Abstract
Purpose
Compare 12-month (12 M) safety and efficacy of endoscopic cyclophotocoagulation (ECP) + cataract surgery (Group 1) versus ECP + cataract surgery + iStent inject trabecular micro-bypass implantation (Group 2) in Brazilian patients with open-angle glaucoma (OAG).
Methods
This prospective, multicenter, comparative case series included patients with OAG and cataract who were randomized to receive treatment in Group 1 or Group 2. Outcomes included mean and percent reduction versus preoperative in intraocular pressure (IOP) and number of glaucoma medications; visual acuity; occurrence of adverse events; and rate of secondary surgeries.
Results
Preoperatively, Groups 1 and 2 had similar mean IOP (mean ± standard deviation 22.1 ± 3.6 and 22.0 ± 2.5 mmHg, respectively) and mean number of medications (3.3 ± 0.6 and 3.4 ± 0.6 medications, respectively). At all follow-up timepoints through 12 M, both groups achieved significant IOP and medication reductions versus preoperative (IOP p < 0.001 and number of medications p < 0.001 for both groups). At 12 M, IOP reductions were 24.2% (Group 1) and 43.6% (Group 2) (p < 0.001); mean medication reductions were 50.2% and 71.5%, respectively. Mean postoperative IOP and number of medications were higher in Group 1 than Group 2 (IOP p < 0.01 all visits, medication p < 0.01 at 6 M and 12 M). Adverse events were generally mild and infrequent in both groups.
Conclusion
Both treatment groups (ECP + phacoemulsification, with/without iStent inject implantation) achieved significant and safe reductions in IOP and medications versus preoperative in Brazilian OAG patients. Percent reductions were significantly greater, and mean IOP and medications were significantly lower, in the group receiving iStent inject.
Clinical trial registration (CTR)
CAAE project identification #20053019.5.0000.5078. Protocol #3.587.147. Clinical Trial Database of the Federal University of Goiás, Brazil. Registration Date: September 19, 2019.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide [1]. A meta-analysis of 50 population-based studies encompassing over a quarter of a million individuals estimated the global prevalence of glaucoma for those aged 40 to 80 years at 3.5%. The number of people in this age group with glaucoma was estimated at 64.3 million in 2013 and is anticipated to increase to 111.8 million in 2040 [1].
Medical and surgical therapies for treating glaucoma focus on lowering intraocular pressure (IOP), currently the only known modifiable risk factor [2]. Although medications are moderately effective and safe, chronic treatment with topical ocular hypotensive agents can be associated with deleterious effects to the ocular surface, issues with patient adherence and persistence to their prescribed regimens, and reduced chance for success with subsequent surgical procedures [3,4,5,6,7,8].
In the surgical realm, phacoemulsification alone often is associated with modest IOP reduction due to an increase in outflow facility [9]; however, these effects appear to be modest and impermanent [10]. Traditional filtration surgeries (e.g., trabeculectomy and tube implants) can dramatically reduce IOP, but often are associated with considerable morbidity [11,12,13]. In the past decade, a new class of procedures, micro-invasive glaucoma surgeries (MIGS), has been developed. These procedures provide moderate IOP reduction and have a more favorable safety profile than filtering surgeries. MIGS procedures can be combined with phacoemulsification or with other non-filtering glaucoma procedures, such as endoscopic cyclophotocoagulation (ECP; BVI Endo Optiks, Waltham, MA, USA) [2].
The iStent inject trabecular micro-bypass (Glaukos Corp., San Clemente, CA, USA), often considered the most micro-invasive and safest of the MIGS implant devices, consists of two pre-loaded injectable titanium stents implanted via an ab-interno approach through the trabecular meshwork into Schlemm’s canal [14]. Once in position, the stents facilitate trabecular outflow. The prospective, randomized, multicenter, Phase 3 pivotal trial evaluating the device showed that iStent inject provides a ≥ 20% IOP reduction in 75.8% of OAG eyes at 24 months when used in combination with cataract surgery compared with 61.9% eyes undergoing cataract surgery alone [15]. For the treatment responders, 84% and 67% of the treated and control eyes, respectively, were medication-free at 23 months. Furthermore, an analysis of these iStent inject patients found improvements in quality of life in terms of ocular symptoms and vision-related activities compared with cataract surgery alone [16]. A large real-world cohort of Australian eyes, which included pseudoexfoliative (PXG) and pigmentary (PG) glaucoma, found clinically meaningful reductions in IOP and ocular hypotensive medication use at 1 year and 2 years [17, 18].
