Abstract
Purpose
To synthesize existing evidence on adverse events, complications, and unfavorable outcomes of current treatment modalities for treatment-requiring retinopathy of prematurity (TR-ROP).
Methods
PubMed, Cochrane Central Register of Controlled Trials, Scopus, EMBASE, Trip Database, and the gray literature available were searched. Randomized Clinical Trials and observational studies comparing the adverse events of intravitreal anti-VEGF injections (bevacizumab, ranibizumab, aflibercept, pegaptanib, conbercept) and laser photocoagulation (LPC) as treatment modalities for infants with TR-ROP were included. The main outcomes compared between the two treatment modalities were: 1. Refractive Errors and Biometry Parameters, 2. Adverse events, complications, and unfavorable outcomes, 3. Disease Recurrence/Disease Regression/Need for retreatment, 4. Neurodevelopmental Outcomes.
Results
Higher quality studies concluded that LPC leads to greater rates of myopia than intravitreal anti-VEGF treatment while the rate of adverse events and of unfavorable neurodevelopmental outcomes is similar. However, there was controversy among the included studies concerning the rate of ROP recurrence between intravitreal anti-VEGF injections and LPC.
Conclusion
There is need for future primary studies assessing the adverse events of intravitreal anti-VEGF injections compared with LPC as treatment modalities for infants with TR-ROP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Retinopathy of prematurity (ROP), the leading cause of infants’ blindness all over the world [1], is a disorder of the retinal vasculature, with pathologic vessels growing into the vitreous instead of the retina [2]. Before introducing the use of anti-vascular endothelial growth factor (anti-VEGF) agents in infants, clinical trials had confirmed the efficacy and safety of ablating the avascular peripheral retina to achieve the regression of preretinal neovascularization and prevent ensuing fibrovascular retinal detachments [2]. Treatment-warranted ROP was defined as a set of characteristics that resulted in a 50% chance of an unfavorable outcome, and cryotherapy was the first treatment modality that proved to lower that risk when compared to no treatment. [3]. Subsequently, ET-ROP study showed the efficacy of the laser treatment for a less severe form of treatment-warranting ROP (type 1 ROP), in which the risk of an unwanted outcome was approximately 15% [4].
The BEAT-ROP study [5] was the first prospective, multicenter, stratified, randomized controlled trial (RCT) that attempted a comparison of efficacy between intravitreal bevacizumab (IVB) monotherapy and conventional laser photocoagulation (LPC) for zone 1 or zone 2 posterior stage 3 + ROP. The study reached the conclusion that IVB was beneficial for infants with stage 3 + ROP for zone 1(P = 0.003), but not for zone 2 disease (P = 0.270). Although BEAT-ROP encouraged many clinicians worldwide to use IVB as first-line treatment for ROP, IVB still remains an off-label modality. A second RCT evaluating ranibizumab followed: RAINBOW study [6], an open-label, multicenter, randomized, three-arm, parallel group, superiority trial, assigned infants with treatment-requiring ROP (TR-ROP) in three groups of 0.2 mg intravitreal ranibizumab (IVR), 0.1 mg IVR and LPC, concluding that ranibizumab 0.2 mg is superior to LPC, while having less unfavorable ocular outcomes. In September 2019, the European Medicines Agency (EMA) approved ranibizumab 0.2 mg as an on-label treatment for infants with TR-ROP. The RAINBOW study provided evidence on the drug’s efficacy as well as on short-term safety issues [6]. However, more information is needed concerning the adverse events (AEs), complications, and unfavorable functional and structural outcomes of the two treatment modalities in the long term.
The aim of this systematic review was to capture the current knowledge regarding the adverse events, the complications and the unfavorable structural and functional outcomes of intravitreal anti-VEGF agents and LPC as treatment modalities for TR-ROP, so as to guide clinical ophthalmologists in their choice of the preferred treatment modality for each case of TR-ROP.
Methods
Study characteristics
This study is a systematic review of RCTs and observational studies that compared intravitreal anti-VEGF injections and LPC as treatment modalities in infants with TR-ROP, in terms of adverse events, complications, and unfavorable structural and functional outcomes. Literature search was carried out until 25/7/2020 without restrictions. The study had been registered to PROSPERO with the following registration number: CRD42020189408.
Eligibility criteria
Inclusion criteria
-
The included studies were either RCTs or observational studies.
-
The participants of each study were infants with ROP that required treatment.
-
The included studies evaluated one of the following intravitreal anti-VEGF agents as monotherapy: ranibizumab, bevacizumab, aflibercept, pegaptanib, conbercept, and compared its adverse events, complications, or unfavorable outcomes with one of the following types of LPC, also used as a monotherapy: diode laser, argon laser, Yttrium aluminum garnet (YAG) laser.
