Abstract
Purpose
To quantitatively assess postoperative rotational stability and visual acuity with the DFT/DATx15 extended depth of focus (EDOF) toric intraocular lens (IOL).
Methods
In this prospective case series, thirty-five patients with a calculated IOL power between + 15.0 D and + 25.0 D, corneal astigmatism between 0.75 D and 2.25 D, and no significant ocular pathology underwent cataract surgery. Primary outcome was rotational stability of the IOL at 1 month post-operatively. Secondary outcomes included residual refractive astigmatism, absolute residual astigmatism prediction error, and monocular distance and intermediate visual acuities.
Results
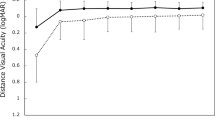
Mean absolute postoperative IOL rotation was 1.1 ± 0.2 degrees, with no rotation of more than 3 degrees at the final visit. Monocular mean best spectacle-corrected distance visual acuity (BSCDVA) improved from logMAR 0.27 ± 0.030 to 0.078 ± 0.017 (P < .001). Monocular uncorrected distance visual acuity (UCDVA) improved from 0.93 ± 0.096 to 0.18 ± 0.022 (P < .001). Best spectacle-corrected intermediate visual acuity (DSCIVA) was 0.17 ± 0.025, and uncorrected intermediate visual acuity (UCIVA) was 0.27 ± 0.040. Residual regular astigmatic refractive error was 0.21 ± 0.047 D.
Conclusions
The toric DFT/DATx15 EDOF lens showed excellent rotational stability and effective and predictable correction of astigmatism. Its refractive outcomes and safety profile were similar to those identified in prior studies of the non-toric DFT/DAT015 EDOF IOL. A small difference in monocular BSCDVA, of uncertain clinical significance, was found when comparing these outcomes with prior DFT/DAT015 data. The trial was retrospectively registered on November 5, 2021 (TRN NCT05119127).
Plain english summary
In cataract surgery, the natural lens of the eye is replaced with an artificial lens implant. In many cases, the patient’s glasses prescription in the operated eye can be reduced or eliminated through careful choice of a lens implant. There are many types of lens implants available. Toric lens implants are used to reduce one component of the glasses prescription, called regular astigmatism (or often just “astigmatism”). To maintain the full astigmatism-reducing effect of the toric lens, the lens implant must not rotate significantly within the eye after the surgery. The DFT/DATx15 (Vivity™) is a relatively new type of lens implant designed to offer patients good spectacle-free vision at far distances and improved glasses-free vision at arm’s length (“intermediate”) compared to a more traditional lens implant that is designed to maximize spectacle-free distance vision only. This study reports one surgeon’s experience with measuring the amount of rotation of DFT/DATx15 lenses after surgery. This study also assessed the ability of the DFT/DATx15 to reduce regular astigmatism and improve glasses-free vision at far and intermediate distances. The results show that this lens did not rotate significantly within the eye and was effective at reducing the regular astigmatism as intended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with clinically significant regular astigmatism typically require spectacle correction to achieve optimal visual acuity. Regular astigmatism can often be reduced or eliminated at the time of cataract surgery by using a toric intraocular lens (IOL) to compensate for corneal astigmatism. Successful use of a toric IOL requires maintaining precise alignment of the marked flat meridian of the IOL with the steep meridian of the patient’s corneal astigmatism. Postoperative rotational stability of the IOL is therefore of great interest to the cataract surgeon. The efficacy of toric IOLs in correcting corneal astigmatism has been demonstrated in numerous studies; a 2016 meta-analysis [1] of 13 randomized controlled trials comparing toric vs. non-toric IOLs found that use of a toric IOL was associated with higher postoperative uncorrected visual acuity and a higher fraction of patients reporting postoperative spectacle independence for distance vision. Rates of repositioning surgery have been found to be low [2,3,4,5]. The AcrySof® toric IOL has been found to be associated with a lower degree of rotation than the TECNIS® toric IOL in some [4, 6,7,8,9] but not all [10] studies.
Numerous approaches are available for improving or preserving near and intermediate vision after cataract surgery, [11,12,13,14,15] each with its own advantages and trade-offs. These include spectacles and contact lenses, monovision, multifocal and enhanced depth of focus IOLs, pinhole IOLs, pharmacologic miosis, and corneal inlays. The DFT/DATx15 (Acrysof Vivity™; Alcon, Fort Worth, Texas, USA) intraocular lens is a single-piece soft hydrophobic acrylic lens featuring a proprietary non-diffractive anterior surface geometry [16]. It is designed to yield improved uncorrected visual acuity at intermediate distances, as compared to a traditional monofocal design, without sacrificing uncorrected distance visual acuity and while minimizing visual artifacts and loss of contrast sensitivity. Premarketing approval (PMA) data submitted to the FDA for the non-toric model (DFT/DAT015) of this lens demonstrated [17, 18] superiority of monocular distance-corrected intermediate visual acuity and noninferiority of best-corrected distance visual acuity to within 0.1 logMAR units, under photopic conditions, compared with a monofocal IOL. A recent study [19] under real-world conditions confirmed these results.
