Abstract
Purpose
To compare the aqueous humor (AH) and serum levels of 4-hydroxynenal (4-HNE) and 8-hydroxy-2′-deoxyguanosine (8-OhdG) in patients with pseudoexfoliation syndrome (PES) and pseudoexfoliation glaucoma (PEG) with each other and with age- and sex-matched control group.
Methods
This prospective study included 66 patients divided into three groups: PES (n = 24), PEG (n = 21), and a control group (n = 21). 4-HNE and 8-OhdG levels were analyzed using the enzyme-linked immunosorbent assay.
Results
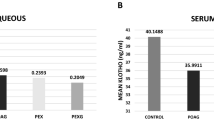
Aqueous and serum 4-HNE levels were significantly higher in the PEG (466.52 ± 62.12 pg/mL and 313.47 ± 47.41 pg/mL) and PES (290.69 ± 63.63 pg/mL and 201.53 ± 39.57 pg/mL) groups than the control group (144.02 ± 39.58 pg/mL and 99.10 ± 16.96 pg/mL; p < 0.001, for all). Both aqueous and serum levels of 4-HNE in the PEG group were significantly higher than in the PES group (p < 0.001, for both). Similar to 4-HNE, the AH 8-OhdG levels were higher in the PEG group (21.18 ± 2.23 ng/mL) compared to the PES (14.90 ± 3.37 ng/mL) and control (4.86 ± 1.94 ng/mL) groups (p < 0.001, for all). Serum 8-OhdG levels were significantly higher in the PEG and PES groups than the control (p < 0.001, for both); however, there was no significant difference between the PES and PEG groups (p = 0.097). There were strong significant correlations between the aqueous and serum levels of 4-HNE (p < 0.001, r = 0.857) and 8-OhdG (p < 0.001, r = 0.807) among all the patients.
Conclusions
Aqueous humor and serum levels of 4-HNE and 8-OhdG increased in the PES and PEG patients. These findings are potentially significant and add to the growing body of evidence concerning oxidative stress in PES and PEG.




Similar content being viewed by others
References
Schlötzer-Schrehardt U, Naumann GO (2006) Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol 141(5):921–937. https://doi.org/10.1016/j.ajo.2006.01.047
Streeten BW, Li Z-Y, Wallace RN, Eagle RC, Keshgegian AA (1992) Pseudoexfoliative fibrillopathy in visceral organs of a patient with pseudoexfoliation syndrome. Arch Ophthalmol 110(12):1757–1762. https://doi.org/10.1001/archopht.1992.01080240097039
Ritch R (2002) Exfoliation syndrome: more than meets the eye. Acta Ophthalmol Scand 80(5):465–467. https://doi.org/10.1034/j.1600-0420.2002.800502.x
Koliakos GG, Befani CD, Mikropoulos D, Ziakas NG, Konstas AG (2008) Prooxidant-antioxidant balance, peroxide and catalase activity in the aqueous humour and serum of patients with exfoliation syndrome or exfoliative glaucoma. Graefes Arch Clin Exp Ophthalmol 246(10):1477–1483. https://doi.org/10.1007/s00417-008-0871-y
Schlötzer-Schrehardt U, Lommatzsch J, Küchle M, Konstas AG, Naumann GO (2003) Matrix metalloproteinases and their inhibitors in aqueous humor of patients with pseudoexfoliation syndrome/glaucoma and primary open-angle glaucoma. Invest Ophthalmol Vis Sci 44(3):1117–1125. https://doi.org/10.1167/iovs.02-0365
Ergan E, Ozturk F, Beyazyildiz E, Elgin U, Sen E, Cankaya AB, Celik T (2016) Oxidant/antioxidant balance in the aqueous humor of patients with glaucoma. Int J Ophthalmol 9(2):249–252. https://doi.org/10.18240/ijo.2016.02.12
Tetikoğlu M, Sağdik HM, Aktas S, Uçar F, Özcura F (2016) Serum prolidase activity and oxidative stress in patients with pseudoexfoliation syndrome. Graefes Arch Clin Exp Ophthalmol 254(7):1339–1343. https://doi.org/10.1007/s00417-016-3338-6
Dursun F, Vural Ozec A, Aydin H, Topalkara A, Dursun A, Toker MI, Erdogan H, Arici MK (2015) Total oxidative stress, paraoxonase and arylesterase levels at patients with pseudoexfoliation syndrome and pseudoexfoliative glaucoma. Int J Ophthalmol 8(5):985–990. https://doi.org/10.3980/j.issn.2222-3959.2015.05.24
Can Demirdöğen B, Demirkaya-Budak S, Özge G, Mumcuoğlu T (2020) Evaluation of tear fluid and aqueous humor concentration of clusterin as biomarkers for early diagnosis of pseudoexfoliation syndrome and pseudoexfoliative glaucoma. Curr Eye Res 45(7):805–813. https://doi.org/10.1080/02713683.2019.1698055
Simavli H, Tosun M, Bucak YY, Erdurmus M, Ocak Z, Onder HI, Acar M (2015) Serum and aqueous xanthine oxidase levels, and mRNA expression in anterior lens epithelial cells in pseudoexfoliation. Graefes Arch Clin Exp Ophthalmol 253(7):1161–1167. https://doi.org/10.1007/s00417-015-3043-x