Endoscopic cyclophotocoagulation reduces aqueous production, thereby decreasing the inflow component of the inflow/outflow balance that contributes to IOP [19,20,21]. ECP is performed using an ab-interno approach wherein a power-titratable 810 nm laser probe is inserted through a limbal incision to allow visualization and continuous photocoagulation of the ciliary process epithelium over approximately 270° [19]. Our group previously reported that phacoemulsification with ECP was safe and effective as a primary procedure for combined cataract and glaucoma [22]. A prospective study by Francis et al. showed significant IOP reductions with cataract surgery plus ECP compared with cataract surgery alone [23]. ECP utility has been shown in mild-to-moderate glaucoma [19,20,21], as well as in refractory glaucoma [24].
Both ECP and iStent inject implantation have been shown to be effective at lowering IOP and topical ocular hypotensive medication burdens in OAG [15, 17, 18, 20, 21, 23]. ECP in combination with cataract surgery is a common treatment modality performed by surgeons in Brazil. With the increasing utilization of iStent inject trabecular micro-bypass, the combination of ECP and stent implantation has emerged as a potential treatment option, allowing the surgeon to target both outflow and inflow components of patients’ disease. Further, this study aims to address the paucity of clinical evidence in a Latin American population with glaucoma and cataract.
The present prospective multicenter study compares 1 year outcomes following phacoemulsification + ECP (Group 1) or phacoemulsification + ECP + iStent inject implantation (Group 2) in a Brazilian population The report supplies some of the first data on this treatment combination, with outcomes observed in a Latin American patient population that historically has been under-represented in the literature.
Material and methods
Study design
This was a prospective, comparative, multicenter case series that evaluated ECP with cataract surgery versus ECP plus iStent inject with cataract surgery in Brazilian patients with OAG. The study was performed in line with the principles of the Declaration of Helsinki [25]; all participants provided informed consent for their enrollment. The study was completed at two clinical sites: Centro Brasileiro De Cirurgia De Olhos (CBCO), Goiânia, GO, Brazil; and Centro Brasileiro Da Visão (CBY), Brasília, DF, Brazil. The study was reviewed and approved by the Ethics Committees of both hospitals. The study was registered in the Clinical Trial Database of the Federal University of Goiás, Brazil (CAAE ID# 20,053,019.5.0000.5078, Protocol #3.587.147, registered September 19, 2019).
Once enrolled, patients were prospectively randomized on a 1:1 basis to either phacoemulsification plus ECP or phacoemulsification plus ECP plus iStent inject implantation. An MS Excel (Microsoft, WA, USA) random number generator was used to provide the master randomization list. They were allowed to have one or both eyes treated as part of the study.
This study was pragmatic in nature, with the aim of gathering evidence for real-world treatment of glaucoma. Therefore, no preoperative or postoperative ocular hypotensive medication washout was required. Postoperative management of patients including medication reintroduction, management of adverse events, postoperative visit scheduling, and procedures were managed at the discretion of the treating physician using standard care.
Inclusion criteria
Adults with OAG [primary open-angle glaucoma (POAG), pseudoexfoliation glaucoma (PXG), or pigmentary glaucoma (PG)] were included. The patients had to be candidates for ECP and iStent inject as judged by the investigators.
Exclusion criteria
Eyes with prior glaucoma filtration surgery, angle-closure glaucoma, traumatic, malignant, uveitic, or neovascular glaucoma or other discernible congenital anomalies of the anterior chamber angle were not allowed to participate. Patients with retrobulbar tumor, thyroid eye disease, Sturge-Weber Syndrome or any other type of condition that may have caused elevated episcleral venous pressure were excluded. Study participation also was not allowed for any patient having a contraindicated ocular or systemic condition.