The DFT/DATx15 lens also is available with a toric posterior surface similar to that of other Alcon toric IOLs, such as the SN6AT series, whose safety, efficacy, and rotational stability have been previously evaluated [20,21,22,23,24,25,26,27,28,29]. Because of this physical similarity, clinical testing specifically of the toric model of the DFT/DATx15 lens was not required for FDA approval, and data regarding its rotational stability have not previously been available.
This study was therefore undertaken to assess the refractive performance of the toric models of the DFT/DATx15 EDOF IOL, with particular attention to rotational stability. To our knowledge, this report provides some of the first real-world data regarding rotational stability and visual outcomes in patients with regular corneal astigmatism implanted with this type of lens.
Materials and methods
Study design
Patients were recruited between September 30, 2020, and February 28, 2021. Informed consent was obtained from all patients. This study conformed to the principles of the Declaration of Helsinki, ISO 14155:2011, and all other applicable regulations, and was approved and monitored by the Institutional Review Board as protocol number 63171943. This trial was registered at www.clinicaltrials.gov with registration number TRN NCT05119127. Individual deidentified patient data will not be externally shared. The following description includes information from the unpublished study protocol.
Inclusion criteria were as follows: eligible subjects were those at least 45 years of age undergoing cataract surgery with intraocular lens implantation who elected placement of a DFTx15 or DATx15 toric EDOF IOL. Only eyes requiring a calculated IOL power between + 15.0 D to + 25.0 D and having regular corneal astigmatism correctable with one of the study lenses (corresponding to keratometric astigmatism values of approximately 0.75–2.25 D) were included. Finally, subjects had to be willing and able to adhere to all scheduled visits and undergo all other study procedures. For patients with two eligible eyes, only the first eye to undergo cataract surgery was included in the study.
Subjects were required to have no other identifiable ocular pathology potentially compromising visual acuity. Only subjects with a postoperative visual potential of 0.2 logMAR (Snellen equivalent 20/32) or better in both eyes, in the opinion of the investigators, were considered eligible. Other exclusion criteria included clinically significant corneal dystrophies or a history of corneal refractive surgery, abnormalities of the pupil, uveitis (whether infectious or noninfectious), or a history of chronic intraocular inflammation. Patients with any macular disease affecting vision were considered ineligible. Patients with a history of glaucoma or retinal detachment were also specifically excluded from the study, regardless of visual prognosis.
The primary outcome was the magnitude of net postoperative rotation of the toric IOL, measured at each scheduled postoperative study visit. Secondary endpoints of interest included the proportion of eyes with final net postoperative rotation of 5 degrees or less; the proportion of eyes with absolute residual astigmatism prediction error ≤ 0.5 D; the proportion of eyes with residual astigmatism ≤ 0.5 D and ≤ 1.00 D; and visual acuity outcomes, including monocular uncorrected distance (UCDVA) and intermediate (UCIVA), best spectacle-corrected distance (BSCDVA), and best distance spectacle-corrected intermediate (DSCIVA) visual acuities. Post hoc subgroup analysis was performed to investigate the effect of large (greater than 5 degree) intraoperative IOL rotations prompted by intraoperative aberrometry (IA).
Preoperative workup
All patients underwent a complete ophthalmic history and exam, including subjective manifest refraction, intraocular pressure (IOP) measurement, slit lamp exam, and dilated fundoscopic exam. Digital alignment data using limbal registration were also captured preoperatively for all patients. Biometry was performed with a LENSTAR 900 (Haag-Streit USA, Mason, OH) optical biometer. Best corrected and uncorrected photopic visual acuity without glare, manifest refraction, intraocular pressure, slit lamp exam, dilated fundoscopic exam, and IOL orientation were obtained at all scheduled postoperative clinic visits; these were conducted approximately 1 day, 1–2 weeks, and 1 month after surgery (hereafter POD#1, POW#1, and POM#1). Postoperative IOL orientation was measured using digital photography with a slit lamp-mounted iPhone (Apple; Cupertino, California, USA), utilizing the toric reticle on the toriCAM app (Graham Barrett; version 4.0) as a reference mark. Photographs were then analyzed to determine the IOL axis. Patient medications, adverse events, device deficiencies, and subject-reported symptoms were documented at each visit during the postoperative period.