Sonowal H, Ramana KV (2019) 4-hydroxy-trans-2-nonenal in the regulation of anti-oxidative and Pro-Inflammatory signaling pathways. Oxid Med Cell Longev 2019:5937326. https://doi.org/10.1155/2019/5937326
Oruc Y, Celik F, Ozgur G, Beyazyildiz E, Ugur K, Yardim M, Sahin I, Akkoc RF, Aydin S (2020) Altered blood and aqueous humor levels of asprosin, 4-hydroxynonenal, and 8-hydroxy-deoxyguanosine in patients with diabetes mellitus and cataract with and without diabetic retinopathy. Retina 40(12):2410–2416. https://doi.org/10.1097/iae.0000000000002776
Liu H, Gambino F Jr, Algenio CS, Wu C, Gao Y, Bouchard CS, Qiao L, Bu P, Zhao S (2020) Inflammation and oxidative stress induced by lipid peroxidation metabolite 4-hydroxynonenal in human corneal epithelial cells. Graefes Arch Clin Exp Ophthalmol 258(8):1717–1725. https://doi.org/10.1007/s00417-020-04647-2
Sano I, Kaidzu S, Tanito M, Hara K, Okuno T, Ohira A (2013) 4-Hydroxyhexenal- and 4-hydroxynonenal-modified proteins in pterygia. Oxid Med Cell Longev 2013:602029. https://doi.org/10.1155/2013/602029
Chang D, Sha Q, Zhang X, Liu P, Rong S, Han T, Liu P, Pan H (2011) The evaluation of the oxidative stress parameters in patients with primary angle-closure glaucoma. PLoS ONE 6(11):e27218. https://doi.org/10.1371/journal.pone.0027218
Izzotti A, Bagnis A, Saccà SC (2006) The role of oxidative stress in glaucoma. Mutat Res 612(2):105–114. https://doi.org/10.1016/j.mrrev.2005.11.001
Saccà SC, Izzotti A, Rossi P, Traverso C (2007) Glaucomatous outflow pathway and oxidative stress. Exp Eye Res 84(3):389–399. https://doi.org/10.1016/j.exer.2006.10.008
Cheng KC, Cahill DS, Kasai H, Nishimura S, Loeb LA (1992) 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G––T and A––C substitutions. J Biol Chem 267(1):166–172. https://doi.org/10.1016/S0021-9258(18)48474-8
Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A (2006) Biomarkers of oxidative damage in human disease. Clin Chem 52(4):601–623. https://doi.org/10.1373/clinchem.2005.061408
Chylack LT Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, Friend J, McCarthy D, Wu SY (1993) The lens opacities classification system III the longitudinal study of cataract study group. Arch Ophthalmol 111(6):831–836. https://doi.org/10.1001/archopht.1993.01090060119035
Ritch R, Schlötzer-Schrehardt U, Konstas AG (2003) Why is glaucoma associated with exfoliation syndrome? Prog Retin Eye Res 22(3):253–275. https://doi.org/10.1016/s1350-9462(02)00014-9
Yağci R, Gürel A, Ersöz I, Keskin UC, Hepşen IF, Duman S, Yiğitoğlu R (2006) Oxidative stress and protein oxidation in pseudoexfoliation syndrome. Curr Eye Res 31(12):1029–1032. https://doi.org/10.1080/02713680601001319
Wiggs JL (2015) Glaucoma genes and mechanisms. Prog Mol Biol Transl Sci 134:315–342. https://doi.org/10.1016/bs.pmbts.2015.04.008
Erdurmuş M, Yağcı R, Atış Ö, Karadağ R, Akbaş A, Hepşen IF (2011) Antioxidant status and oxidative stress in primary open angle glaucoma and pseudoexfoliative glaucoma. Curr Eye Res 36(8):713–718. https://doi.org/10.3109/02713683.2011.584370
Kondkar AA, Sultan T, Azad TA, Tabussum L, Osman EA, Al-Obeidan SA (2019) Increased plasma levels of 8-hydroxy-2’-deoxyguanosine (8-OHdG) in patients with pseudoexfoliation glaucoma. J Ophthalmol 2019:8319563. https://doi.org/10.1155/2019/8319563
Sorkhabi R, Ghorbanihaghjo A, Javadzadeh A, Rashtchizadeh N, Moharrery M (2011) Oxidative DNA damage and total antioxidant status in glaucoma patients. Mol Vis 17:41–46
Mohanty K, Dada R, Dada T (2017) Oxidative DNA damage and reduced expression of DNA repair genes: role in primary open angle glaucoma (POAG). Ophthalmic Genet 38(5):446–450. https://doi.org/10.1080/13816810.2016.1261904
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by NK, MT, BA, and MS. The first draft of the manuscript was written by NK, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study protocol was approved by the Ondokuzmayıs University Ethics Committee (Ethical approval number: 2021000018).
Consent to participate
Informed consent was obtained from all individual participants involved in the study.
Consent to publish
Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koçak, N., Can, E., Yeter, V. et al. Aqueous humor and serum levels of 4-hydroxynonenal and 8-hydroxy-2′deoxyguanosine in pseudoexfoliation syndrome and glaucoma. Int Ophthalmol 43, 1395–1404 (2023). https://doi.org/10.1007/s10792-022-02539-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-022-02539-4