Abstract
Purpose
We aimed to investigate the correlation between the color Doppler imaging (CDI) results and parameters determined by spectral domain optical coherence tomography (SD-OCT) in cases with pseudoexfoliation syndrome (XFS).
Methods
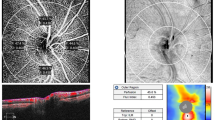
99 participants were included in this prospective study. Retinal nerve fiber layer (RNFL), ganglion cell complex (GCC), optic nerve head (ONH) measurements were recorded. Perfusions of the ophthalmic artery (OA) and central retinal artery (CRA) were determined and resistivity indices (RI) were calculated.
Results
No statistically significant differences were determined between the groups regarding the RNFL and ONH parameters. Only the minimum GCC thickness value was determined to be reduced in XFS group (n = 49) when compared to the controls (n = 50) (p = 0.018). The OA-RI and CRA-RI values of XFS group were significantly higher than the control group (p < 0.001 and p = 0.001). In XFSs, negative correlations were present between OA-RI and the minimum, average, inferior and inferotemporal regions of GCC thickness (r = − 0.448 p = 0.001, r = − 0.275 p = 0.040, r = − 0.295 p = 0.027, r = − 0.304 p = 0.024, respectively), and also between CRA-RI and minimum GCC values (r = − 0.317, p = 0.017). While a significant relationship was present between age and OA-RI and CRA-RI values in controls, no such correlation was present in XFSs.
Conclusions
The vascular resistance increased with age in controls, whereas it was independent of age in XFS group. In XFSs, RIs correlated significantly with certain GCC values, but not with RNFL and ONH parameters. It would be beneficial to follow the XFS with CDI as it provides supportive parameters to GCC in order to recognize early changes in XFS.


Similar content being viewed by others
References
Ritch R, Schlotzer-Schrehardt U, Konstas AG (2003) Why is glaucoma associated with exfoliation syndrome? Prog Retin Eye Res 22(3):253–275
Alay C, Tekeli O, Yanik Odabas O, Calis KF (2019) Evaluation of the retinal nerve fiber layer and ganglion cell complex thicknesses in patients with exfoliation syndrome. Turk J Med Sci 49(1):272–278
Puska PM (2002) Unilateral exfoliation syndrome: conversion to bilateral exfoliation and to glaucoma: a prospective 10-year follow-up study. J Glaucoma 11(6):517–524
Yüksel N, Karabaş VL, Arslan A et al (2001) Ocular hemodynamics in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Ophthalmology 108(6):1043–1049
Goktas S, Sakarya Y, Ozcimen M et al (2014) Choroidal thinning in pseudoexfoliation syndrome detected by enhanced depth imaging optical coherence tomography. Eur J Ophthalmol 24(6):879–884
Prata TS, Rozenbaum I, de Moraes CG et al (2010) Retinal vascular occlusions occur more frequently in the more affected eye in exfoliation syndrome. Eye (Lond) 24(4):658–662
Ocakoglu O, Koyluoglu N, Kayiran A et al (2004) Microvascular blood flow of the optic nerve head and peripapillary retina in unilateral exfoliation syndrome. Acta Ophthalmol Scand 82(1):49–53
Harju M, Vesti E (2001) Blood flow of the optic nerve head and peripapillary retina in exfoliation syndrome with unilateral glaucoma or ocular hypertension. Graefes Arch Clin Exp Ophthalmol 239(4):271–277
Fujimoto J, Swanson E (2016) The development, commercialization, and impact of optical coherence tomography. Invest Ophthalmol Vis Sci 57(9):OCT1–OCT13
Nakatani Y, Higashide T, Ohkubo S et al (2011) Evaluation of macular thickness and peripapillary retinal nerve fiber layer thickness for detection of early glaucoma using spectral domain optical coherence tomography. J Glaucoma 20(4):252–259
Mwanza JC, Durbin MK, Budenz DL et al (2012) Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: comparison with nerve fiber layer and optic nerve head. Ophthalmology 119(6):1151–1158
Jeoung JW, Choi YJ, Park KH, Kim DM (2013) Macular ganglion cell imaging study: glaucoma diagnostic accuracy of spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 54(7):4422–4429
Na JH, Sung KR, Baek S et al (2012) Detection of glaucoma progression by assessment of segmented macular thickness data obtained using spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 53(7):3817–3826
Novak Laus K, Tomic Z, Simic Prskalo M et al (2017) Structure-function relationship of changes in visual field indices with quadrant and average retinal nerve fiber layer thickness in the eyes with exfoliation. Acta Clin Croat 56(4):609–617
Kerrigan-Baumrind LA, Quigley HA, Pease ME et al (2000) Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci 41(3):741–748
Detorakis ET, Achtaropoulos AK, Drakonaki EE, Kozobolis VP (2007) Hemodynamic evaluation of the posterior ciliary circulation in exfoliation syndrome and exfoliation glaucoma. Graefes Arch Clin Exp Ophthalmol 245(4):516–521