Outcome measures
Outcome measures included IOP, topical ocular hypotensive medication burden, and safety. Data were collected and descriptive analyses were performed using MS Excel. The following measures were analyzed at postoperative study timepoints and compared to preoperative outcomes as appropriate: mean IOP; percentage of eyes with IOP ≤ 18 mmHg and with ≤ 15 mmHg; mean medication burden (number of medications) and percentage of eyes with categorical medication burden; percentage of eyes with more, fewer or the same medication burden at postoperative study timepoints compared to preoperative; Snellen best-corrected visual acuity; and percentage of eyes with intraoperative or postoperative complications. The schedule of visits and assessments through 12 months postoperative is shown in Supplemental Table S1.
Statistics
Statistical calculations included mean, standard deviation, percentage of total, and categorical counts. Two-tailed t-tests were performed to compare postoperative results with preoperative results. A p-value of < 0.05 was considered significant.
Sample size
Given the statistical assumptions (Supplemental Table S2) and a significance level of 0.05, 30 subjects per arm provided 80% power to detect a treatment difference that was statistically significant.
Results
Study participants
There were 35 eyes from 33 patients in the Phacoemulsification + ECP group (Group 1) and 36 eyes from 33 patients in the Phacoemulsification + ECP + iStent inject group (Group 2) (Table 1). Eyes in both treatment groups had generally comparable preoperative characteristics, including mean age, racial distribution, cup:disk ratio, visual fields, and retinal nerve fiber layer thickness. Nearly all eyes in both groups had moderate to severe primary open-angle glaucoma. More eyes in Group 2 had undergone laser iridotomy prior to the study. Both groups had mean preoperative IOP values of approximately 22 mmHg on 3.3–3.4 topical ocular hypotensive medications.
Intraocular pressure
Eyes in both groups achieved significant reductions in IOP at all postoperative data points versus preoperative (p < 0.001) (Fig. 1); IOP values were significantly lower in Group 2 versus Group 1 at all study visits through Year 1 (p < 0.01). At Year 1, eyes in Group 2 achieved significantly greater mean percent reductions in IOP from preoperative levels compared to Group 1 (43.6% versus 24.2%, respectively; p < 0.001) (Fig. 1).
Both treatment groups demonstrated clinically meaningful categorical reductions in IOP (Table 2). For Group 1, 11.4% and 0% of eyes had preoperative IOP ≤ 18 mmHg and ≤ 15 mmHg, respectively. At Year 1 these proportions had increased to 97.0% and 33.3%, respectively. Meanwhile, Group 2 had 2.8% and 0% of eyes with preoperative IOP ≤ 18 mmHg and ≤ 15 mmHg, respectively. Following surgery, 100% of Group 2 eyes achieved IOP ≤ 18 mmHg at all study visits through Year 1, and 100% achieved IOP ≤ 15 mmHg at visits from Month 3 to Year 1.
Glaucoma medications
Both treatment groups achieved significant reductions in topical ocular medication use at all postoperative time points (p < 0.001) (Fig. 2). At Month 6 and Year 1, the mean numbers of medications were significantly lower in Group 2 than in Group 1 (p < 0.01). At Year 1, eyes in Group 2 achieved significantly greater medication reduction versus preoperative than those in Group 1 (71.5% versus 50.2%, respectively; p < 0.001) (Fig. 2).
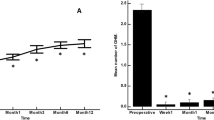
At the preoperative visit in Group 1, no eyes were receiving 0 or 1 medication, while 94.2% of eyes were using 3–4 medications (Fig. 3). At Year 1, 6.1% and 18.2% of eyes were receiving 0 or 1 medication, respectively, while only 3.0% of eyes were on 3 medications; no eyes were receiving 4 medications. At the preoperative visit in Group 2, no eyes were receiving 0 or 1 medication, while 97.2% of eyes were using 3–4 medications (Fig. 4). At Year 1, 28.6% and 48.6% of eyes were receiving 0 or 1 medication, respectively, while only 2.9% of eyes were on 3 medications; no eyes were receiving 4 mediations.