Surgical technique
The Barrett Universal 2 toric formula was used for IOL power calculations and served as the basis for calculation of absolute residual astigmatism prediction error. A plano refractive target was chosen for all eyes. All surgery was performed by an experienced cataract surgeon (KB). An intraocular lens model number DFT315 or DAT315; DFT415 or DAT415; or DFT515 or DAT515, hereafter referred to as “T3,” “T4,” or “T5,” was implanted into the capsular bag using standard small-incision phacoemulsification techniques with a temporal clear corneal incision. No relaxing incisions were performed. The VERION™ digital marking system was used intraoperatively to guide and confirm alignment of the toric IOL. Intraoperative aberrometry (IA) using the ORA System® (Alcon) was also used to guide selection of toric IOL power and to verify optimal alignment of the IOL. If the IOL orientation was changed based on IA, the final orientation (“implantation axis”) was recorded with the VERION™ system and used as the baseline from which to assess postoperative rotation. Absolute postoperative rotation of an IOL at a particular point in time was defined as the absolute value of the difference between the implantation axis and the axis measured at the specified time point.
Data analysis
Data were analyzed using Wizard 2.0.5 on OS X. All statistical tests were two-tailed, with a P value of 0.05 chosen as the definition of statistical significance. Data were approximately normally distributed except where noted. IOP data did not appear to be normally distributed and were evaluated using the Friedman non-parametric test for unequal ranks. Visual acuity and refractive outcome time series were characterized using a one-way ANOVA test. Data regarding the proportion of eyes achieving final refractive endpoints were evaluated with a chi-square test. Post hoc subgroup analysis was performed using a t test for equal means except as noted. Results are presented as value ± standard error unless otherwise specified. Visual acuity is presented in units of logMAR, except where otherwise specified.
Results
Demographics
A total of 35 eyes of 35 patients were recruited. Data for the POM#1 visit were unavailable for one patient. All other enrolled patients completed all study visits. Preoperative uncorrected visual acuity data were unavailable for two patients. Demographic data are reported in Table 1. There was a trend toward female predominance that did not reach statistical significance (P = 0.063). The axial length of all enrolled eyes fell within the range of 22–26 mm. A majority of eyes (74%) received a T3 lens, with smaller numbers of eyes receiving T4 (17%) and T5 (8.6%) lenses.
Safety
Median intraocular pressure (IOP) at baseline was 15. Statistically significant differences (P = 0.004) were found among the median postoperative IOP values: median IOP at POD#1 was slightly higher than baseline, by 1.5 mm Hg, but normalized on subsequent visits.
There were no major adverse events during the study. Any complications were minor and consistent in severity and frequency with those expected to occur with routine cataract surgery. No patient required return to the operating room for rotation of a toric IOL.
Rotational stability
The mean absolute postoperative IOL rotation at POM#1 was 1.1 ± 0.2 degrees. This was stable throughout the postoperative period (ANOVA, P = 0.58) when measured at POD#1 and POW#1 (see Table 2). The maximum observed value of postoperative IOL rotation at POM#1 was 3.0 degrees. Therefore, one of the secondary endpoints of the study, the proportion of IOLs undergoing less than 5 degrees of net postoperative rotation, was met by 100% of eyes for which POM#1 data were available.
When eyes were grouped based on whether the amount of intraoperative IOL rotation performed as a result of guidance provided by IA was greater than, or less than or equal to, 5 degrees (see Table 3), there was no significant difference in mean absolute postoperative rotation between the two subgroups (1.57 ± 0.57 degrees vs. 0.96 ± 0.23 degrees, respectively; P = 0.26).
Visual acuity
Mean BSCDVA improved from 0.27 ± 0.030 (Snellen 20/37) preoperatively to 0.078 ± 0.017 (Snellen 20/24) at POM#1 (ANOVA, P < 0.001) (Table 4). Mean UCDVA improved from 0.93 ± 0.096 (Snellen 20/170) to 0.18 ± 0.022 (Snellen 20/30) at POM#1 (ANOVA, P < 0.001). Preoperative intermediate visual acuity was not assessed in this study; however, mean postoperative DSCIVA was 0.17 ± 0.025 (Snellen 20/30), and mean postoperative UCIVA was 0.27 ± 0.040 (Snellen 20/37).
Subgroup analysis based on the amount of IA-guided rotation found no statistically significant differences in any measured visual acuity outcome between the two subgroups. (see Table 3).