Jimenez-Aragon F, Garcia-Martin E, Larrosa-Lopez R et al (2013) Role of color Doppler imaging in early diagnosis and prediction of progression in glaucoma. Biomed Res Int 2013:871689
Dayanir V, Topaloglu A, Ozsunar Y et al (2009) Orbital blood flow parameters in unilateral pseudoexfoliation syndrome. Int Ophthalmol 29(1):27–32
Kocaturk T, Isikligil I, Uz B et al (2016) Ophthalmic artery blood flow parameters in pseudoexfoliation glaucoma. Eur J Ophthalmol 26(2):124–127
European Glaucoma Society Terminology and Guidelines for Glaucoma (2017) 4th edition—part 1 supported by the EGS foundation Br J Ophthalmol 101(4): 1–72 (published online 2017 Mar 21). https://doi.org/10.1136/bjophthalmol-2016-EGSguideline.001
Medeiros FA, Sample PA, Weinreb RN (2003) Corneal thickness measurements and frequency doubling technology perimetry abnormalities in ocular hypertensive eyes. Ophthalmology 110(10):1903–1908
Planiol T, Pourcelot L, Pottier JM, Degiovanni E (1972) Study of carotid circulation by means of ultrasonic methods and thermography. Rev Neurol (Paris) 126:127–141
Vesti E, Kivela T (2000) Exfoliation syndrome and exfoliation glaucoma. Prog Retin Eye Res 19(3):345–368
Schlotzer-Schrehardt U, Naumann GO (2006) Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol 141(5):921–937
Dagdelen K, Dirican E (2018) The assessment of structural changes on optic nerve head and macula in primary open angle glaucoma and ocular hypertension. Int J Ophthalmol 11(10):1631–1637
Eltutar K, Acar F, Kayaarasi Ozturker Z et al (2016) Structural changes in pseudoexfoliation syndrome evaluated with spectral domain optical coherence tomography. Curr Eye Res 41(4):513–520
Demircan S, Yilmaz U, Kucuk E et al (2017) The effect of pseudoexfoliation syndrome on the retinal nerve fiber layer and choroid thickness. Semin Ophthalmol 32(3):341–347
Lisboa R, Paranhos A Jr, Weinreb RN et al (2013) Comparison of different spectral domain OCT scanning protocols for diagnosing preperimetric glaucoma. Invest Ophthalmol Vis Sci 54(5):3417–3425
RAO, Harsha L et al (2010) Comparison of different spectral domain optical coherence tomography scanning areas for glaucoma diagnosis. Ophthalmology 117.9:1692–1699. e1
Mwanza JC, Oakley JD, Budenz DL, Anderson DR (2011) Cirrus optical coherence tomography normative database study G. Ability of cirrus HD-OCT optic nerve head parameters to discriminate normal from glaucomatous eyes. Ophthalmology 118(2):241–248.e1
Puska P, Harju M, Liebkind R (2004) Peripapillary atrophy in the unilateral exfoliation syndrome. Graefes Arch Clin Exp Ophthalmol 242(4):301–305
Özbaş M, Kuğu S, Yılmaz İ et al (2013) Psödoeksfoliasyon Sendromlu Gözlerin Heidelberg Retinal Tomografi İle Değerlendirilmesi. Med J Bakırköy 9(3):126–130
Seong M, Sung KR, Choi EH et al (2010) Macular and peripapillary retinal nerve fiber layer measurements by spectral domain optical coherence tomography in normal-tension glaucoma. Invest Ophthalmol Vis Sci 51(3):1446–1452
Kim NR, Lee ES, Seong GJ et al (2010) Structure-function relationship and diagnostic value of macular ganglion cell complex measurement using Fourier-domain OCT in glaucoma. Invest Ophthalmol Vis Sci 51(9):4646–4651
Aydin D, Kusbeci T, Uzunel UD et al (2016) Evaluation of retinal nerve fiber layer and ganglion cell complex thickness in unilateral exfoliation syndrome using optical coherence tomography. J Glaucoma 25(6):523–527
Galassi F, Sodi A, Ucci F et al (2003) Ocular hemodynamics and glaucoma prognosis: a color Doppler imaging study. Arch Ophthalmol 121(12):1711–1715
Magureanu M, Stanila A, Bunescu LV, Armeanu C (2016) Color Doppler imaging of the retrobulbar circulation in progressive glaucoma optic neuropathy. Rom J Ophthalmol 60(4):237–248
Martinez A, Sanchez M (2008) Retrobulbar hemodynamic parameters in pseudoexfoliation syndrome and pseudoexfoliative glaucoma. Graefes Arch Clin Exp Ophthalmol 246(9):1341–1349
Lam AK, Chan ST, Chan H, Chan B (2003) The effect of age on ocular blood supply determined by pulsatile ocular blood flow and color Doppler ultrasonography. Optom Vis Sci 80(4):305–311
Quigley HA, Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90(3):262–267
Tham YC, Li X, Wong TY et al (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 121(11):2081–2090
Shiga Y, Aizawa N, Tsuda S et al (2018) Preperimetric glaucoma prospective study (PPGPS): predicting visual field progression with basal optic nerve head blood flow in normotensive PPG eyes. Trans Vis Sci Technol 7(1):11
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdullayev, Ö.K., Kocatürk, T., Abdullayev, O. et al. Correlation of optical coherence tomography and Doppler ultrasonography findings in pseudoexfoliation syndrome. Int Ophthalmol 42, 549–558 (2022). https://doi.org/10.1007/s10792-021-02026-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-02026-2