Both treatment groups demonstrated high proportions of eyes with reductions in topical ocular hypotensive medication use throughout follow-up (Fig. 5). All eyes in both groups maintained or reduced their medication burden versus preoperative at all points in follow-up. None of the eyes in either group showed an increase in medication use.
Safety
Best-corrected visual acuity was dramatically improved in both treatment groups (Fig. 6), consistent with expectations following standard cataract surgery. No eye in either group had BCVA of 20/20 or 20/25 preoperatively, but by Year 1, 68.6% and 80.0% of eyes had achieved BCVA of 20/20 to 20/25 in Group 1 and Group 2, respectively.
Intraoperative complications were few in both groups. These included one eye with transitory bleeding in each group and one eye in Group 2 with an over-implanted stent (Table 3). Postoperatively, there were modest numbers of eyes with mild cells and flare at Day 1 and Week 1 in both groups, consistent with levels expected after standard cataract surgery. Adverse events were generally minimal, few, and resolved without sequelae.
Cup:disk ratios, visual fields, and retinal nerve fiber layer and corneal thicknesses remained stable over the course of the 1-year study in both groups (Table 4).
Discussion
A considerable shift from traditional glaucoma surgeries to MIGS procedures has occurred in recent years [26, 27]. A study from the American Academy of Ophthalmology Intelligent Research in Sight (IRIS®) Registry data found that, from 2013 to 2018, MIGS surgeries rose to comprise nearly half of all glaucoma surgeries in the US. The most common concurrent procedures were ECP and iStent/iStent inject implantation, which accounted for 55.4% of all concurrent glaucoma procedures [26]. These trends underscore the relevance and value of the current study.
The present report provides safety and effectiveness outcomes of phacoemulsification and ECP, either with or without iStent inject implantation, in Brazilian patients with cataract and glaucoma. Substantial IOP-lowering and reductions in ocular hypotensive medication use were observed for both treatment groups and were sustained through 1 year. Both procedures were generally well-tolerated with few procedure-related adverse events.
The combination of micro-bypass stent, cataract surgery, and ECP leverages different mechanisms of action in glaucoma management [28]. Not unexpectedly, the eyes receiving the iStent inject in combination with phacoemulsification and ECP demonstrated significantly greater IOP reductions throughout the 1 year of follow-up (p < 0.01). Similarly, although both treatment groups achieved clinically significant categorical reductions in IOP versus preoperative, reductions were more marked in the group undergoing iStent inject implantation (Group 2), including a three-fold higher percentage of eyes achieving IOP ≤ 15 mmHg in Group 2 versus Group 1 at Year 1. Additionally, the IOP reductions in Group 2 appeared to be maintained with less upward drift over time than for those undergoing the single ECP procedure.
These findings are consistent with the literature on ECP and trabecular micro-bypass. A retrospective consecutive case series by Ferguson et al. [28] demonstrated significantly greater IOP reductions in eyes receiving ECP + iStent than those undergoing iStent implantation alone. Similarly, a longitudinal retrospective 12 month study by Pantalon and colleagues [29] in eyes with early-to-moderate OAG reported significantly larger IOP reductions from baseline for eyes undergoing phacoemulsification + ECP + 2 iStents versus phacoemulsification + 2 iStents. These results demonstrate the value of pairing procedures and are consistent with the current study in which the combined procedure provided greater IOP-lowering efficacy than cataract surgery with ECP alone.
Favorable trends also were observed in medication reduction in the current study, with the vast majority of eyes in both groups reducing their number of medications through Year 1. As with IOP, the medication reductions were significantly greater in the group undergoing iStent inject implantation (Group 2). This included a three-fold higher proportion of Group 2 versus Group 1 eyes on 0–1 medication at Year 1, and a threefold to fourfold lower proportion of Group 2 eyes on ≥ 2 medications at Year 1. These outcomes are aligned with those of Pantalon and colleagues, who reported significantly greater 1-year medication reductions after phacoemulsification + ECP + iStent inject than after phacoemulsification + iStent inject [29].
