. https://doi.org/10.1097/01.ico.0000177835.93130.d4
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funds, grants or other supports were received.
Author information
Authors and Affiliations
Contributions
AAE performed data acquisition, analyzing the data, statistics and results, interpreting the findings and drafting of the manuscript. MFK did data collection, writing the manuscript and statistics and results. AFG done conceptualization and study design, study supervision and writing & reviewing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or nonfinancial interests to disclose.
Ethics approval and consent to participate
Approval was obtained from the Institutional Review Board and Ethics committee.
Consent for publication
Obtained from the patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gabr, A.F., Kamel, M.F. & Elbarawy, A.A. Topical bromfenac as adjunctive treatment with intravitreal ranibizumab for diabetic macular edema. Int Ophthalmol 43, 3219–3226 (2023). https://doi.org/10.1007/s10792-023-02722-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-023-02722-1