Exclusion criteria
-
The studies that did not involve humans as subjects.
-
The studies that reported early-stage outcomes that are also reported in the complete version of the study. In this case, the most complete version was included to avoid duplication of our results.
-
The studies that reported the outcome of the interventions conducted to treat an adverse event of a treatment modality for ROP, rather than comparing the outcomes of intravitreal anti-VEGF as monotherapy with LPC, also used as a monotherapy, as treatment modalities for ROP.
Study outcomes
The outcomes of interest were the comparison of adverse events, complications, and unfavorable structural and functional outcomes between intravitreal anti-VEGF treatment and LPC, and were categorized as follows:
-
1.
Refractive Errors and Biometry Parameters This subsection evaluated refractive spherical power, spherical equivalent (SE), cylinder power, best-corrected visual acuity (BCVA), rates of myopia and high myopia, rates of anisometropia, rates of astigmatism and biometric results (e.g., anterior chamber depth (ACD), lens thickness (LT), axial length (AL), and central choroidal thickness (CCT)).
-
2.
Adverse events, complications, and unfavorable outcomes This subsection evaluated both ocular and systemic unfavorable outcomes: rates of retinal detachment, vitreous hemorrhage, macular dragging, retinal fold, macular ectopia, endophthalmitis, ocular inflammations, cataract formation, glaucoma, corneal opacity requiring transplantation, and death.
-
3.
Disease Recurrence/Disease Regression/Need for retreatment There is not a universal definition of ROP recurrence and its difference from treatment failure. Therefore, the definition of ROP recurrence, treatment failure, or treatment success that each study used, are provided when the respective results are reported.
-
4.
Neurodevelopmental Outcomes Reported Bayley-3 scores of cognition, language and motor composite were assessed.
-
5.
Optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA) Measurements OCT assessment of the posterior part of the eye, including inner foveal thickness (IFT), outer foveal thickness (OFT), subfoveal choroid thickness (CT), foveal avascular zone (FAZ), foveal vessel density (VD), parafoveal VD, perifoveal VD and macular volume, is presented.
-
6.
Other reported outcomes Comparison of serum-free VEGF levels, serum insulin-like growth factor–1 (IGF-1) levels and cardiovascular assessment between infants with TR-ROP that were treated with either intravitreal anti-VEGF or LPC, are included in this subsection.
Searches and search strategy
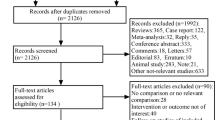
The following databases were searched: PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, EMBASE and Trip Database. A search in the gray literature, such as ClinicalTrials.gov and in available conference proceedings of American Academy of Ophthalmology (AAO), European Paediatric Ophthalmological Society (EPOS), American Association for Pediatric Ophthalmology and Strabismus (AAPOS) and EURETINA, has also been conducted. Furthermore, to ensure a systematic search of the existing literature, reference lists of any included study were scanned rigorously to find eligible studies that the search may had missed. The basic search terms that corresponded to each element of the research question were used for the search strategies in all databases. Every step of the systematic review process was performed by two independent researchers. In the case of disagreements, the final decision was determined by the senior author. After duplicates were removed, all studies were searched by title and abstract. Studies that did not satisfy the research question were excluded. Full-text screening was performed in the remaining, potentially eligible studies. Whenever a study had been published in different versions, the latest and most complete version was selected. The PRISMA flow diagram is presented in Fig. 1 [7].
The PRISMA flow diagram of this systematic review. (from: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6: e1000097. https://doi.org/10.1371/journal.pmed1000097)
Risk-of-bias (quality) assessment
The risk-of-bias (quality) assessment for the RCTs and non-randomized studies of interventions (NRSI) was conducted with the use of the RoB 2.0 [8] and ROBINS-I [9] tools, respectively.
Patient consent form
No patient consent forms were needed as this is a systematic review.
Results
Refractive errors and biometry
Comparison between three groups (IVB, IVR and diode LPC)
The observational studies that compared the refractive errors and biometric measurements between IVB, IVR and diode LPC groups reported no differences between the three groups [10,11,12], as it is shown in Table 1.
Comparison between 2 groups
All RCTs [13, 14] and observational studies [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] that compared intravitreal anti-VEGF injections with LPC for refractive errors and biometry are shown in Table 2. Raghuram et al. [15] reported a more myopic median refraction and a greater rate of myopia ≤ − 0.25 diopters (D) in diode LPC-treated eyes than in IVB-treated eyes at 18–24 months of age (P = 0.020, P = 0.040, respectively). These outcomes are at moderate overall risk of bias and of the highest quality among the observational studies.