These medication reductions represent significant benefits at the level of each patient, given the well-known personal, physical, social, and financial consequences of chronic medication exposure. For example, the cost of chronic medication can be a financial burden for patients; not surprisingly, numerous studies have shown the cost effectiveness of the iStent device compared with the use of chronic topical medications [30,31,32,33,34,35,36]. Topical ocular hypotensive medications also can contain preservatives, such as benzalkonium chloride, which are known to cause adverse effects on the ocular surface [4, 11]. In addition, patient adherence to topical ocular hypotensive drops is typically less than ideal [7, 9, 10, 37, 38] for a number of potential reasons (e.g., complex medication regimens, difficulty with drop instillation, or social and environmental limitations) [9]. Poor adherence to glaucoma medication therapy can put a patient at risk for glaucoma progression with irreversible optic nerve damage and vision loss. Thus the implantation of micro-bypass stents can provide a treatment alternative that reduces preservative load to the ocular surface [18, 39,40,41,42,43,44,45] and lessens reliance on patient adherence, thereby helping to preserve visual function.
Both ECP and iStent inject implantation were associated with few intraoperative complications or postoperative adverse events in our study. The majority of events occurred in the initial days following surgery and resolved soon thereafter. Cup:disk ratios, visual fields, and retinal nerve fiber layer thickness remained stable over the course of the 1 year study in both groups. Best-corrected visual acuity improved dramatically at Year 1 versus preoperative, consistent with what would be expected after cataract surgery alone; there was no indication that the addition of iStent inject or ECP detracted from patients’ overall visual potential.
These visual acuity findings are consistent with prior evaluations of phacoemulsification + ECP and/or iStent implantation [22, 29, 46,47,48,49,50,51]. For example, a previous study from our group demonstrated improvements in the logMAR visual acuity (p = 0.01) for up to 2 years in eyes receiving phacoemulsification + ECP [22]. Morales and colleagues [50] found improvements in corrected distance visual acuity of 2 Snellen lines or more at 1 year in 73% of patients undergoing ECP and phacoemulsification. In another study of eyes undergoing ECP with phacoemulsification, Clement et al. [47] reported 94% of eyes achieved stable or improved vision after 1 year. Kang et al. [48] demonstrated maintenance or improvement in visual acuity for 95% of their ECP + phacoemulsification group with a mean follow-up of 21 months. Several studies have shown improvements in visual acuity 1 year or more following iStent implantation, either as a stand-alone procedure [51], or when combined with cataract surgery [46, 49]. Finally, Pantalon et al. [29] reported similar 1 year BCVA results in eyes undergoing phacoemulsification plus either iStent inject + ECP or iStent inject. All of these studies, as well as the current one, demonstrate favorable outcomes with ECP, iStent inject implantation, or the combination of the two procedures in combination with phacoemulsification in terms of preserving the visual improvements expected after cataract surgery alone.
This study is limited by its modest sample size, 1 year follow-up duration, and inclusion of data from 2 surgeons at 2 sites. Patients with other forms of glaucoma than OAG were excluded, as well as those who had undergone previous glaucoma filtration surgery. The majority of patients in the study population were White, Hispanic, or Black, so results may not be directly applicable to other demographic groups (e.g., Asians). Not all data were available for all parameters at all time points. A future study could include a greater number of patients, longer duration of follow-up, data from more sites, or a broader range of glaucoma subtypes.
Conclusion
When combined with cataract surgery, both ECP and ECP + iStent inject implantation procedures were safe and effective in lowering IOP, reducing topical ocular hypotensive medication use, and preserving the visual improvements experienced after cataract surgery. The IOP and medication reductions were greater in the group undergoing iStent inject implantation alongside phacoemulsification + ECP than in the group receiving phacoemulsification + ECP only. Thus, the results demonstrate favorable outcomes with these two procedures when combined with cataract surgery, as well as confirmation of the additional benefit of stent implantation, through one year postoperative in a Brazilian patient cohort.