Refractive outcomes
Mean residual regular astigmatic refractive error at POM#1 (Table 3) was 0.21 ± 0.047 D and was not statistically distinguishable at any time point (ANOVA, P = 0.91) from the mean residual astigmatism predicted during treatment planning. 94% of eyes achieved a final residual astigmatic refractive error of less than or equal to 0.5 D, and 100% had less than or equal to 1.0 D (Table 3). 94% of eyes were found to have a residual astigmatic refractive error at POM#1 that was within 0.5 D of the value predicted during treatment planning. There were no statistically significant differences in these proportions at any postoperative time point. Pre- and postoperative astigmatic refractive outcomes were visualized in a double-angle plot (Fig. 1).
Preoperative and postoperative refractive regular astigmatism. The cylindrical component of preoperative and postoperative (month 1) manifest refractions were plotted [30] on a single-angle plot in positive cylinder notation
When refractive outcome data were stratified on the amount of IA-guided rotation (Table 3), there were no statistically significant differences in residual refractive astigmatism (0.20 ± 0.055 vs. 0.25 ± 0.094; P = 0.70) or absolute residual astigmatism prediction error less than or equal to 0.5 D (96% vs. 86%; P = 0.28) between the low rotation (< 5 degree) and the high rotation (> 5 degree) subgroups, respectively.
Discussion
To our knowledge, this is the first publication that reports real-world performance of the toric model of the DFT/DATx15 EDOF IOL.
A number of previous reports using physically similar, monofocal AcrySof® toric IOLs found mean absolute postoperative rotation of approximately 3.5–4 degrees [20,21,22,23,24, 26, 27], although a handful of studies have reported smaller values, such as 1.6 degrees [28] or 2.66 degrees [29], or reported a median (2 degrees [25]) rather than a mean. Our corresponding figure was 1.1 degrees, with a 95% CI of 0.7–1.5 degrees. Differences in inclusion criteria, patient population, surgical technique, and/or IOL orientation measurement technique, as discussed below, could account for the smaller mean postoperative rotations seen in this study compared to prior work. Our result supports the hypothesis that the rotational stability of the toric DFT/DATx15 is noninferior to that of other AcrySof® toric IOLs.
It is of clinical interest to compare this study's refractive outcome data to previous data [17,18,19] regarding the (non-toric) DFT/DAT015 EDOF IOL with the understanding that differences in inclusion criteria and methodology limit the validity of this comparison. The PMA study [17] and the more recent report by Bala et al [19] examined the DFT/DAT015 in patients without significant corneal astigmatism. Mean monocular BSCDVA in this study (0.078 ± 0.017; approx. Snellen 20/24) was slightly less than that reported for the PMA data (0.016 ± 0.0091; Snellen 20/21) and that of Bala et al [19] (–0.008 ± 0.0076; Snellen 20/20). Monocular UCDVA was not reported by either study. Monocular DSCIVA values were similar among the studies (this study, 0.169 ± 0.025; Bala et al., [19] 0.161 ± 0.0136; PMA data [17], 0.148 ± 0.0120). Small BSCDVA disparities could reflect characteristics of the different study populations. In addition to intrinsic differences in the amount of corneal astigmatism, the populations could also plausibly differ in the prevalence of comorbidities such as higher-order corneal aberrations. Inclusion and exclusion criteria were also dissimilar; for example, the PMA study excluded all “clinically significant ocular surface disease that would affect study measurements,” [17] whereas such disease was excluded in our study only if it limited visual prognosis. The time interval between our final visit, at 1 month, and the defined endpoints of the other two studies, at 6 months, could allow visual changes to occur due to postoperative evolution of the ocular surface or neuroadaptation. Other differences in data collection and reporting methodology, surgical planning and technique, and demographics, as well as any hypothetical differences attributable to use of the lens itself, could also explain this result. Additional studies would be needed to confirm the existence of this small numerical monocular BSCDVA difference, evaluate monocular UCDVA, and assess any clinical significance.
One strength of this study was the use of digital marking to maximize measurement accuracy of IOL orientation. There are several potential sources of error in the quantitative assessment of IOL rotational stability, and no gold standard methodology exists. The implantation axis can be defined by the intended placement axis or can be measured either intraoperatively or in the immediate postoperative period. Definition of implantation axis by postoperative measurement carries the risk that rotation that occurs between the time of implantation and the time of axis measurement will not be recorded; indeed, one study [31] found that rotation during the first postoperative hour constituted the largest component of the final net postoperative rotation. Preoperative definition and intraoperative measurement of the reference axis both rely on accurate marking of the orientation of the eye and measurement of the orientation of the IOL relative to the marks. Several studies (reviewed by Panagiotopoulou et al [32]) have found intraoperative digital marking systems, including the VERION™, to be equivalent or superior to manual marking.
