Abstract
Purpose
We aimed to evaluate choroidal thickness (CT) in patients who have recovered from COVID-19 by using enhanced depth imaging optical coherence tomography (EDI-OCT).
Methods
We included fifty-eight patients who have recovered from COVID-19 (group 1) and fifty healthy control subjects (group 2) in this prospective study. Best corrected visual acuity, anterior segment and posterior segment examinations of all subjects were performed. CT scan and measurements were taken with the EDI mode of the Spectral Domain OCT device.
Results
Of the 108 subjects included in this study, 57 were female and 51 were male. The mean age was similar in both groups (36.10 ± 7.12 and 35.58 ± 7.29, respectively, p = 0.276). Group 1 had the following characteristics: the mean time since diagnosis was 53.18 ± 2.84; it had been 38.48 ± 4.07 days since the PCR test was negative; and all subjects were outpatients. It was detected that the CT of the patients in group 1 decreased in all areas compared to group 2, and this decrease was significant in subfoveal, temporal and inferior areas (257.48 ± 32.79, 273.62 ± 45.04, p = 0.04; 232.96 ± 41.79, 252.76 ± 46.09, p = 0.02, and 245.22 ± 44.58, 271.54 ± 55.07, p = 0.01, respectively). In the retinal nerve fiber layer analysis for group 1, thickening was detected in all areas, although it was not statistically significant, except in the temporal area where it was (superotemporal, superonasal, nasal, inferonasal, inferotemporal, temporal, and global [p = 0 .08, p = 0.45, p = 0.73, p = 0.64, p = 0.74, p = 0.02, and p = 0.10, respectively]).
Conclusion
For individuals who had recovered from COVID-19, it was found that CT decreased in all areas in these patients. Therefore, this study in which we have demonstrated the decrease in the thickness of the choroidal tissue, a tissue with high blood flow, may contribute to the understanding of the systemic microvascular waste of this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which can be potentially fatal and causes coronavirus disease (COVID-19), a novel disease that affects lungs and airways and is often manifested by fever, cough, malaise, muscle pain, and shortness of breath, is thought to be transmitted mainly by droplets in the environment as a result of contact with infected patients [1,2,3].
It has been shown that SARS-CoV-2 infects cells with the angiotensin converting enzyme-2 receptor, which has been reported to be expressed in eye tissues, such as the cornea and conjunctiva, and the presence of RNA of this virus in tear and retinal biopsies has been shown in multiple studies [4,5,6].
The choroid, which is the vascular layer of the eye, is the region with the highest blood supply in the posterior segment of the eye. It has been reported that choroidal thickness (CT), which is reported to vary depending on age, refractive error, axial length, and diurnal variations, may also change in various retinal diseases and systemic diseases, such as glaucoma and Alzheimer’s disease [7,8,9].
Vasculitis and microembolism have been reported in COVID-19 patients, and studies have also shown that retinal circulation is affected in these patients. Therefore, determining the changes in microcirculation due to COVID-19, especially in tissues with high blood supply in the posterior segment of the eye, such as the choroid, and detecting possible changes in the perfusion of ocular tissues in these patients may contribute to the literature.
Enhanced depth imaging optical coherence tomography (EDI-OCT), which is a noninvasive diagnostic method, enables the evaluation of choroidal morphological features in a cross-sectional manner and in high resolution by reducing the signal strength behind the retinal pigment epithelium [10].
The aim of this study was to evaluate the CT of patients recovering from COVID-19 by EDI-OCT.
Methods
Study design and subject
This prospective study included 58 patients who had recovered from COVID-19 (group 1) and 50 healthy control patients (group 2) of a similar age and gender. We obtained approval from the ethics committee. All study participants gave written consent before measurements were taken, and the study was carried out according to the Declaration of Helsinki.
The study group consisted of people who had contracted COVID-19 and had recovered from it for at least one month (group 1) and individuals who applied to our outpatient clinic for a routine eye examination and did not have any eye disease (group 2).
All subjects in the study had best corrected visual acuity, refractive error detection, anterior segment, and posterior segment examinations performed. In both groups, subjects with previous ocular surgery, spherical equivalent diopters of more than ± 4 diopters, other ocular diseases (corneal opacity, glaucoma, uveitis, amblyopia, retinal disease, etc.), and systemic diseases, such as diabetes and hypertension, were not included in the study.
Optical coherence tomography measurement
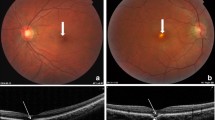
In all included subjects, measurements were taken at the same time interval to prevent diurnal changes. CT was obtained with the EDI mode of the Spectral Domain OCT device (Heidelberg Engineering, Heidelberg, Germany). In the horizontal section image passing through the fovea, measurements were taken in the nasal and temporal regions at a distance of 1500 μm from the central fovea and fovea center. The measurement of CT, which was manually measured and defined as the vertical distance from the outer surface of the retinal pigment epithelium to the choroid-scleral interface, was performed by a single trained technician (Fig. 1). A circular scan centered on the optic disk was used to evaluate the RNFL thickness measurement of the patients (Fig. 2).