Data availability
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
References
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121(11):2081–2090
The American Academy of Ophthalmology (2020) Primary open-angle glaucoma preferred practice pattern. San Francisco, CA
Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM (2005) Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol 140(4):598–606
Steven DW, Alaghband P, Lim KS (2018) Preservatives in glaucoma medication. Br J Ophthalmol 102(11):1497–1503
Friedman DS, Quigley HA, Gelb L et al (2007) Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the glaucoma adherence and persistency study (GAPS). Invest Ophthalmol Vis Sci 48(11):5052–5057
Tsai JC (2009) A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology 116(11 Suppl):S30-36
Tsai JC, McClure CA, Ramos SE, Schlundt DG, Pichert JW (2003) Compliance barriers in glaucoma: a systematic classification. J Glaucoma 12(5):393–398
Su CC, Lee YC, Lee PRC (2021) Assessment of ocular surface disease in glaucoma patients with benzalkonium chloride-preserved latanoprost eye drops: a short-term longitudinal study. Graefes Arch Clin Exp Ophthalmol 259(5):1243–1251
Jampel HD, Solus JF, Tracey PA, Gilbert DL, Loyd TL, Jefferys JL, Quigley HA (2012) Outcomes and bleb-related complications of trabeculectomy. Ophthalmology 119(4):712–722
Rulli E, Biagioli E, Riva I, Gambirasio G, De Simone I, Floriani I, Quaranta L (2013) Efficacy and safety of trabeculectomy vs nonpenetrating surgical procedures: a systematic review and meta-analysis. JAMA Ophthalmol 131(12):1573–1582
Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC (2012) Tube versus trabeculectomy study group. Postoperative complications in the tube versus trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol 153:804–814
Alaghband P, Beltran-Agullo L, Galvis EA, Overby DR, Lim KS (2018) Effect of phacoemulsification on facility of outflow. Br J Ophthalmol 102(11):1520–1526
Slabaugh MA, Bojikian KD, Moore DB, Chen PP (2014) The effect of phacoemulsification on intraocular pressure in medically controlled open-angle glaucoma patients. Am J Ophthalmol 157(1):26–31
Glaukos® Corporation iStent inject® Trabecular micro-bypass system. [Directions for use]. Glaukos Corp. San Clemente, CA. 2020
Samuelson TW, Sarkisian SR Jr, Lubeck DM et al (2019) Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology 126(6):811–821
Samuelson TW, Singh IP, Williamson BK et al (2021) Quality of life in primary open-angle glaucoma and cataract: an analysis of VFQ-25 and OSDI from the iStent inject pivotal trial. Am J Ophthalmol 229:220–229
Clement C, Howes F, Ioannidis AS et al (2020) Two-year multicenter outcomes of iStent inject trabecular micro-bypass stents combined with phacoemulsification in various types of glaucoma and ocular hypertension. Clin Ophthalmol 14:3507–3517
Clement CI, Howes F, Ioannidis AS, Shiu M, Manning D (2019) One-year outcomes following implantation of second-generation trabecular micro-bypass stents in conjunction with cataract surgery for various types of glaucoma or ocular hypertension: multicenter, multi-surgeon study. Clin Ophthalmol 13:491–499
Seibold LK, SooHoo JR, Kahook MY (2015) Endoscopic cyclophotocoagulation. Middle East Afr J Ophthalmol 22(1):18–24
Sarkisian SR Jr, Radcliffe N, Harasymowycz P et al (2021) Erratum to: visual outcomes of combined cataract surgery and minimally invasive glaucoma surgery. J Cataract Refract Surg 47(2):286
Sarkisian SR Jr, Radcliffe N, Harasymowycz P et al (2020) Visual outcomes of combined cataract surgery and minimally invasive glaucoma surgery. J Cataract Refract Surg 46(10):1422–1432
Lima FE, Carvalho DM, Avila MP (2010) Phacoemulsification and endoscopic cyclophotocoagulation as primary surgical procedure in coexisting cataract and glaucoma. Arq Bras Oftalmol 73(5):419–422