Our study defined the implantation axis as that measured by the VERION™, thereby eliminating any effects attributable to inaccuracy of manual axis marking. Thus, the accuracy of the implantation axis measurement in our study was limited only by the accuracy of the VERION™ Digital Marker. In the absence of a gold standard methodology, the absolute accuracy of any given marking device is difficult to determine. One study [33] comparing two different intraoperative digital marking systems, including the VERION™, found alignment discrepancies between the two devices of 3 degrees or more in 47% of cases; however, absolute accuracy was not assessed, and it is not known which device, if either, was superior.
Postoperative measurement of IOL orientation can be accomplished with slit lamp techniques, optionally incorporating digital photography, digital image analysis [29], and/or use of custom software [28, 34]. In our study, postoperative IOL axis was measured by analyzing digital images taken from a slit lamp-mounted smartphone running the toriCAM app. In the setting of a dilated pupil, this software can acquire an image of the IOL orientation markings and overlay a toric reticle oriented by gravity. This procedure does not account for cyclorotation and is dependent on the accuracy of the accelerometer and camera hardware on the individual smartphone used for the measurement; no study in the open literature, to our knowledge, has assessed performance of the toriCAM app for postoperative measurement of IOL orientation. However, the accuracy of preoperative marking of the eye using the toriCAM app has been formally evaluated [35], using the iTrace wavefront aberrometer/topographer as a reference. Mean absolute error was found to be 1.28 ± 1.34 degrees, which could plausibly be interpreted as an upper bound on the error associated with reticle placement when using the toriCAM app.
For the purpose of toric IOL alignment, some recent studies have found advantages to the use of IA; however, not all studies concur, and the benefit may depend on the IOL formula against which it is compared [36,37,38,39,40,41,42,43,44] (reviewed in Kane [45]). Our methodology allowed us to capture all rotations prompted by IA. We defined a subgroup of eyes for which this rotation was more than 5 degrees and detected no outcome differences between this subgroup and the remainder of the study population. Because IA was utilized for all patients, and the sample size of the high rotation subgroup was particularly limited, the effect of IA cannot be reliably inferred from our data set alone. However, malrotation of a toric IOL by 5 degrees corresponds to a theoretical loss of approximately 17% of the astigmatic effect [46, 47]. This corresponds to roughly 0.25 D of lost astigmatism correction for a T3 lens, although in general the baseline residual astigmatism and lost astigmatism correction do not share a common axis and do not add linearly. Taking note of this estimate and the actual mean residual astigmatism of only 0.25 ± 0.094 D in the high rotation subgroup, it seems plausible to speculate that there could have been a clinically significant increase in residual astigmatism in this subgroup if IA had not been used. It therefore bears mentioning that the results of this study with regard to uncorrected visual acuity and astigmatic refractive outcomes may not be fully generalizable to settings in which IA is not routinely employed.
Limitations of this study include the following: in the absence of a control group, caution must be used when comparing our outcome data with results obtained using other lens options. The ability to detect rare adverse events and to perform subgroup analysis was limited by sample size. Patients with visually or surgically significant ocular comorbidities were excluded, so evaluation of the performance of the study IOL in such patients will require further investigation. Data regarding contrast sensitivity, mesopic visual acuity, and visual acuity in the presence of glare could also be collected in future studies. Subjective information regarding patient experience was not collected in a systematic manner, and the single-surgeon design and systematic use of IA and digital marking could limit generalizability with regard to refractive outcomes. Finally, although this study had no specific exclusion criteria based on biometric parameters other than corneal astigmatism, there were no eyes with extreme values of axial length enrolled in the study, which could also limit generalizability.
Conclusion
The DFT/DATx15 toric EDOF IOL displayed excellent postoperative rotational stability, as anticipated given its strong physical similarity to other toric intraocular lenses from the same manufacturer. When used in combination with a digital marking system and IA, it yielded effective and predictable correction of astigmatism. No lens rotated postoperatively by more than 3 degrees at the final visit. Cautious comparison of visual acuity outcomes with outcomes reported in other studies for the non-toric DFT/DAT015 EDOF IOL noted similar monocular DSCIVA but a possible small disparity in monocular BSCDVA that is of uncertain origin and clinical significance and could readily be attributed to differences in patient population and/or study parameters. The toric DFT/DATx15 IOL had a good safety profile, consistent with previous data regarding the DFT/DAT015 IOL.
Value statement
What was known.