Statistical analysis
SPSS version 26.0 (Chicago, IL, USA) was used for all statistical analyses. Demographic data were calculated using descriptive statistics. Mean and standard deviations were used to describe the data. After all measurements were entered into the SPSS program, a normality test was applied. The student t test was used to compare the continuous variables of the two groups according to the normality test results. Comparison of categorical variables was made using the chi-square test.
Results
This study included 57 females and 51 males. The mean age was similar in both groups (36.10 ± 7.12 and 35.58 ± 7.29, respectively, p = 0.276). For group 1, the mean time since diagnosis of COVID-19 was 54.25 ± 6.36 days, the number of days since the PCR test was negative was 38.45 ± 6.87, and all the subjects were outpatients (Table 1).
It was detected that the CT of the patients in group 1 decreased in all areas compared to group 2, and this decrease was significant in subfoveal, temporal and inferior areas (257.48 ± 32.79, 273.62 ± 45.04, p = 0.04; 232.96 ± 41.79, 252.76 ± 46.09, p = 0.02, and 245.22 ± 44.58, 271.54 ± 55.07, p = 0.01, respectively). (Table 2).
In the RNFL analysis of group 1, thickening was detected in all quadrants, although it was not statistically significant, except in the temporal area where it was (superotemporal, superonasal, nasal, inferonasal, inferotemporal, temporal, and global [p = 0 0.08, p = 0.45, p = 0.73, p = 0.64, p = 0.74, p = 0.02, and p = 0.10, respectively]) (Table 3).
Discussion
For group 1, it was found that CT decreased in all areas, especially in the subfoveal, temporal, and inferior areas. In addition, although it was not statistically significant in the RNFL analysis of these patients, thickening was detected in all quadrants.
Although the potential transmission of SARS-CoV-2 from the ocular surface is thought to be important, the route of virus transmission to ocular tissue is still controversial because the presence of SARS-CoV-2 RNA in blood samples has been demonstrated. It has also been reported that conjunctival symptoms range from 0.8 to 32% [11, 12].
Studies have reported that COVID-19 caused by SARS-CoV-2 causes tissue hypoperfusion and thrombosis by causing endothelitis, resulting in neurovascular events and pulmonary artery thrombosis [13,14,15,16]. In addition, the presence of cotton wool exudates associated with retinal vascular occlusion and retinal ischemia has been reported in patients with COVID-19 [17,18,19]. There are studies examining the retinal microcirculation changes in these patients with optical coherence tomography (OCTA). In a study examining patients recovering from COVID-19, significant decreases in superficial capillary plexus vessel density (SCP-VD) and deep capillary plexus vessel density (DCP-VD) were reported in both foveal and parafoveal regions of the macula. Another study reported that moderate and severe COVID-19 patients had decreased central retinal VD [20, 21]. Similarly, patients with COVID-19 have been reported to have lower radial peripapillary plexus perfusion density [22]. However, one study reported that the SCP-VD and DCP-VD of patients recovering from moderate COVID-19 were similar to those of healthy controls. Possible reasons for this change in VD were that the study may have been limited only to patients severely affected by COVID-19 or a possible return of damage to the macular capillary plexuses after acute infection [23].
It has been reported that hypoxia may increase circulating proinflammatory cytokine levels, leading to vascular leakage and consequently edema [24]. In addition, it has been reported that this value increased in four out of five patients whose RNFL thicknesses before and after COVID-19 infection were compared [25]. The result of this study may have resulted from the hypoxia-related inflammation and edema caused by the decrease in the choroidal blood supply that we obtained in our study.
The limitations of this study were the relatively small number of participants, the use of a cross-sectional study conducted in a single center, and a lack of long-term follow-up of choroidal thickness change as an indicator of choroidal blood supply with the subjects. The potential strength of this study is that, to our knowledge, it is the first study to examine choroidal blood supply, which plays an important role in eye blood supply in patients recovering from COVID-19 using EDI-OCT.
In conclusion, individuals in group 1 showed decreased CT in all areas in these patients. Therefore, the results of our study may contribute to the understanding of the systemic microvascular waste of this disease. However, these findings should be demonstrated and supported by multicenter, long-term, high case series studies.
Data availability
Data and material are available.