Adverse events, complications, and unfavorable structural outcomes
Comparison between three groups
RCTs
In the RAINBOW trial [6], unfavorable structural outcomes, defined as structural abnormalities that have potential effects on visual acuity were found in all three arms: 1 infant in the 0.2 mg IVR arm, 5 in the 0.1 mg IVR arm, and 7 infants in the LPC arm. At 24 weeks after initial treatment, death, serious adverse events (SAEs) and non-serious systemic AEs were similar between the treatment groups as 4 deaths occurred in each group. In the 0.2 mg IVR group, one infant had a moderate cataract formation, while in the 0.1 mg IVR group, one infant developed endophthalmitis in one eye. These outcomes are at low overall risk of bias.
Comparison between 2 groups
The results of all the RCTs [5, 6, 13, 37,38,39], and the observational studies [10, 19, 22, 27, 31, 33, 36, 40,41,42,43,44,45,46,47], that compared the adverse events, complications, and unfavorable outcomes between intravitreal anti-VEGF injections and LPC, are displayed in Table 3.
Disease recurrence/disease regression/need for retreatment
In the RAINBOW study [6], treatment success, defined as alive and without treatment switch and unfavorable structural outcome or active ROP at day 169, was reported as odds ratio (OR) and 95% Confidence Interval (CI) in pairwise comparisons of the treatment arms: OR = 2.19 (CI 0.99 to 4.82; P = 0.050) of 0.2 mg.
IVR compared to LPC, and OR = 1.57 (95% CI: 0.76 to 3.26) of 0.1 mg IVR compared to LPC. This outcome is at low overall risk of bias.
The results of all the other RCTs (except RAINBOW), and the observational studies, that compared the rates of disease recurrence, disease regression, need for retreatment between IVB, IVR and LPC groups, are displayed in Table 4 [5, 6, 10, 13, 19, 22, 29, 33, 36,37,38,39,40, 42, 44,45,46, 48,49,50,51].
Neurodevelopmental outcomes
Observational studies
The observational studies [15, 17, 22, 36, 44, 47, 52,53,54,55] that compared neurodevelopmental outcomes between intravitreal anti-VEGF and LPC for TR-ROP were of moderate or serious overall risk of bias and found many similarities between the two treatment modalities, while in case a significant difference existed, LPC had the better results in terms of neurodevelopmental outcomes (Table 5).
In the RCT of Kennedy et al. [56], 16 infants of the BEAT-ROP study were evaluated for medical and neurodevelopmental outcomes at 18–28 months corrected age (CA). The authors reported similar results in all outcomes when comparing the two treatment groups at follow-up (median cognitive score P = 0.060, language score P = 0.180, motor composite score P = 0.220, gross motor function level P = 0.850, rate of cerebral palsy P = 1.000, median CA P = 0.100, median weight percentile for age P = 0.270, median length percentile for age at follow-up P = 0.390, median head circumference percentile for age at follow-up P = 0.460). These outcomes are at medium overall risk of bias. This is the only RCT that we found concerning the comparison of neurodevelopmental outcomes between intravitreal anti-VEGF and LPC treatment modalities for TR-ROP.
OCT and OCTA measurements
The observational studies [16, 20], that compared the macular OCT and OCTA measurements between intravitreal anti-VEGF and LPC in infants with TR-ROP were of critical overall risk of bias and in case a significant difference existed, IVB treatment showed lower mean foveal, parafoveal, perifoveal, and inner foveal thickness (Table 6).
Finally, studies that compared other reported outcomes [54, 57,58,59], such as serum-free VEGF levels, serum Insulin-like growth factor 1 (IGF-1) levels, tricuspid E-wave values, odds of returning to respiratory baseline by 48 h, number of diagnoses at time of discharge, hospitalization days, days for oxygen requirement, and duration of hospitalization, are summarized in Table 7.
Discussion
This systematic review of the literature regarding treatment of ROP revealed that higher quality studies concluded that LPC leads to greater rates of myopia than intravitreal anti-VEGF treatment, while the rate of adverse events and of unfavorable neurodevelopmental outcomes is similar. However, there was controversy among the included studies concerning the rate of ROP recurrence. Studies agree on findings regarding the refractive outcome, the rates of adverse events, and the neurodevelopmental outcomes, while they differ in disease recurrence rates. Notably, authors defined their outcomes differently and results are thus not directly comparable. Furthermore, most studies are observational and of moderate risk of bias, so safe conclusions cannot be drawn.