Francis BA, Berke SJ, Dustin L, Noecker R (2014) Endoscopic cyclophotocoagulation combined with phacoemulsification versus phacoemulsification alone in medically controlled glaucoma. J Cataract Refract Surg 40(8):1313–1321
Lima FE, Magacho L, Carvalho DM, Susanna R Jr, Avila MP (2004) A prospective, comparative study between endoscopic cyclophotocoagulation and the Ahmed drainage implant in refractory glaucoma. J Glaucoma 13(3):233–237
World Medical Association (2013) World Medical Association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310(20):2191–2194
Yang SA, Mitchell W, Hall N et al (2021) Trends and usage patterns of minimally invasive glaucoma surgery in the United States: IRIS(R) registry analysis 2013–2018. Ophthalmol Glaucoma 29(4):443–451
Rathi S, Andrews CA, Greenfield DS, Stein JD (2021) Trends in glaucoma surgeries performed by glaucoma subspecialists versus nonsubspecialists on Medicare beneficiaries from 2008 through 2016. Ophthalmology 128(1):30–38
Ferguson TJ, Swan R, Sudhagoni R, Berdahl JP (2017) Microbypass stent implantation with cataract extraction and endocyclophotocoagulation versus microbypass stent with cataract extraction for glaucoma. J Cataract Refract Surg 43(3):377–382
Pantalon AD, Barata ADO, Georgopoulos M, Ratnarajan G (2020) Outcomes of phacoemulsification combined with two iStent inject trabecular microbypass stents with or without endocyclophotocoagulation. Br J Ophthalmol 104(10):1378–1383
Iordanous Y, Kent JS, Hutnik CM, Malvankar-Mehta MS (2014) Projected cost comparison of trabectome, iStent, and endoscopic cyclophotocoagulation versus glaucoma medication in the Ontario health insurance plan. J Glaucoma 23(2):e112-118
Le K, Saheb H (2014) iStent trabecular micro-bypass stent for open-angle glaucoma. Clin Ophthalmol 8:1937–1945
Ngan K, Fraser E, Buller S, Buller A (2018) A cost minimisation analysis comparing iStent accompanying cataract surgery and selective laser trabeculoplasty versus topical glaucoma medications in a public healthcare setting in New Zealand. Graefes Arch Clin Exp Ophthalmol 256(11):2181–2189
Nieland K, Labbe A, Schweitzer C et al (2021) A cost-effectiveness analysis of iStent inject combined with phacoemulsification cataract surgery in patients with mild-to-moderate open-angle glaucoma in France. PLoS ONE 16(6):e0252130
Ordonez JE, Ordonez A, Osorio UM (2019) Cost-effectiveness analysis of iStent trabecular micro-bypass stent for patients with open-angle glaucoma in Colombia. Curr Med Res Opin 35(2):329–340
Resende AF, Patel NS, Waisbourd M, Katz LJ (2016) iStent® trabecular microbypass stent: an update. J Ophthalmol 2016:2731856
Tan SZ, Au L (2016) Manchester iStent study: 3-year results and cost analysis. Eye (London) 30(10):1365–1370
Guven S, Koylu MT, Mumcuoglu T (2021) Adherence to glaucoma medication, illness perceptions, and beliefs about glaucoma: attitudinal perspectives among Turkish population. Eur J Ophthalmol 31(2):469–476
Wolfram C, Stahlberg E, Pfeiffer N (2019) Patient-reported nonadherence with glaucoma therapy. J Ocul Pharmacol Ther 35(4):223–228
Voicu L, Salim S (2021) New strategies for the management of ocular surface disease in glaucoma patients. Curr Opin Ophthalmol 32(2):134–140
Ahmed IIK, Fea A, Au L et al (2020) A prospective randomized trial comparing hydrus and iStent microinvasive glaucoma surgery implants for standalone treatment of open-angle glaucoma: the COMPARE study. Ophthalmology 127(1):52–61
Gallardo MJ, Supnet RA (2019) Three-year outcomes of combined trabecular micro-bypass and phacoemulsification in a predominantly Hispanic population with primary open-angle glaucoma. Clin Ophthalmol 13:869–879