-
Toric intraolcular lenses (IOL) can help correct regular corneal astigmatism at the time of cataract surgery
-
The DFT/DATx15 lens has been shown to yield excellent visual acuity at results in both distance and intermediate distance vision
-
IOL rotation following IOL implantation has been documented to varying degrees
What this paper adds
-
The DFT/DATx15 Toric IOL is rotationally stable
-
The DFT/DATx15 Toric IOL effectively and predictably corrected regular corneal astigmatism
-
The DFT/DATx15 Toric IOL yielded comparable uncorrected, intermediate visual acuities
Change history
19 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10792-023-02750-x
References
Kessel L, Andresen J, Tendal B, Erngaard D, Flesner P, Hjortdal J (2016) Toric intraocular lenses in the correction of astigmatism during cataract surgery: a systematic review and meta-analysis. Ophthalmology 123(2):275–286. https://doi.org/10.1016/j.ophtha.2015.10.002
Oshika T, Fujita Y, Hirota A et al (2020) Comparison of incidence of repositioning surgery to correct misalignment with three toric intraocular lenses. Eur J Ophthalmol 30(4):680–684. https://doi.org/10.1177/1120672119834469
Oshika T, Inamura M, Inoue Y et al (2018) Incidence and outcomes of repositioning surgery to correct misalignment of toric intraocular lenses. Ophthalmology 125(1):31–35. https://doi.org/10.1016/j.ophtha.2017.07.004
Chang DF (2009) Repositioning technique and rate for toric intra-ocular lenses. J Cataract Refract Surg 35:1315–1316
Ruíz-Mesa R, Carrasco-Sánchez D, Díaz-Alvarez SB, Ruíz-Mateos MA, Ferrer-Blasco T, Montés-Micó R (2009) Refractive lens exchange with foldable toric intraocular lens. Am J Ophthalmol 147(6):990-996.e1. https://doi.org/10.1016/j.ajo.2009.01.004
Lee BS, Chang DF (2018) Comparison of the rotational stability of two toric intraocular lenses in 1273 consecutive eyes. Ophthalmology 125:1325–1331. https://doi.org/10.1016/j.ophtha.2018.02.012
Kramer BA, Berdahl J, Gu X, Merchea M (2021) Real world incidence of monofocal toric iol repositioning: analysis of the American Academy of Ophthalmology IRIS registry. J Cataract Refract Surg. https://doi.org/10.1097/j.jcrs.0000000000000748
Kramer BA, Hardten DR, Berdahl JP (2020) Rotation characteristics of three toric monofocal intraocular lenses. Clin Ophthalmol 14:4379–4384. https://doi.org/10.2147/OPTH.S285818
Potvin R, Kramer BA, Hardten DR, Berdahl JP (2016) Toric intraocular lens orientation and residual refractive astigmatism: an analysis. Clin Ophthalmol 10:1829–1836. https://doi.org/10.2147/OPTH.S114118
Yang JJ, Qin YZ, Qin L, Li JM (2020) Comparison of the clinical efficacy of AcrySof® IQ and TECNIS® toric intraocular lenses: a real-world study. Exp Ther Med 20(5):25. https://doi.org/10.3892/etm.2020.9153
McDonald MB, Mychajlyszyn A, Mychajlyszyn D, Klyce SD (2021) Advances in corneal surgical and pharmacological approaches to the treatment of presbyopia. J Refract Surg 37(S1):S20–S27. https://doi.org/10.3928/1081597X-20210408-04
Yoo SH, Zein M (2021) Vision restoration: cataract surgery and surgical correction of myopia, hyperopia, and presbyopia. Med Clin North Am 105(3):445–454. https://doi.org/10.1016/j.mcna.2021.01.002
Schallhorn JM, Pantanelli SM, Lin CC et al (2021) Multifocal and accommodating intraocular lenses for the treatment of presbyopia: a report by the American Academy of Ophthalmology. Ophthalmology. https://doi.org/10.1016/j.ophtha.2021.03.013
Katz JA, Karpecki PM, Dorca A et al (2021) Presbyopia — A review of current treatment options and emerging therapies. Clin Ophthalmol 15:2167–2178. https://doi.org/10.2147/OPTH.S259011
Mercer RN, Milliken CM, Waring GO 4th, Rocha KM (2021) Future trends in presbyopia correction. J Refract Surg 37(S1):S28–S34. https://doi.org/10.3928/1081597X-20210408-06
Rampat R, Gatinel D (2020) Multifocal and extended depth-of-focus intraocular lenses in 2020. Ophthalmology. https://doi.org/10.1016/j.ophtha.2020.09.026