The RCTs [14] and the observational studies [15, 19] of the highest quality concluded that eyes treated with LPC developed more myopic refraction than the eyes treated with intravitreal anti-VEGF injections, a finding shared with the majority of observational studies as well [16, 20,21,22,23, 27,28,29, 33,34,35,36]. Rates of adverse events, complications and unfavorable outcomes were similar between intravitreal anti-VEGF agents and LPC in high quality RCTs like RAINBOW [6] and BEAT-ROP [5]. Results in lower-quality RCTs [13, 37,38,39] and observational studies [10, 19, 22, 27, 31, 33, 36, 40,41,42,43,44,45,46,47] did not generally differ.
Disease recurrence, disease regression and need for retreatment were similar between intravitreal anti-VEGF injections and LPC in the RAINBOW study [6], the highest-quality RCT available in our systematic review concerning that outcome. Some of the lower-quality RCTs concluded that the rate of disease recurrence was greater in the intravitreal anti-VEGF injection group [37,38,39], while others like BEAT-ROP [5] concluded the exact opposite. Finally, other RCTs [13] agreed with the findings of RAINBOW [6]. Some of the observational studies of higher quality concluded that rates of ROP recurrence were greater in the intravitreal anti-VEGF injection group [19], while others reported similar ROP recurrence rates between the two treatment groups [29, 45, 48], and others concluded that rates of ROP regression were greater in eyes treated with intravitreal anti-VEGF injections [50].
The only RCT that conducted a comparison of neurodevelopmental outcomes between intravitreal anti-VEGF and LPC, which was at medium overall risk of bias, found no differences in the neurodevelopmental outcomes between the two treatment modalities [56]. Most of the observational studies of higher quality also report this result [15, 36, 52, 54]. However, some high-quality observational studies supported that the intravitreal anti-VEGF group had worse neurodevelopmental outcomes in the Language-Social domain Developmental Quotient (DQ) at 18 months CA [53], motor composite score at 18 months CA [55], or more neurodevelopmental disabilities [55]. This may be due to the fact that there is only one RCT comparing neurodevelopmental outcomes between intravitreal anti-VEGF and LPC, and due to the observational design of the other studies.
The studies that reported OCT and OCTA measurements were of very low quality due to critical overall risk of bias and generally concluded that foveal thickness was lower, while mean foveal avascular zone (FAZ) was higher in the IVB group [16, 20].
Finally, an RCT with high overall risk of bias that compared the serum levels of free VEGF and IGF-1 between IVB and LPC treatment groups found lower serum levels of these two biochemical markers in the IVB group [57]. The observational studies that reported different outcomes related to the wide spectrum of adverse events were of very low quality and therefore analyzing these studies is out of the scope of this systematic review [54, 58, 59].
Intravitreal anti-VEGF treatment leads to lower rates of myopia, having a similar rate of adverse events and unfavorable neurodevelopmental outcomes as LPC. Therefore, intravitreal anti-VEGF treatment seems to have the preferable outcomes overall. However, no safe conclusions can be drawn concerning the rates of disease recurrence. More primary studies need to be conducted to give a definite answer to which treatment modality has greater rates of disease recurrence and to verify the aforementioned findings.
Strengths and weaknesses
This study captured the comparison of all the adverse events, complications, and unfavorable structural and functional outcomes between intravitreal anti-VEGF injections and LPC that have been reported in the literature concerning the treatment of TR-ROP. Due to its systematic nature, this study aimed to summarize all current knowledge in view of facilitating clinical decisions. The adverse event comparison was stratified in sections that correlated with clinical significance, distinguishing different clinical entities of interest. Finally, the critical appraisal of the included studies was thoroughly conducted by assessing the risk of bias of each outcome of each individual study. Two independent researchers conducted the risk-of-bias assessment to limit bias as much as possible.
On the other hand, it should be mentioned that there is high heterogeneity between the included studies due to the very wide spectrum of the outcomes of our interest. This is the reason for not performing a quantitative synthesis of the results (meta-analysis).
Furthermore, some reported outcomes have been defined in different ways from study to study. For example, in the result section of disease recurrence or regression and need for retreatment, RAINBOW study defined treatment success as: alive and without treatment switch and unfavorable structural outcome or active ROP at day 169 [6], while BEAT-ROP study defined treatment failure as: the recurrence of neovascularization in one or both eyes arising from the retinal vessels and requiring retreatment by 54 weeks’ postmenstrual age [5]. That is, an obstacle encountered in many other outcomes of interest and therefore the included studies were synthesized in a descriptive way to succeed in providing the reader with conclusions that reflect everyday clinical practice. Lastly, an endogenous limitation that is related to the pathophysiology of ROP is that it is not clear if ROP recurrence is an adverse event or a failure of the respective treatment modality; therefore, ROP recurrence and adverse events were analyzed in different result sections.