Hengerer FH, Auffarth GU, Riffel C, Conrad-Hengerer I (2018) Prospective, non-randomized, 36-month study of second-generation trabecular micro-bypass stents with phacoemulsification in eyes with various types of glaucoma. Ophthalmol Ther 7(2):405–415
Hooshmand J, Rothschild P, Allen P, Kerr NM, Vote BJ, Toh T (2019) Minimally invasive glaucoma surgery: comparison of iStent with iStent inject in primary open angle glaucoma. Clin Exp Ophthalmol 47(7):898–903
Katz LJ, Erb C, Carceller GA et al (2015) Prospective, randomized study of one, two, or three trabecular bypass stents in open-angle glaucoma subjects on topical hypotensive medication. Clin Ophthalmol 9:2313–2320
Gallardo MJ, Supnet RA, Giamporcaro JE, Hornbeak DM (2016) Outcomes of combined trabecular micro-bypass and phacoemulsification in a predominantly Hispanic patient population. Clin Ophthalmol 10:1931–1937
Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, Morales-Fernandez L, Fernandez-Perez C, Garcia-Feijoo J (2016) Glaukos iStent inject® trabecular micro-bypass implantation associated with cataract surgery in patients with coexisting cataract and open-angle glaucoma or ocular hypertension: a long-term study. J Ophthalmol 2016:1056573
Clement CI, Kampougeris G, Ahmed F, Cordeiro MF, Bloom PA (2013) Combining phacoemulsification with endoscopic cyclophotocoagulation to manage cataract and glaucoma. Clin Exp Ophthalmol 41(6):546–551
Kang S, Luk S, Han H et al (2017) Refractive outcome of combined phacoemulsification and endoscopic cyclophotocoagulation. Int Ophthalmol 37(6):1311–1317
Lindstrom R, Lewis R, Hornbeak DM et al (2016) Outcomes following implantation of two second-generation trabecular micro-bypass stents in patients with open-angle glaucoma on one medication: 18-month follow-up. Adv Ther 33(11):2082–2090
Morales J, Al Qahtani M, Khandekar R et al (2015) Intraocular pressure following phacoemulsification and endoscopic cyclophotocoagulation for advanced glaucoma: 1-year outcomes. J Glaucoma 24(6):e157-162
Voskanyan L, Garcia-Feijoo J, Belda JI et al (2014) Prospective, unmasked evaluation of the iStent(R) inject system for open-angle glaucoma: synergy trial. Adv Ther 31(2):189–201
Funding
No monetary nor product support was received for the work in this study. Editorial assistance (Dana M. Hornbeak, MD, MPH) and publication fees were provided by Glaukos Corporation.
Author information
Authors and Affiliations
Contributions
FL, JG, MA guided study design and wrote protocol. FL, JG, MA completed data collection. FL, JG, MA contributed to data analysis and manuscript preparation (including text, figures, and tables). FL, JG, MA reviewed and approved the manuscript.
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Consent to participate
Medical writing and editorial support were provided by Julie Crider, PhD (Collaborative Medical Writing, LLC), and Dana Hornbeak, MD, MPH (Glaukos Corporation).
Ethical approval
The study was performed according to the tenets of the Declaration of Helsinki; all participants provided their informed consent for enrollment into the study. The study was reviewed and approved by the Ethics Committees of both hospitals involved: Centro Brasileiro De Cirurgia De Olhos (CBCO), Goiânia, GO, Brazil; and Centro Brasileiro Da Visão (CBY), Brasília, DF, Brazil. The study was registered in the Clinical Trial Database of the Federal University of Goiás, Brazil (CAAE ID# 20053019.5.0000.5078, Protocol #3.587.147, registered September 19, 2019).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lima, F.E., Geraissate, J.C. & Ávila, M.P. A multicenter prospective comparative study evaluating cataract surgery and endoscopic cyclophotocoagulation either with or without iStent inject implantation in Brazilian patients with glaucoma. Int Ophthalmol 43, 1665–1676 (2023). https://doi.org/10.1007/s10792-022-02563-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-022-02563-4