U.S. Food and Drug Administration, Center for Devices and Radiological Health (CDRH) (2020) Summary of safety and effectiveness data: AcrySof™ IQ Vivity™ extended vision, toric extended vision, extended vision UV Absorbing, and Toric Extended Vision UV Absorbing IOLs. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf/P930014S126B.pdf. Accessed May 26, 2021
Schallhorn JM (2021) Multifocal and extended depth of focus intraocular lenses: a comparison of data from the United States Food and Drug Administration premarket approval trials. J Refract Surg 37(2):98–104. https://doi.org/10.3928/1081597X-20201111-02
Bala C, Poyales F, Guarro M et al (2021) Multi-country clinical outcomes of a new nondiffractive presbyopia-correcting intraocular lens. J Cataract Refract Surg. https://doi.org/10.1097/j.jcrs.0000000000000712
Mendicute J, Irigoyen C, Aramberri J, Ondarra A, Montés-Micó R (2008) Foldable toric intraocular lens for astigmatism correction in cataract patients. J Cataract Refract Surg 34(4):601–607. https://doi.org/10.1016/j.jcrs.2007.11.033
Bauer NJ, de Vries NE, Webers CA, Hendrikse F, Nuijts RM (2008) Astigmatism management in cataract surgery with the AcrySof toric intraocular lens. J Cataract Refract Surg 34(9):1483–1488. https://doi.org/10.1016/j.jcrs.2008.05.031
Gayton JL, Seabolt RA (2011) Clinical outcomes of complex and uncomplicated cataractous eyes after lens replacement with the AcrySof toric IOL. J Refract Surg 27(1):56–62. https://doi.org/10.3928/1081597X-20100325-01
Holland E, Lane S, Horn JD, Ernest P, Arleo R, Miller KM (2010) The AcrySof Toric intraocular lens in subjects with cataracts and corneal astigmatism: a randomized, subject-masked, parallel-group, 1-year study. Ophthalmology 117(11):2104–2111. https://doi.org/10.1016/j.ophtha.2010.07.033
Chang DF (2008) Comparative rotational stability of single-piece open-loop acrylic and plate-haptic silicone toric intraocular lenses. J Cataract Refract Surg 34(11):1842–1847. https://doi.org/10.1016/j.jcrs.2008.07.012
Hoffmann PC, Auel S, Hütz WW (2011) Results of higher power toric intraocular lens implantation. J Cataract Refract Surg 37(8):1411–1418. https://doi.org/10.1016/j.jcrs.2011.02.028
Chua WH, Yuen LH, Chua J, Teh G, Hill WE (2012) Matched comparison of rotational stability of 1-piece acrylic and plate-haptic silicone toric intraocular lenses in Asian eyes. J Cataract Refract Surg 38(4):620–624. https://doi.org/10.1016/j.jcrs.2011.10.037
U.S. Food and Drug Administration, Center for Devices and Radiological Health (CDRH) (2005) Summary of Safety and Effectiveness Data: ACRYSOF® Single-Piece Posterior Chamber Intraocular Lenses With Toric Optic. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf/P930014S015b.pdf. Accessed Sept. 2, 2021
Shah GD, Praveen MR, Vasavada AR, Vasavada VA, Rampal G, Shastry LR (2012) Rotational stability of a toric intraocular lens: influence of axial length and alignment in the capsular bag. J Cataract Refract Surg 38(1):54–59. https://doi.org/10.1016/j.jcrs.2011.08.028
Koshy JJ, Nishi Y, Hirnschall N et al (2010) Rotational stability of a single-piece toric acrylic intraocular lens. J Cataract Refract Surg 36(10):1665–1670. https://doi.org/10.1016/j.jcrs.2010.05.018
Gauvin M, Wallerstein A (2018) AstigMATIC: an automatic tool for standard astigmatism vector analysis. BMC Ophthalmol 18(1):255. https://doi.org/10.1186/s12886-018-0920-1
Inoue Y, Takehara H, Oshika T (2017) Axis misalignment of toric intraocular lens: placement error and postoperative rotation. Ophthalmology 124(9):1424–1425. https://doi.org/10.1016/j.ophtha.2017.05.025
Panagiotopoulou EK, Ntonti P, Gkika M et al (2019) Image-guided lens extraction surgery: a systematic review. Int J Ophthalmol 12(1):135–151. https://doi.org/10.18240/ijo.2019.01.21
Hura AS, Osher RH (2017) Comparing the Zeiss Callisto eye and the alcon verion image guided system toric lens alignment technologies. J Refract Surg 33(7):482–487. https://doi.org/10.3928/1081597X-20170504-02
Kasthurirangan S, Feuchter L, Smith P, Nixon D (2014) Software-based evaluation of toric IOL orientation in a multicenter clinical study. J Refract Surg 30(12):820–826. https://doi.org/10.3928/1081597X-20141117-01