Clinical implications
Almost all the included studies, and most importantly, the higher quality studies like RAINBOW [6], agreed that LPC treatment leads to greater refractive errors and greater rates of myopia than intravitreal anti-VEGF treatment modalities. Similarly, almost all included studies reported no differences in the rates of adverse events, complications, unfavorable structural outcomes, and unfavorable neurodevelopmental outcomes between LPC and anti-VEGF. Findings of different high-quality studies, in terms of ROP recurrence/regression and need for retreatment, are overall controversial. This may be partly due to different definitions of ROP recurrence in the different studies that investigated this outcome or due to the vague nature of the outcome itself, because some authors interpret ROP recurrence as an adverse event, while others as failure of the applied treatment.
Eyes treated with LPC for TR-ROP tend to have more myopic refraction than the eyes treated with intravitreal anti-VEGF injections, while the rates of adverse events, complications, unfavorable structural outcomes, and unfavorable neurodevelopmental outcomes between the two treatment modalities seem to be similar. The RAINBOW study is designed to follow up participants until the age of five years so hopefully more data will become available soon [6]. There is a need for more primary studies, and a consensus needs to be agreed upon concerning the definition of the outcomes of interest. This would help in lowering the heterogeneity of future systematic reviews and in providing clinical ophthalmologists with more precise and more high-quality evidence about the comparison of intravitreal anti-VEGF injections and LPC in the treatment of TR-ROP.
References
Darlow BA, Gilbert C (2019) Retinopathy of prematurity: a world update. Semin Perinatol 43:315–316. https://doi.org/10.1053/j.semperi.2019.05.001
Hartnett ME (2020) Retinopathy of prematurity: evolving treatment with anti-vascular endothelial growth factor. Am J Ophthalmol 218:208–213. https://doi.org/10.1016/j.ajo.2020.05.025
Tasman W (1988) Multicenter trial of cryotherapy for retinopathy of prematurity. Arch Ophthalmol 106:463–464
Good WV, Hardy RJ (2001) Guest editorial: the multicenter study of early treatment for retinopathy of prematurity (ETROP). Ophthalmology 108:1013–1014. https://doi.org/10.1016/S0161-6420(01)00540-1
Mintz-Hittner HA, Kennedy KA, Chuang AZ (2011) Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 364:603–615. https://doi.org/10.1056/NEJMoa1007374
Stahl A, Lepore D, Fielder A, Fleck B, Reynolds JD, Chiang MF et al (2019) Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet 394:1551–1559. https://doi.org/10.1016/S0140-6736(19)31344-3
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. https://doi.org/10.1371/journal.pmed.1000097
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al (2019) RoB 2: a revised Cochrane risk-of-bias tool for randomized trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. https://doi.org/10.1136/bmj.i4919
Kang HG, Kim TY, Han J, Han S-H (2019) Refractive outcomes of 4-year-old children after intravitreal anti-vascular endothelial growth factor versus laser photocoagulation for retinopathy of prematurity. Korean J Ophthalmol 33:272. https://doi.org/10.3341/kjo.2019.0011
Kabataş EU, Kurtul BE, Altıaylık Özer P, Kabataş N (2017) Comparison of intravitreal bevacizumab, intravitreal ranibizumab and laser photocoagulation for treatment of type 1 retinopathy of prematurity in Turkish preterm children. Curr Eye Res 42:1054–1058. https://doi.org/10.1080/02713683.2016.1264607
Gunay M, Sukgen EA, Celik G, Kocluk Y (2017) Comparison of bevacizumab, ranibizumab, and laser photocoagulation in the treatment of retinopathy of prematurity in Turkey. Curr Eye Res 42:462–469. https://doi.org/10.1080/02713683.2016.1196709
Roohipoor R, Torabi H, Karkhaneh R, Riazi-Eafahani M (2019) Comparison of intravitreal bevacizumab injection and laser photocoagulation for type 1 zone II retinopathy of prematurity. J Curr Ophthalmol 31:61–65. https://doi.org/10.1016/j.joco.2018.10.008