References
Epidemiology Unit S lanka (2020) Novel coronavirus (2019-nCoV) - Situation report – 2020.01.28. Situat Rep pp 1–7. https://www.epid.gov.lk/web/index.php?option=com_content&view=article&id=225&lang=en
Fan Q, Zhang W, Li B et al (2020) Association between ABO Blood group system and COVID-19 susceptibility in Wuhan. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2020.00404
Van Doremalen N, Bushmaker T, Morris DH et al (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 382:1564–1567
Xia J, Tong J, Liu M et al (2020) Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol 92:589–594. https://doi.org/10.1002/jmv.25725
Colavita F, Lapa D, Carletti F et al (2020) SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann Intern Med 173:242–243. https://doi.org/10.7326/M20-1176
Casagrande M, Fitzek A, Püschel K et al (2020) Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul Immunol Inflamm 28:721–725. https://doi.org/10.1080/09273948.2020.1770301
Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y (2010) Choroidal thickness in healthy Japanese subjects. Investig Ophthalmol Vis Sci 51:2173–2176. https://doi.org/10.1167/iovs.09-4383
Tan CS, Ouyang Y, Ruiz H, Sadda SR (2012) Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Investig Ophthalmol Vis Sci 53:261–266. https://doi.org/10.1167/iovs.11-8782
Bayhan HA, AslanBayhan S, Celikbilek A et al (2015) Evaluation of the chorioretinal thickness changes in Alzheimer’s disease using spectral-domain optical coherence tomography. Clin Exp Ophthalmol 43:145–151. https://doi.org/10.1111/ceo.12386
Spaide RF, Koizumi H, Pozonni MC (2008) Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 146:496–500. https://doi.org/10.1016/j.ajo.2008.05.032
Seah I, Agrawal R (2020) Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm 28:391–395. https://doi.org/10.1080/09273948.2020.1738501
Chang L, Zhao L, Gong H et al (2020) Severe acute respiratory syndrome coronavirus 2 RNA detected in blood donations. Emerg Infect Dis 26:1631–1633. https://doi.org/10.3201/eid2607.200839
Becker RC (2020) COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis 50:54–67. https://doi.org/10.1007/s11239-020-02134-3
Carnevale S, Beretta P, Morbini P (2021) Direct endothelial damage and vasculitis due to SARS-CoV-2 in small bowel submucosa of COVID-19 patient with diarrhea. J Med Virol 93:61–63. https://doi.org/10.1002/jmv.26119
Hess DC, Eldahshan W, Rutkowski E (2020) COVID-19-related stroke. Transl Stroke Res 11:322–325. https://doi.org/10.1007/s12975-020-00818-9
Xu Z, Shi L, Wang Y et al (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8:420–422. https://doi.org/10.1016/S2213-2600(20)30076-X
Gaba WH, Ahmed D, Al Nuaimi RK et al (2020) Bilateral central retinal vein occlusion in a 40-year-old man with severe coronavirus disease 2019 (COVID-19) pneumonia. Am J Case Rep 21:e9276911-1-e927691-5
Sheth JU, Narayanan R, Goyal J, Goyal V (2020) Retinal vein occlusion in COVID-19: a novel entity. Indian J Ophthalmol 68:2291–2293. https://doi.org/10.4103/ijo.IJO_2380_20
Landecho MF, Yuste JR, Gándara E et al (2021) COVID-19 retinal microangiopathy as an in vivo biomarker of systemic vascular disease? J Intern Med 289:116–120. https://doi.org/10.1111/joim.13156
Abrishami M, Emamverdian Z, Shoeibi N et al (2020) Optical coherence tomography angiography analysis of the retina in patients recovered from COVID-19: a case-control study. Can J Ophthalmol 56:24–30. https://doi.org/10.1016/j.jcjo.2020.11.006
Zapata MÁ, Banderas García S, Sánchez A et al (2020) Retinal microvascular abnormalities in patients after COVID-19 depending on disease severity. Br J Ophthalmol. https://doi.org/10.1136/bjophthalmol-2020-317953
Savastano A, Crincoli E, Savastano M et al (2020) Peripapillary retinal vascular involvement in early post-COVID-19 patients. J Clin Med 9:2895. https://doi.org/10.3390/jcm9092895
Savastano MC, Gambini G, Cozzupoli GM et al (2021) Retinal capillary involvement in early post-COVID-19 patients: a healthy controlled study. Graefe’s Arch Clin Exp Ophthalmol. https://doi.org/10.1007/s00417-020-05070-3
Eltzschig HK, Carmeliet P (2011) Hypoxia and Inflammation. N Engl J Med 364:656–665. https://doi.org/10.1056/nejmra0910283
Burgos-Blasco B, Güemes-Villahoz N, Donate-Lopez J et al (2021) Optic nerve analysis in COVID-19 patients. J Med Virol 93:190–191. https://doi.org/10.1002/jmv.26290
Funding
No funding was required for the study.
Author information
Authors and Affiliations
Contributions
Study design: S.E., B.B.Ş., M.K., S.A., M.E.D.; Study intervention: S.E., B.B.Ş., M.E.D., M.K., U.K., B.D Methodology: S.E., B.B.Ş, M.K., U.K.; Formal Analysis: S.E., S.A., M.K., U.K, B.D., B.B.Ş.; Writing–Original Draft Preparation: S.E., B.B.Ş, M.K., B.D., S.A. Writing–Review and Editing: S.E., U.K., L.H., M.E.D., S.A.; Visualization: S.E, B.D., B.B.Ş., U.K., L.H., S.A.; Supervision: S.E., M.E.D., B.B.Ş., L.H., M.K., U.K., M.E.D.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Consent to participate
Informed consents were obtained from the participants.
Consent to publish
Participants signed informed consent regarding publishing their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Erdem, S., Karahan, M., Ava, S. et al. Evaluation of choroidal thickness in patients who have recovered from COVID-19. Int Ophthalmol 42, 841–846 (2022). https://doi.org/10.1007/s10792-021-02049-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-02049-9