Pallas A, Yeo TK, Trevenen M, Barrett G (2018) Evaluation of the accuracy of two marking methods and the novel toriCAM application for toric intraocular lens alignment. J Refract Surg 34(3):150–155. https://doi.org/10.3928/1081597X-20180115-03
Raufi N, James C, Kuo A, Vann R (2020) Intraoperative aberrometry vs modern preoperative formulas in predicting intraocular lens power. J Cataract Refract Surg 46(6):857–861. https://doi.org/10.1097/j.jcrs.0000000000000173
Blaylock JF, Hall B (2020) Astigmatic results of a diffractive trifocal toric IOL following intraoperative aberrometry guidance. Clin Ophthalmol 14:4373–4378. https://doi.org/10.2147/OPTH.S285711
Hovanesian JA (2021) Comparison of preoperative measurements with intraoperative aberrometry in predicting need for correction in eyes with low astigmatism undergoing cataract surgery. Clin Ophthalmol 15:2189–2196. https://doi.org/10.2147/OPTH.S314618
Christopher KL, Patnaik JL, Ifantides C et al (2021) Time utilization and refractive prediction enhancement associated with intraoperative aberrometry use during cataract surgery. Clin Ophthalmol 15:531–539. https://doi.org/10.2147/OPTH.S287573
Solomon KD, Sandoval HP, Potvin R (2019) Evaluating the relative value of intraoperative aberrometry versus current formulas for toric IOL sphere, cylinder, and orientation planning. J Cataract Refract Surg 45(10):1430–1435. https://doi.org/10.1016/j.jcrs.2019.05.023
Solomon KD, Sandoval HP, Potvin R (2019) Correcting astigmatism at the time of cataract surgery: Toric IOLs and corneal relaxing incisions planned with an image-guidance system and intraoperative aberrometer versus manual planning and surgery. J Cataract Refract Surg 45(5):569–575. https://doi.org/10.1016/j.jcrs.2018.12.002
Potvin R, Kramer BA, Hardten DR, Berdahl JP (2018) Factors associated with residual astigmatism after toric intraocular lens implantation reported in an online toric intraocular lens back-calculator. J Refract Surg 34(6):366–371. https://doi.org/10.3928/1081597X-20180327-01
Hatch KM, Woodcock EC, Talamo JH (2015) Intraocular lens power selection and positioning with and without intraoperative aberrometry. J Refract Surg 31(4):237–242. https://doi.org/10.3928/1081597X-20150319-03
Woodcock MG, Lehmann R, Cionni RJ, Breen M, Scott MC (2016) Intraoperative aberrometry versus standard preoperative biometry and a toric IOL calculator for bilateral toric IOL implantation with a femtosecond laser: one-month results. J Cataract Refract Surg 42(6):817–825. https://doi.org/10.1016/j.jcrs.2016.02.048
Kane JX, Chang DF (2020) Intraocular lens power formulas, biometry, and intraoperative aberrometry: a review. Ophthalmology. https://doi.org/10.1016/j.ophtha.2020.08.010
Novis C (2000) Astigmatism and toric intraocular lenses. Curr Opin Ophthalmol 11(1):47–50. https://doi.org/10.1097/00055735-200002000-00007
Holladay JT, Koch DD (2020) Residual astigmatism with toric intraocular lens misalignment. J Cataract Refract Surg 46(8):1208–1209. https://doi.org/10.1097/j.jcrs.0000000000000273
Acknowledgements
Eric D. Rosenberg, DO, MSE, performed the statistical analysis. Michael S. C. Hemond, MD, PhD, prepared the draft manuscript and figures for subsequent editing by the authors.
Funding
This study was supported by an investigator-initiated trial by Alcon.
Author information
Authors and Affiliations
Contributions
KB, PM, AC and BJ designed and conducted the study. Eric Rosenberg DO, MSE performed the statistical analysis. Michael Hemond MD, PhD prepared the draft manuscript and figures. SO edited and finalized the manuscript and submitted it for publishing.
Corresponding author
Ethics declarations
Competing interests
This study was supported by an investigator-initiated trial by Alcon.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barber, K.M., O’Connor, S., Mackinder, P. et al. Rotational stability and refractive outcomes of the DFT/DATx15 toric, extended depth of focus intraocular lens. Int Ophthalmol 43, 2737–2747 (2023). https://doi.org/10.1007/s10792-023-02673-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-023-02673-7