Geloneck MM, Chuang AZ, Clark WL, Hunt MG, Norman AA, Packwood EA et al (2014) Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: A randomized clinical trial. JAMA Ophthalmol 132:1327–1333. https://doi.org/10.1001/jamaophthalmol.2014.2772
Raghuram K, Isaac M, Yang J, AlAli A, Mireskandari K, Ly LG et al (2019) Neurodevelopmental outcomes in infants treated with intravitreal bevacizumab versus laser. J Perinatol 39:1300–1308. https://doi.org/10.1038/s41372-019-0420-z
Chen YC, Chen SN (2020) Foveal microvasculature, refractive errors, optical biometry and their correlations in school-aged children with retinopathy of prematurity after intravitreal antivascular endothelial growth factors or laser photocoagulation. Br J Ophthalmol 104:691–696. https://doi.org/10.1136/bjophthalmol-2019-314610
Therani N, Isaac M, Raghuram K, Mireskandari K, Shah P (2019) Neurodevelopmental and visual outcomes in infants with retinopathy of prematurity treated with bevacizumab versus laser. In: 2019 AAPOS abstract book, pp 240
Manuchian S, Isaac M, Mireskandari K, Tehrani N, Vincer M, Hatchette J et al (2019) Binocularity outcomes following treatment for retinopathy of prematurity. In: 2019 AAPOS abstract book, pp 244
Roohipoor R, Karkhaneh R, Riazi-Esfahani M, Farahani AD, Khodabandeh A, Adib NE et al (2018) Comparison of intravitreal bevacizumab and laser photocoagulation in the treatment of retinopathy of prematurity. Ophthalmol Retin 2:942–948. https://doi.org/10.1016/j.oret.2018.01.017
Lee YS, See LC, Chang SH, Wang NK, Hwang YS, Lai CC et al (2018) Macular structures, optical components, and visual acuity in preschool children after intravitreal bevacizumab or laser treatment. Am J Ophthalmol 192:20–30. https://doi.org/10.1016/j.ajo.2018.05.002
Mueller B, Salchow DJ, Waffenschmidt E, Joussen AM, Schmalisch G, Czernik C et al (2017) Treatment of type I ROP with intravitreal bevacizumab or laser photocoagulation according to retinal zone. Br J Ophthalmol 101:365–370. https://doi.org/10.1136/bjophthalmol-2016-308375
Kong L, Dinh K, Schechet S, Coats D, Voigt R, Demny A et al (2015) Comparison of ocular and developmental outcomes in laser-and bevacizumab-treated infants with retinopathy of prematurity. Ophthalmol Res An Int J 3:13–22. https://doi.org/10.9734/OR/2015/13281
Vujanović MS, Stanković-Babić GL, Oros A, Zlatanović GD, Jovanović P, Cekić SP et al (2017) Refractive errors in premature infants with retinopathy of prematurity after anti-vascular endothelial growth factor (anti-VEGF) therapy. Vojnosanit Pregl 74:323–328. https://doi.org/10.2298/VSP150831191V
Lolas M, Tuma A, Zanolli M, Agurto R, Stevenson R, Ossandón D (2017) Anatomical and refractive outcomes in patients with treated retinopathy of prematurity. Arch Soc Esp Oftalmol 92:472–476. https://doi.org/10.1016/j.oftal.2016.12.007
Gunay M, Sekeroglu MA, Bardak H, Celik G, Esenulku CM, Hekimoglu E et al (2016) Evaluation of refractive errors and ocular biometric outcomes after intravitreal bevacizumab for retinopathy of prematurity. Strabismus 24:84–88. https://doi.org/10.3109/09273972.2016.1159232
Gunay M, Celik G, Tuten A, Karatekin G, Bardak H, Ovali F (2016) Characteristics of severe retinopathy of prematurity in infants with birth weight above 1500 grams at a referral center in Turkey. PLoS ONE 11:e0161692. https://doi.org/10.1371/journal.pone.0161692
Gunay M, Celik G, Gunay BO, Aktas A, Karatekin G, Ovali F (2015) Evaluation of 2-year outcomes following intravitreal bevacizumab (IVB) for aggressive posterior retinopathy of prematurity. Arq Bras Oftalmol 78:300–304. https://doi.org/10.5935/0004-2749.20150079
Li AL, Rahman EZ, Voigt RG, Coats DK, Steinkuller PG, Kong L (2015) Long-term vision and neurodevelopmental outcomes in laser and bevacizumab-treated infants with retinopathy of prematurity. Investig Ophthalmol Vis Sci 56:4321
Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR (2015) Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity: a 5-year retrospective analysis. Ophthalmology 122:1008–1015. https://doi.org/10.1016/j.ophtha.2014.12.017
Kuo HK, Sun IT, Chung MY, Chen YH (2015) Refractive error in patients with retinopathy of prematurity after laser photocoagulation or bevacizumab monotherapy. Ophthalmologica 234:211–217. https://doi.org/10.1159/000439182
Isaac M, Mireskandari K, Tehrani N (2015) Treatment of type 1 retinopathy of prematurity with bevacizumab versus laser. J AAPOS 19:140–144. https://doi.org/10.1016/j.jaapos.2015.01.009
Isaac M, Mireskandari K, Tehrani N (2015) Outcomes following treatment of type 1 retinopathy of prematurity with bevacizumab versus laser: two year follow up. Investig Ophthalmol Vis Sci 56:985
Warren A, Warren K, Weatherstone K (2015) Bevacizumab (AvastinTM) as a treatment alternative for threshold retinopathy of prematurity (ROP). Investig Ophthalmol Vis Sci 56:973
Harder BC, Schlichtenbrede FC, von Baltz S, Jendritza W, Jendritza B, Jonas JB (2013) Intravitreal bevacizumab for retinopathy of prematurity: refractive error results. Am J Ophthalmol 155:1119-1124.e1. https://doi.org/10.1016/j.ajo.2013.01.014
Harder BC, von Baltz S, Schlichtenbrede FC, Jonas JB (2012) Early refractive outcome after intravitreous bevacizumab for retinopathy of prematurity. Arch Ophthalmol 130:800–801. https://doi.org/10.1001/archophthalmol.2012.1
Kang HG, Choi EY, Byeon SH, Kim SS, Koh HJ, Lee SC et al (2019) Intravitreal ranibizumab versus laser photocoagulation for retinopathy of prematurity: efficacy, anatomical outcomes and safety. Br J Ophthalmol 103:1332–1336. https://doi.org/10.1136/bjophthalmol-2018-312272
Lepore D, Quinn GE, Molle F, Orazi L, Baldascino A, Ji MH et al (2018) Follow-up to age 4 years of treatment of type 1 retinopathy of prematurity intravitreal bevacizumab injection versus laser: fluorescein angiographic findings. Ophthalmology 125:218–226. https://doi.org/10.1016/j.ophtha.2017.08.005
Karkhaneh R, Khodabande A, Riazi-Eafahani M, Roohipoor R, Ghassemi F, Imani M et al (2016) Efficacy of intravitreal bevacizumab for zone-II retinopathy of prematurity. Acta Ophthalmol 94:e417–e420. https://doi.org/10.1111/aos.13008
Zhang G, Yang M, Zeng J, Vakros G, Su K, Chen M et al (2017) Comparison of intravitreal injection of ranibizumab versus laser therapy for Zone II treatment-requiring retinopathy of prematurity. Retina 37:710–717. https://doi.org/10.1097/IAE.0000000000001241
Blair M, Gonzalez JMG, Snyder L, Schechet S, Greenwald M, Shapiro M et al (2018) Bevacizumab or laser for aggressive posterior retinopathy of prematurity. Taiwan J Ophthalmol 8:243–248. https://doi.org/10.4103/tjo.tjo_69_18
Walz JM, Bemme S, Reichl S, Akman S, Breuß H, Süsskind D et al (2018) Treated cases of retinopathy of prematurity in Germany: 5-year data from the Retina.net ROP registry. Ophthalmologe 115:476–488. https://doi.org/10.1007/s00347-018-0701-5
Lyu J, Zhang Q, Chen C, Xu Y, Ji X, Zhao P (2019) Ranibizumab injection and laser photocoagulation to treat type 1 retinopathy of prematurity after 40 weeks post menstrual age: a retrospective case series study. BMC Ophthalmol 19:60. https://doi.org/10.1186/s12886-019-1067-4
Shah PK, Subramanian P, Venkatapathy N, Chan RVP, Chiang MF, Campbell JP (2019) Aggressive posterior retinopathy of prematurity in two cohorts of patients in South India: implications for primary, secondary, and tertiary prevention. J AAPOS 23:264. https://doi.org/10.1016/j.jaapos.2019.05.014
Zhang M, Blair MP, Rodriguez SH (2019) Two-year ocular and neurodevelopmental outcomes among infants treated for retinopathy of prematurity using a commercial claims database. In: 2019 AAPOS abstract book, pp 237
Sukgen EA, Koçluk Y (2017) Treatment for stage 4A retinopathy of prematurity: laser and/or ranibizumab. Graefe’s Arch Clin Exp Ophthalmol 255:263–269. https://doi.org/10.1007/s00417-016-3443-6
Barry GP, Tauber KA, Fisher M, Greenberg S, Zobal-Ratner J, Binenbaum G (2019) Short-term retinal detachment risk after treatment of type 1 retinopathy of prematurity with laser photocoagulation versus intravitreal bevacizumab. J AAPOS 23:260. https://doi.org/10.1016/j.jaapos.2019.05.013
Peyton C, Rodriquez S, Andrews B, Schreiber M, Wroblewski K, Msall M (2018) Neurodevelopmental outcomes after extreme prematurity comparing bevacizumab to laser surgery for type 1 retinopathy of prematurity. Dev Med Child Neurol 60:47. https://doi.org/10.1111/dmcn.71_14017
Ling KP, Liao PJ, Wang NK, Chao AN, Chen KJ, Chen TL et al (2020) Rates and risk factors for recurrence of retinopathy of prematurity after laser or intravitreal anti-vascular endothelial growth factor monotherapy. Retina 40:1793–1803. https://doi.org/10.1097/IAE.0000000000002663
Moran S, O’Keefe M, Hartnett C, Lanigan B, Murphy J, Donoghue V (2014) Bevacizumab versus diode laser in stage 3 posterior retinopathy of prematurity. Acta Ophthalmol 92:e496–e497. https://doi.org/10.1111/aos.12339
Nicoară SD, Ștefănuţ AC, Nascutzy C, Zaharie GC, Toader LE, Drugan TC (2016) Regression rates following the treatment of aggressive posterior retinopathy of prematurity with bevacizumab versus laser: 8-year retrospective analysis. Med Sci Monit 22:1192–1209. https://doi.org/10.12659/MSM.897095
Toy BC, Schachar IH, Tan GSW, Moshfeghi DM (2016) Chronic vascular arrest as a predictor of bevacizumab treatment failure in retinopathy of prematurity. Ophthalmology 123:2166–2175. https://doi.org/10.1016/j.ophtha.2016.06.055
Lien R, Yu MH, Hsu KH, Liao PJ, Chen YP, Lai CC et al (2016) Neurodevelopmental outcomes in infants with retinopathy of prematurity and bevacizumab treatment. PLoS ONE 11:e0148019. https://doi.org/10.1371/journal.pone.0148019
Arima M, Akiyama M, Fujiwara K, Mori Y, Inoue H, Seki E et al (2020) Neurodevelopmental outcomes following intravitreal bevacizumab injection in Japanese preterm infants with type 1 retinopathy of prematurity. PLoS ONE 15(3):e0230678. https://doi.org/10.1371/journal.pone.0230678
Chen TA, Schachar IH, Moshfeghi DM (2018) Outcomes of intravitreal bevacizumab and diode laser photocoagulation for treatment-warranted retinopathy of prematurity. Ophthalmic Surg Lasers Imaging Retina 49:126–131. https://doi.org/10.3928/23258160-20180129-07
Morin J, Luu TM, Superstein R, Ospina LH, Lefebvre F, Simard MN et al (2016) Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics 137:e20153218. https://doi.org/10.1542/peds.2015-3218
Kennedy KA, Mintz-Hittner HA, BEAT-ROP Cooperative Group (2018) Medical and developmental outcomes of bevacizumab versus laser for retinopathy of prematurity. J AAPOS 22:61-65.e1. https://doi.org/10.1016/j.jaapos.2017.10.006
Kong L, Bhatt AR, Demny AB, Coats DK, Li A, Rahman EZ et al (2015) Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF-1 in infants with retinopathy of prematurity. Invest Ophthalmol Vis Sci 56:956–961. https://doi.org/10.1167/iovs.14-15842
Cilsal E, Sukgen EA (2020) Cardiovascular assessment after treatment for retinopathy of prematurity: a comparative study between anti-VEGF agent (aflibercept) and laser. Cardiovasc J Afr 31:123–129
Barry G, Tauber K, Afroze F, Finuncane E, Greenberg SH, Oechsner H et al (2019) A comparison of respiratory outcomes after treatment for retinopathy of prematurity (ROP) with pan-retinal photocoagulation (PRP) or bevacizumab. In: 2019 AAPOS abstract book, pp 238
Funding
Open access funding provided by HEAL-Link Greece. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by GNT MD, MSc and AKS MD, MSc. The first draft of the manuscript was written by GNT MD, MSc, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to participate
No consent to participate was needed as the respective study is a systematic review.
Consent to publish
No consent to publish was needed as the respective study is a systematic review.
Ethics approval
No ethics approval was needed as the respective study is a systematic review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsiropoulos, G.N., Seliniotaki, A.K., Haidich, AB. et al. Comparison of adverse events between intravitreal anti-VEGF and laser photocoagulation for treatment-requiring retinopathy of prematurity: a systematic review. Int Ophthalmol 43, 1027–1062 (2023). https://doi.org/10.1007/s10792-022-02480-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-022-02480-6